Abstract
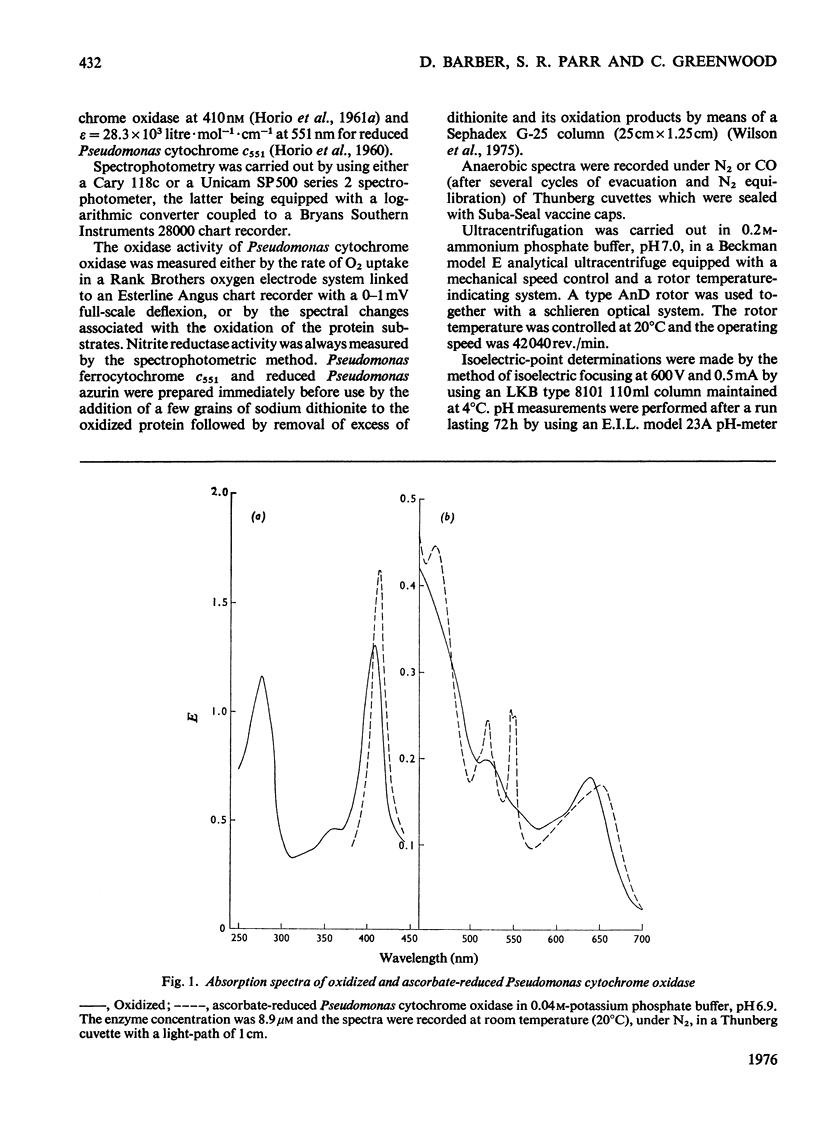
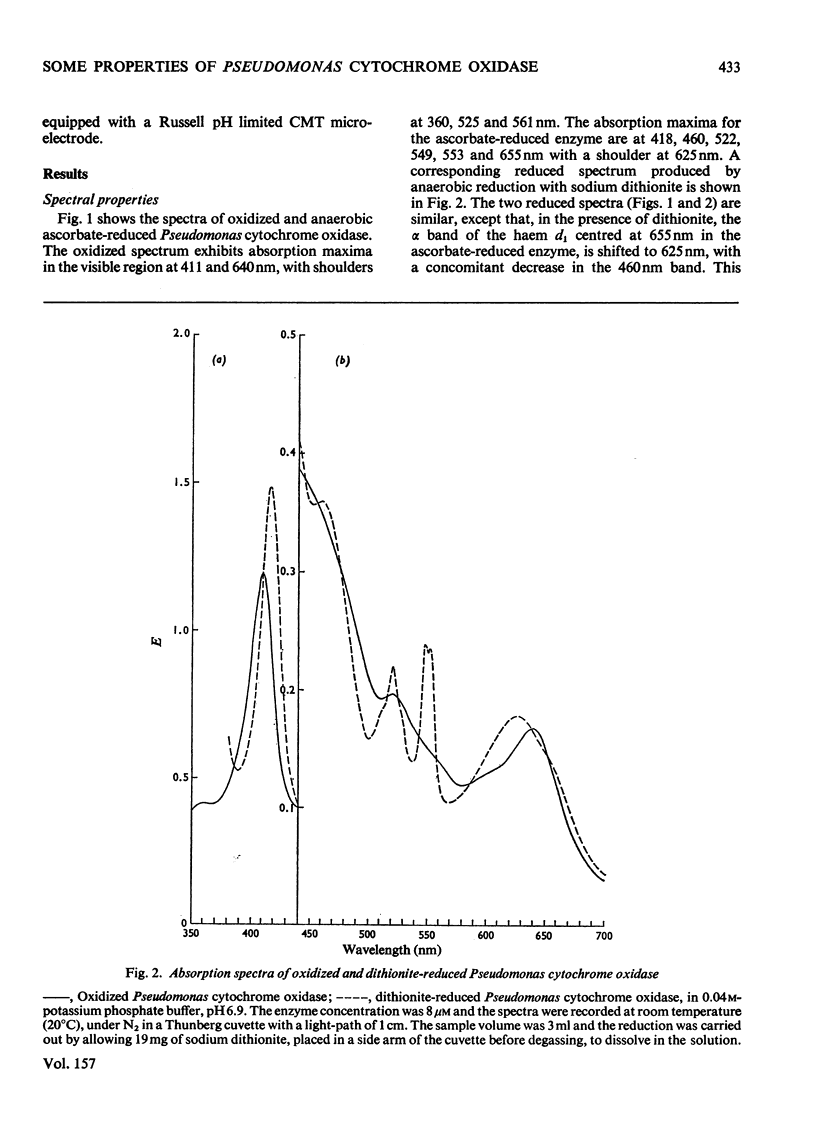
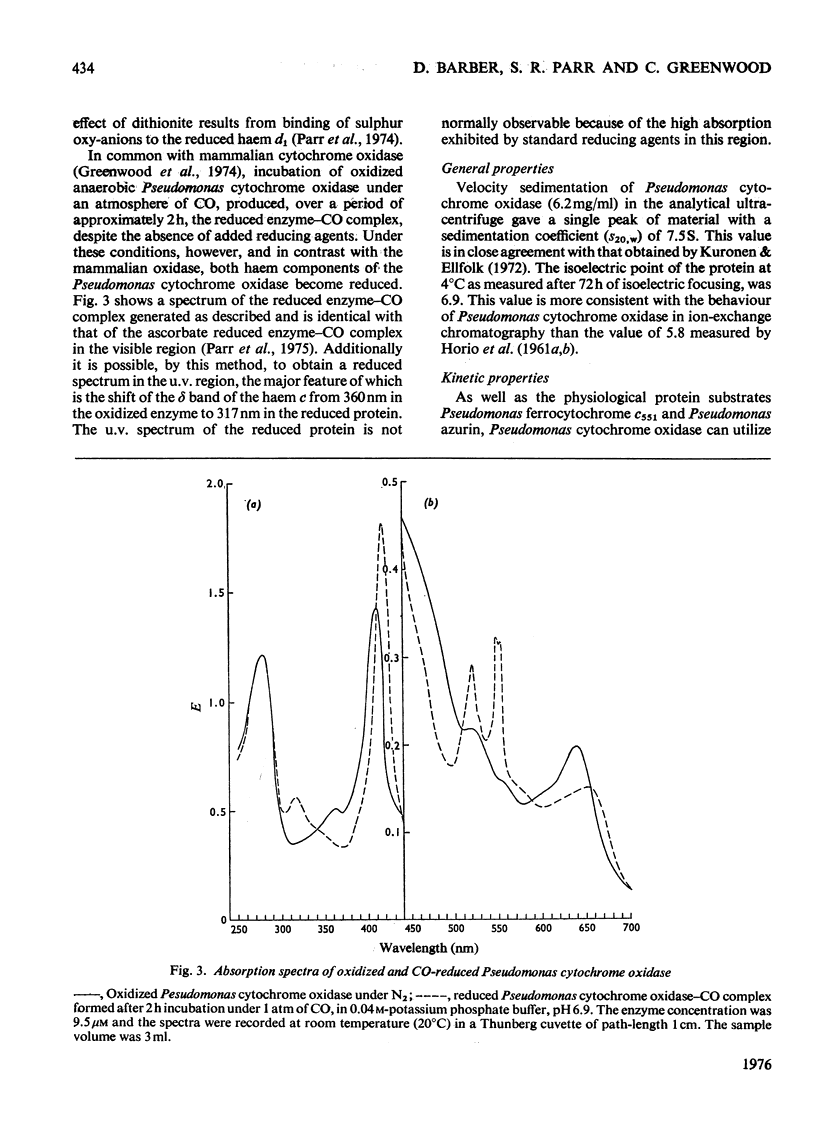
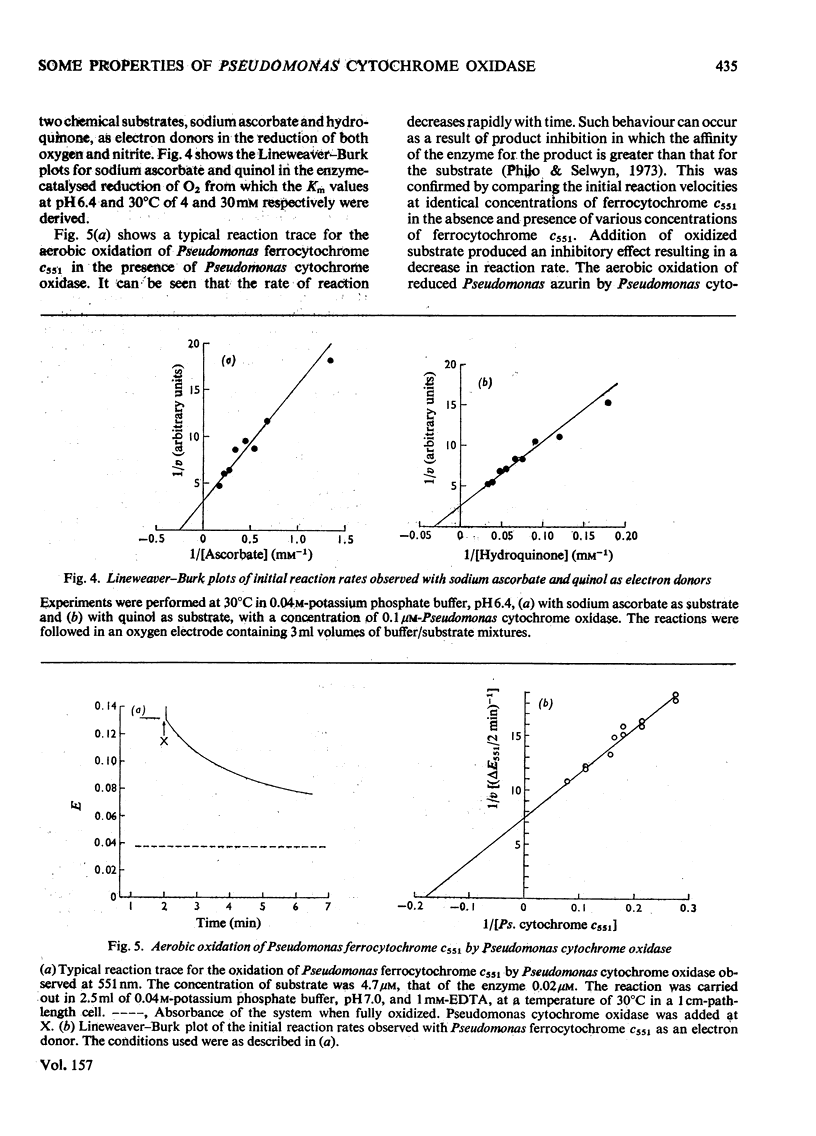
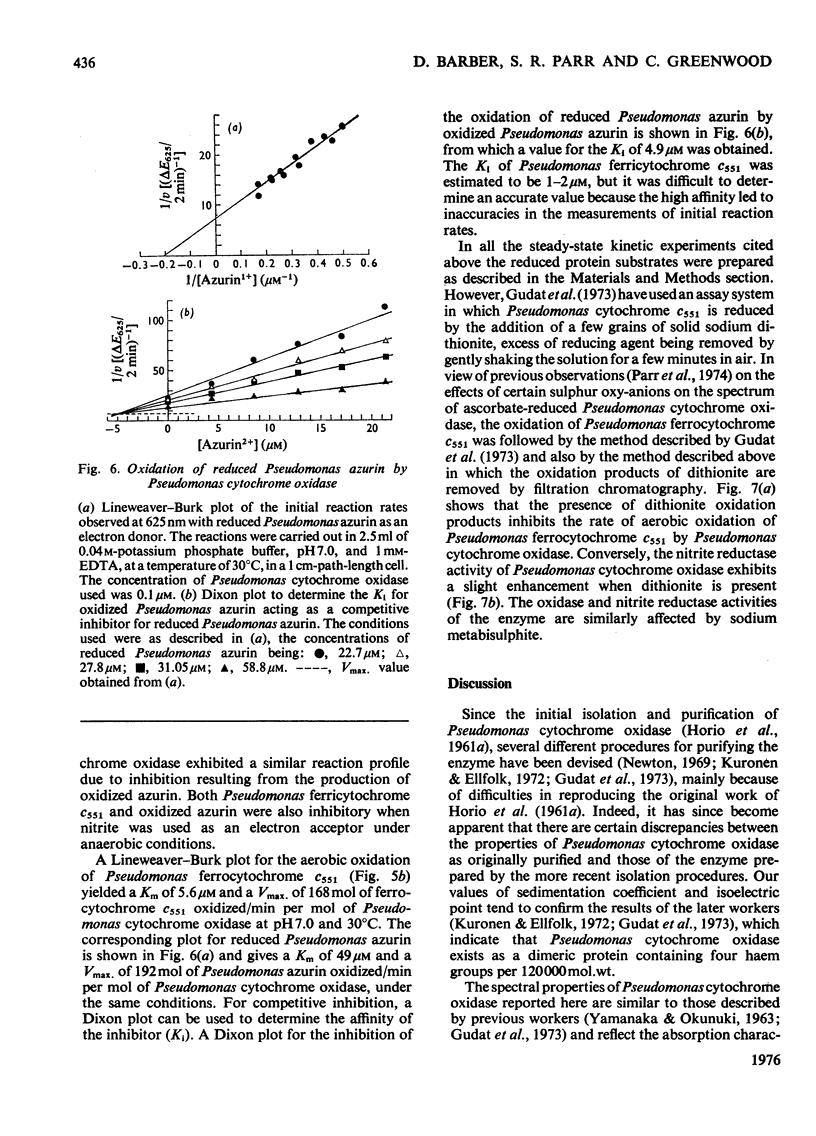
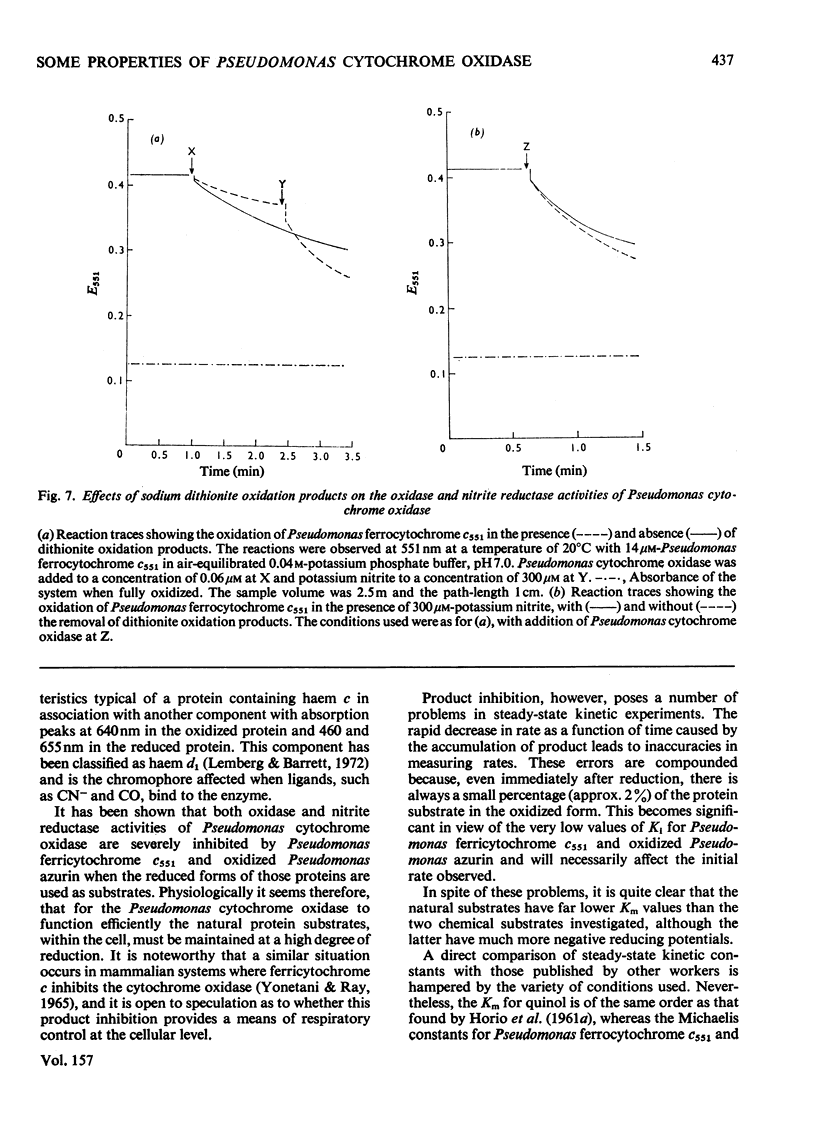
Some spectra of Pseudomonas cytochrome oxidase are reported, both for comparison with those of other workers and to illustrate the differences between the ascorbate- and dithionite-reduced forms of the enzyme. A spectrum of the reduced enzyme-CO complex, prepared in the absence of added reductants by incubation under CO, is also included. Ultracentrifugation studies yielded a value for the sedimentation coefficient (s20,w) of 7.5S, and an isoelectric point of pH6.9 was determined by isoelectric focusing. Steady-state kinetic constants of the electron donors, quinol, sodium ascorbate, reduced Pseudomonas azurin and Pseudomonas ferrocytochrome c551 were investigated giving Km values of 30mM, 4mM, 49muM and 5.6muM respectively. The two protein substrates were observed to be subject to product inhibition and the Ki for oxidized Pseudomonas azurin was evaluated at 4.9muM. Steady-state kinetics were also used to investigate the effects of the oxidation products of dithionite on the oxidase and nitrite reductase activities of Pseudomonas cytochrome oxidase. These experiments showed that whereas the oxidase activity was inhibited, the nitrite reductase activity was slightly enhanced.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brill A. S., Bryce G. F., Maria H. J. Optical and magnetic properties of Pseudomonas azurins. Biochim Biophys Acta. 1968 Feb 19;154(2):342–351. doi: 10.1016/0005-2795(68)90048-2. [DOI] [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudat J. C., Singh J., Wharton D. C. Cytochrome oxidase from Pseudomonas aeruginosa. I. Purification and some properties. Biochim Biophys Acta. 1973 Feb 22;292(2):376–390. doi: 10.1016/0005-2728(73)90044-3. [DOI] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., MATSUBARA H., KUSAI K., NAKAI M., OKUNUKI K. High purification and properties of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1958 Aug;29(2):297–302. doi: 10.1016/0006-3002(58)90188-4. [DOI] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., SASAGAWA M., KUSAI K., NAKAI M., OKUNUKI K. Preparation of crystalline Pseudomonas cvtochrome c-551 and its general properties. Biochem J. 1960 Oct;77:194–201. doi: 10.1042/bj0770194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., YAMANAKA T., MATSUBARA H., OKUNUKI K. Purification and properties of cytochrome oxidase from Pseudomonas aeruginosa. J Biol Chem. 1961 Mar;236:944–951. [PubMed] [Google Scholar]
- Kuronen T., Ellfolk N. A new purification procedure and molecular properties of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1972 Sep 20;275(3):308–318. doi: 10.1016/0005-2728(72)90212-5. [DOI] [PubMed] [Google Scholar]
- Kuronen T., Saraste M., Ellfork N. The subunit structure of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1975 May 30;393(1):48–54. doi: 10.1016/0005-2795(75)90215-9. [DOI] [PubMed] [Google Scholar]
- Newton N. The two-haem nitrite reductase of Micrococcus denitrificans. Biochim Biophys Acta. 1969;185(2):316–331. doi: 10.1016/0005-2744(69)90425-2. [DOI] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C. A purification procedure for the soluble cytochrome oxidase and some other respiratory proteins from Pseudomonas aeruginosa. Biochem J. 1976 Aug 1;157(2):423–430. doi: 10.1042/bj1570423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr S. R., Wilson M. T., Greenwood C. The reaction of Pseudomonas aeruginosa cytochrome c oxidase with carbon monoxide. Biochem J. 1975 Oct;151(1):51–59. doi: 10.1042/bj1510051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr S. R., Wilson M. T., Greenwood C. The reaction of Pseudomonas aeurginosa cytochrome c oxidase with sodium metabisulphite. Biochem J. 1974 Apr;139(1):273–276. doi: 10.1042/bj1390273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo R. D., Selwyn M. J. Use of progress curves to investigate product inhibition in enzyme-catalysed reactions. Application to the soluble mitochondrial adenosine triphosphatase. Biochem J. 1973 Nov;135(3):525–530. doi: 10.1042/bj1350525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. T., Greenwood C., Brunori M., Antonini E. Electron transfer between azurin and cytochrone c-551 from Pseudomonas aeruginosa. Biochem J. 1975 Mar;145(3):449–457. doi: 10.1042/bj1450449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMANAKA T., OKUNUKI K. Crystalline Pseudomonas cytochrome oxidase. II. Spectral properties of the enzyme. Biochim Biophys Acta. 1963 Mar 12;67:394–406. doi: 10.1016/0006-3002(63)91845-6. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OTA A., OKUNUKI K. A nitrite reducing system reconstructed with purified cytochrome components of Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 Oct 28;53:294–308. doi: 10.1016/0006-3002(61)90442-5. [DOI] [PubMed] [Google Scholar]
- YONETANI T., RAY G. S. STUDIES ON CYTOCHROME OXIDASE. VI. KINETICS OF THE AEROBIC OXIDATION OF FERROCYTOCHROME C BY CYTOCHROME OXIDASE. J Biol Chem. 1965 Aug;240:3392–3398. [PubMed] [Google Scholar]


