Abstract
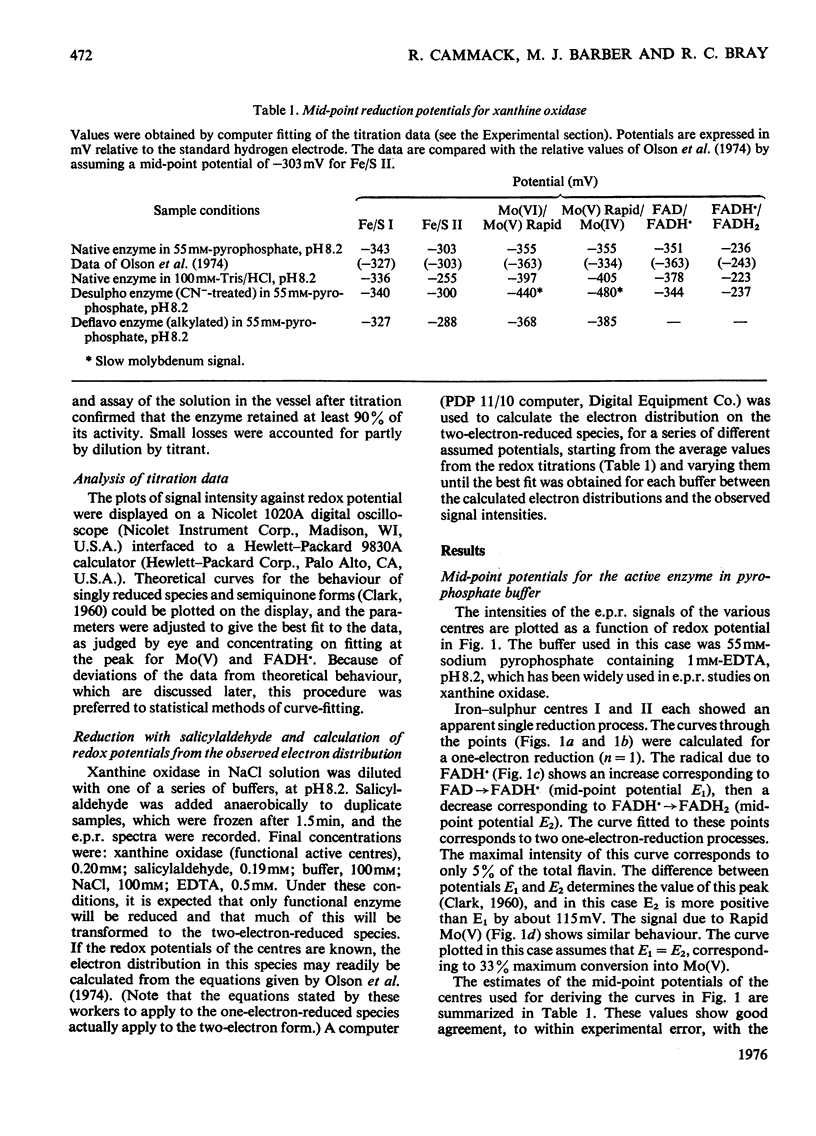
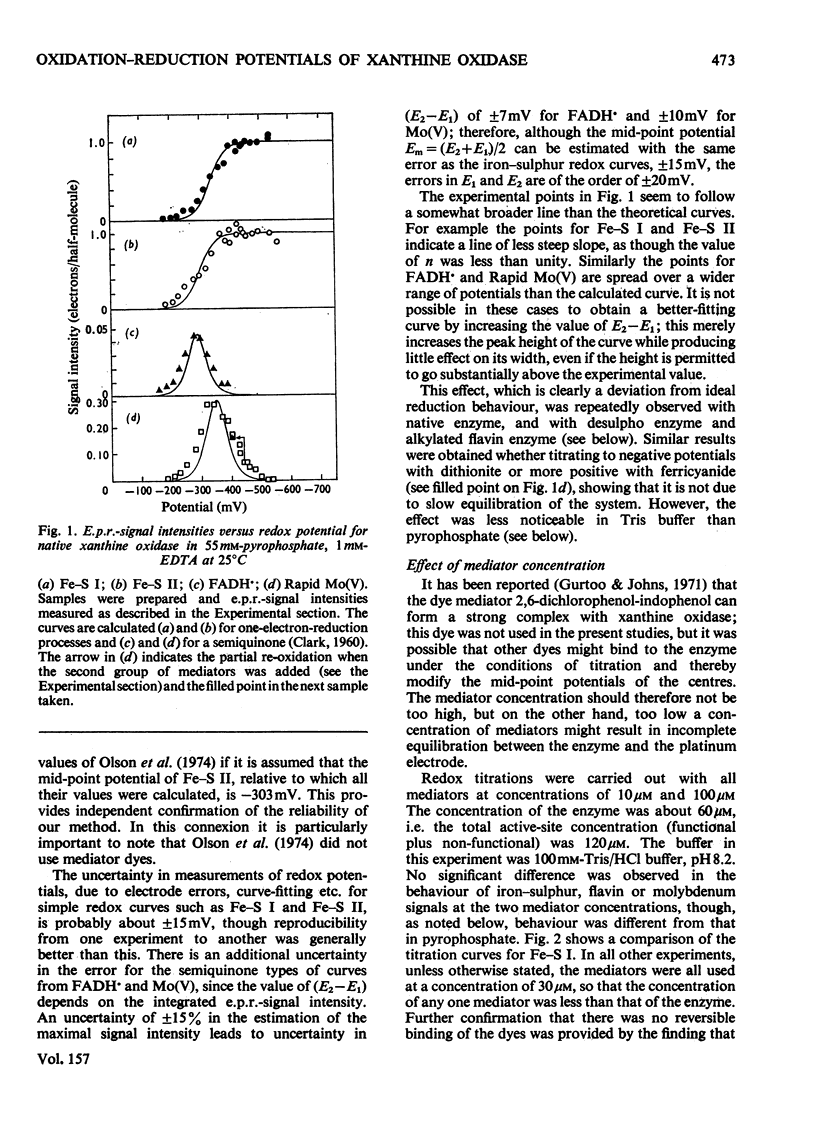
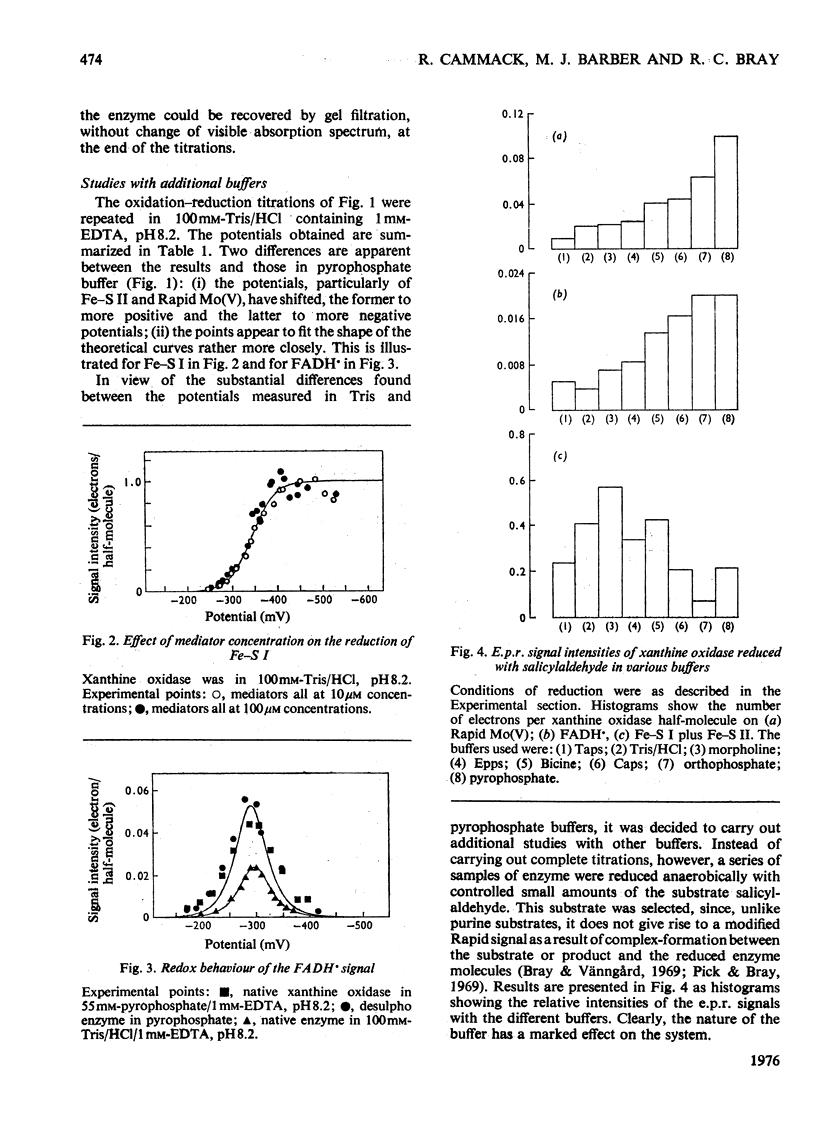
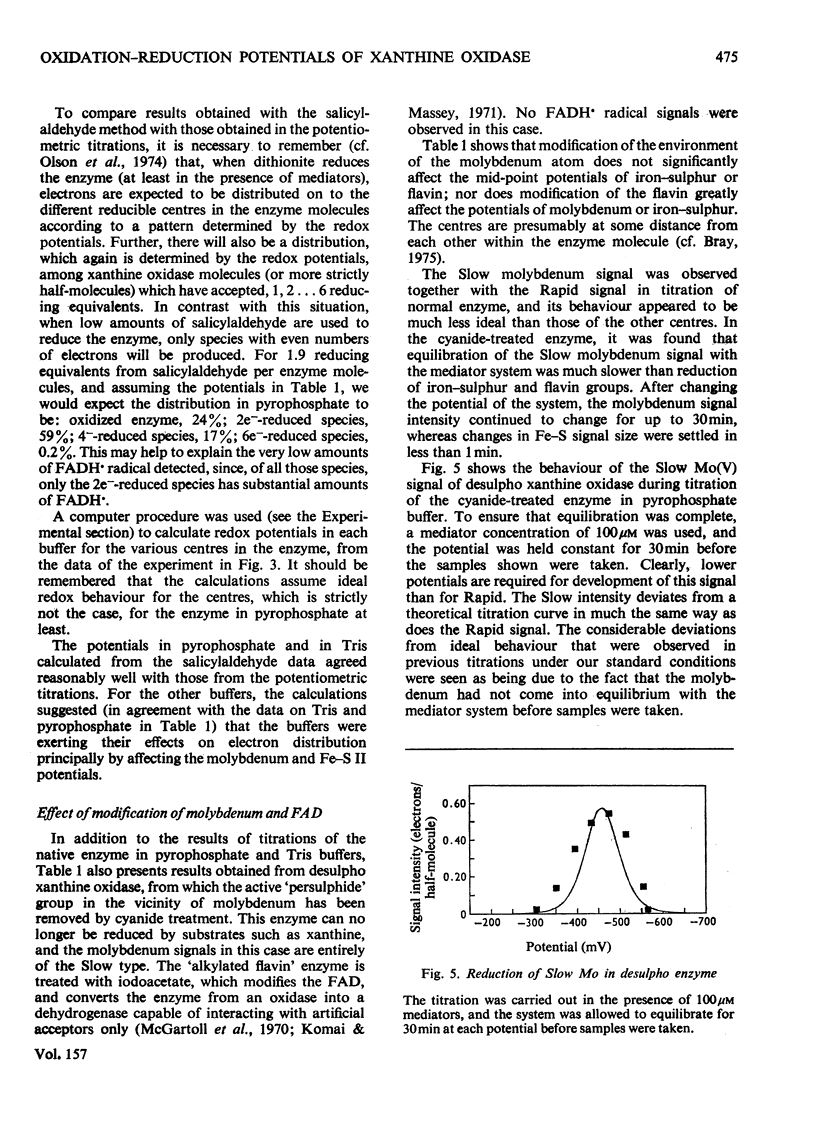
1. The mid-point reduction potentials of the various groups in xanthine oxidase from bovine milk were determined by potentiometric titration with dithionite in the presence of dye mediators, removing samples for quantification of the reduced species by e.p.r. (electron-paramagnetic-resonance) spectroscopy. The values obtained for the functional enzyme in pyrophosphate buffer, pH8.2, are: Fe/S centre I, -343 +/- 15mV; Fe/S II, -303 +/- 15mV; FAD/FADH-; -351 +/- 20mV; FADH/FADH2, -236 +/-mV; Mo(VI)/Mo(V) (Rapid), -355 +/- 20mV; Mo(V) (Rapid)/Mo(IV), -355 +/- 20mV. 2. Behaviour of the functional enzyme is essentially ideal in Tris but less so in pyrophosphate. In Tris, the potential for Mo(VI)/Mo(V) (Rapid) is lowered relative to that in pyrophosphate, but the potential for Fe/S II is raised. The influence of buffer on the potentials was investigated by partial-reduction experiments with six other buffers. 3. Conversion of the enzyme with cyanide into the non-functional form, which gives the Slow molybdenum signal, or alkylation of FAD, has little effect on the mid-point potentials of the other centres. The potentials associated with the Slow signal are: Mo(VI)/Mo(V) (Slow), -440 +/- 25mV; Mo(V) (Slow)/Mo(IV), -480 +/- 25 mV. This signal exhibits very sluggish equilibration with the mediator system. 4. The deviations from ideal behaviour are discussed in terms of possible binding of buffer ions or anti-co-operative interactions amongst the redox centres.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAY R. C., MALMSTROM B. G., VANNGARD T. The chemistry of xanthine oxidase. Electron-spin resonance of xanthine oxidase solutions. Biochem J. 1959 Sep;73:193–197. doi: 10.1042/bj0730193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M. J., Bray R. C., Lowe D. J., Coughlan M. P. Studies by electron-paramagnetic-resonance spectroscopy and stopped-flow spectrophotometry on the mechanism of action of turkey liver xanthine dehydrogenase. Biochem J. 1976 Feb 1;153(2):297–307. doi: 10.1042/bj1530297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman B. G., Tollin G. Flavine-protein interactions in flavoenzymes. Thermodynamics and kinetics of reduction of Azotobacter flavodoxin. Biochemistry. 1972 Dec 5;11(25):4755–4759. doi: 10.1021/bi00775a019. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Vänngård T. "Rapidly appearing" molybdenum electron-paramagnetic-resonance signals from reduced xanthine oxidase. Biochem J. 1969 Oct;114(4):725–734. doi: 10.1042/bj1140725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Watts D. C. The chemistry of xanthine oxidase. Reaction with iodoacetamide. Biochem J. 1966 Jan;98(1):142–148. doi: 10.1042/bj0980142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper R. D., Ingraham L. L. A potentiometric study of the flavin semiquinone equilibrium. Arch Biochem Biophys. 1968 Jun;125(3):802–808. doi: 10.1016/0003-9861(68)90517-1. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Edmondson D., Massey V., Palmer G., Beacham L. M., 3rd, Elion G. B. The resolution of active and inactive xanthine oxidase by affinity chromatography. J Biol Chem. 1972 Mar 10;247(5):1597–1604. [PubMed] [Google Scholar]
- Evans M. C., Reeves S. G., Cammack R. Determination of the oxidation-reduction potential of the bound iron-sulphur proteins of the primary electron acceptor complex of photosystem I in spinach chloroplasts. FEBS Lett. 1974 Dec 1;49(1):111–114. doi: 10.1016/0014-5793(74)80644-7. [DOI] [PubMed] [Google Scholar]
- Gurtoo H. L., Johns D. G. On the interaction of the electron acceptor 2,6-dichlorophenolindophenol with bovine milk xanthine oxidase. J Biol Chem. 1971 Jan 25;246(2):286–293. [PubMed] [Google Scholar]
- Hall D. O., Rao K. K., Cammack R. The iron-sulphur proteins: structure, function and evolution of a ubiquitous group of proteins. Sci Prog. 1975 Summer;62(246):285–317. [PubMed] [Google Scholar]
- Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem J. 1970 Mar;116(5):851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai H., Massey V., Palmer G. The preparation and properties of deflavo xanthine oxidase. J Biol Chem. 1969 Apr 10;244(7):1692–1700. [PubMed] [Google Scholar]
- Lowe D. J., Lynden-Bell R. M., Bray R. C. Spin-spin interaction between molybdenum and one of the iron-sulphur systems of xanthine oxidase and its relevance to the enzymic mechanism. Biochem J. 1972 Nov;130(1):239–249. doi: 10.1042/bj1300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem. 1970 Dec 25;245(24):6595–6598. [PubMed] [Google Scholar]
- Mayhew S. G., Foust G. P., Massey V. Oxidation-reduction properties of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem. 1969 Feb 10;244(3):803–810. [PubMed] [Google Scholar]
- McGartoll M. A., Pick F. M., Swann J. C., Bray R. C. Properties of xanthine oxidase preparations dependent on the proportions of active and inactivated enzyme. Biochim Biophys Acta. 1970 Sep 16;212(3):523–526. doi: 10.1016/0005-2744(70)90264-0. [DOI] [PubMed] [Google Scholar]
- Olson J. S., Ballou D. P., Palmer G., Massey V. The mechanism of action of xanthine oxidase. J Biol Chem. 1974 Jul 25;249(14):4363–4382. [PubMed] [Google Scholar]
- Onishi T. Mechanism of electron transport and energy conservation in the site I region of the respiratory chain. Biochim Biophys Acta. 1973 Dec 7;301(2):105–128. [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. Heterogeneity of paramagnetic species in two iron-sulfur proteins: Clostridium pasteurianum ferredoxin and milk xanthine oxidase. Biochem Biophys Res Commun. 1969 Aug 7;36(3):337–344. doi: 10.1016/0006-291x(69)90569-5. [DOI] [PubMed] [Google Scholar]
- PALMER G., BRAY R. C., BEINERT H. DIRECT STUDIES ON THE ELECTRON TRANSFER SEQUENCE IN XANTHINE OXIDASE BY ELECTRON PARAMAGNETIC RESONANCE SPECTROSCOPY. I. TECHNIQUES AND DESCRIPTION OF SPECTRA. J Biol Chem. 1964 Aug;239:2657–2666. [PubMed] [Google Scholar]
- Palmer G., Massey V. Electron paramagnetic resonance and circular dichroism studies on milk xanthine oxidase. J Biol Chem. 1969 May 25;244(10):2614–2620. [PubMed] [Google Scholar]
- Pick F. M., Bray R. C. Complex-formation between reduced xanthine oxidase and purine substrates demonstrated by electron paramagnetic resonance. Biochem J. 1969 Oct;114(4):735–742. doi: 10.1042/bj1140735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann J. C., Bray R. C. Multiple phases in the reduction of xanthine oxidase by substrates. Eur J Biochem. 1972 Apr 11;26(3):407–415. doi: 10.1111/j.1432-1033.1972.tb01781.x. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Erecinska M., Dutton P. L., Tsudzuki T. The oxidation-reduction potentials of the iron-sulfur proteins in mitochondria. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1273–1278. doi: 10.1016/0006-291x(70)90225-1. [DOI] [PubMed] [Google Scholar]


