Abstract
A tetrameric recombinant major histocompatibility complex (MHC) class II-peptide complex was used to quantitate human immunodeficiency virus type 1 (HIV-1) envelope (Env)-specific CD4+ T cells in vaccinated and in simian/human immunodeficiency virus (SHIV)-infected rhesus monkeys. A rhesus monkey MHC class II DR molecule, Mamu-DR*W201, and an HIV-1 Env peptide (p46) were employed to construct this tetrameric complex. A p46-specific proliferative response was seen in sorted, tetramer-binding, but not nonbinding, CD4+ T cells, directly demonstrating that this response was mediated by the epitope-specific lymphocytes. Although staining of whole blood from 10 SHIV-infected Mamu-DR*W201+ rhesus monkeys failed to demonstrate tetramer-binding CD4+ T cells (<0.02%), p46-stimulated peripheral blood mononuclear cells (PBMCs) from 9 of these 10 monkeys had detectable p46 tetramer-binding cells, comprising 0.5 to 15.2% of the CD4+ T cells. p46-stimulated PBMCs from 7 of 10 Mamu-DR*W201+ monkeys vaccinated with a recombinant canarypox virus–HIV-1 env construct also demonstrated p46 tetramer-binding cells, comprising 0.9 to 7.2% of the CD4+ T cells. Thus, Env p46-specific CD4+ T cells can be detected by tetrameric Mamu-DR*W201–p46 complex staining of PBMCs in both SHIV-infected and vaccinated rhesus monkeys. These epitope-specific cell populations appear to be present in peripheral blood at a very low frequency.
CD4+ T lymphocytes play a number of essential roles in the immune responses that contain viral replication. Their help is needed for antibody production by B cells (22), CD8+ cytotoxic T-lymphocyte (CTL) function is dependent on their activity (19, 27, 31, 33), they secrete antiviral cytokines, and they have sometimes been shown to have antiviral cytotoxic effector function (4). Yet, because of technical difficulties associated with studying these cells in vivo, there are many basic issues concerning virus-specific CD4+ T-lymphocyte biology that remain poorly understood. In particular, it has proven difficult to quantitate epitope-specific CD4+ T-lymphocyte populations in the biologically important settings of infection and disease.
Attention is now being focused on the central importance of virus-specific CD4+ T-lymphocytes in containing human immunodeficiency virus type 1 (HIV-1) replication (28, 29). It has long been appreciated that HIV-1 infection is associated clinically with a gradual decline in CD4+ T-lymphocyte numbers and an impairment of CD4+ T-lymphocyte function (3, 12, 20). Recent studies have shown that control of viral replication in vivo is associated with vigorous HIV-1-specific CD4+ T-lymphocyte proliferative responses (28). It has been suggested that HIV-1-specific CD4+ T lymphocytes may be critically important for vaccine-elicited protective immunity against HIV-1 infection.
Nonhuman primate models have provided powerful tools for exploring AIDS pathogenesis (16, 17) and HIV-1 vaccine strategies (18, 30). Simian/human immunodeficiency virus (SHIV)-infected rhesus monkeys have proven particularly useful for these studies, since they allow the in vivo evaluation in higher primates of immune responses to lentiviruses that express HIV-1 envelope (Env) glycoproteins. The SHIV models also allow the evaluation of infections which result in CD4+ T-lymphocyte loss and AIDS or infections that are nonpathogenic (25, 26).
We have recently begun characterizing HIV-1 Env-specific CD4+ T-lymphocyte responses in SHIV-infected and HIV-1 Env-vaccinated rhesus monkeys. In the course of these studies, Env-specific CD4+ T-lymphocyte lines have been generated, allowing the definition of several CD4+ T-lymphocyte Env epitopes and their restricting major histocompatibility complex (MHC) class II molecules (14, 15). In the present study, we have used one of these MHC class II molecules and its associated CD4+ T-lymphocyte peptide to construct an MHC class II/peptide tetrameric staining reagent for characterizing Env epitope-specific CD4+ T-lymphocyte populations in rhesus monkeys.
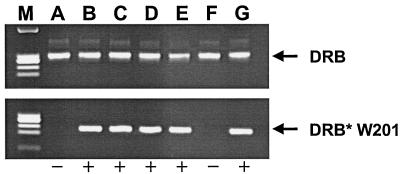
For identifying Mamu-DR*W201-positive rhesus monkeys, PCR, direct sequencing, and a functional proliferation assay were used. DNA from monkey lymphocytes was amplified with allele-specific primer pairs, and the resulting PCR products were directly sequenced. Genomic DNA was amplified by PCR, using the allele-specific 3′ primer DRB*W201/R (5′-CCGCTCCAGGATGTCCTCCC-3′) in conjunction with a 5′ primer for the conserved region of Mamu-DRB, 5′MDRB (5′-GCCTCGAGTGTCCCCCCAGCACGTTTC-3′). One additional PCR, using primers specific for a conserved MHC class II sequence (based on the macaque homologue of HLA-DRB3), was included as a positive control. Primers 5′MDRB (5′-GCC TCG AGT GTC CCC CCA GCA CGT TTC-3′) and 3′MDRB (5′-GCA AGC TTT CAC CTC GCC GCT G-3′) were each used at a final concentration of 680 nM. PCR products were analyzed by electrophoresis in 2% agarose gels (Fig. 1). DNA sequence analysis was then performed on all positive PCR products to confirm nucleotide sequence identity with the published Mamu-DRB*W201 prototype sequence (13). Finally, B-lymphoblastoid cell lines (B-LCL) from monkeys selected as positive for Mamu-DRB*W201 were assessed, following pulsing with the peptide p46, for their ability to induce proliferation of a p46-specific, Mamu-DR*W201-restricted CD4+ T-cell line. Those monkeys whose p46-pulsed B-LCL induced proliferation of this cell line were deemed Mamu-DR*W201 positive. By PCR amplification, sequencing, and functional criteria, we found that 47 of 133 (35%) rhesus monkeys from five different colonies expressed Mamu-DR*W201.
FIG. 1.
Identification of Mamu-DRB*W201 alleles by PCR with allele-specific primers. Shown is the gel electrophoresis profile of PCR products amplified with allele-specific primers from DNA extracted from PBMCs of various rhesus monkeys (A to G). DRB, PCR products amplified by primers specific for a conserved region of the Mamu-DRB sequences; DRB*W201, PCR product amplified by Mamu-DRB*W201 allele-specific primers; −, Mamu-DR*W201 was not amplified from DNA; +, Mamu-DR*W201 was amplified from DNA; M, DNA standard (HaeIII digest of φ174 DNA).
To generate the tetrameric Mamu-DR*W201–p46 complex, Mamu-DR*W201 covalently bound to HIV Env p46 peptide was expressed in a Drosophila melanogaster cell protein expression system. DNAs coding for the soluble domains of Mamu-DRA and Mamu-DRB*W201 were amplified by PCR with the 5′ primer MDRA0101/F (5′-GATTATGGTACCAGGATGGCCGAAAGTGGAGTCCCC 3′) and the 3′ primer MDRA0101/R (5′-ACGTTCACGCGTGTTCTCTGTAGTCTCTGGGAG-3′) for Mamu-DRA or with the 5′ primer W201/F (5′-GATGATGGTACCAGCATGGTGTGTCTGAAG-3′) and the 3′ primer W201-BTtag/R (5′-GGACGTACGCGTTCAACGATGATTCCACACCATTTTCTGTGCATCCAG AATATGATGCAGGGATCCCATCTTGCTCTGTGCAGA- 3′) for Mamu-DRB*W201. The 3′ primer W201-BTtag/R encoded a biotinylation tag (in boldface) followed by a stop codon. Mamu-DRα and Mamu-DRB*W201 plasmid DNAs were employed as templates for these reactions (13). Both PCR products were digested with KpnI and MluI and subcloned into the expression plasmid pMT/V5-His A (Invitrogen, Carlsbad, Calif.). Mamu-DRA was cloned into the pMT/V5-His A vector in frame so that the histidine tag was attached to the C terminus to facilitate purification. The covalently bound peptide YKYKVVKIEPLGV (HIV Env residues 484 to 496) (p46) (15) was designed by making a pair of long primers overlapping by 21 nucleotides. Primers P46/F (5′-GTGCTGAGCTCCCCACTGGCTGTGGCTGGGGACACCTATAAGTATAAGGTGGTGAAGATCGAGCCACTGGGAGTGGGAGGTGGTGGCTCACTAGTG-3′) and P46/R (5′-TGACTTA GCATGCCCCAGGAAACGTGGGGACCCACCTCCTCCAGAGCCCCGTGGCACTAGTGAGCCACCACCTCC-3′) were used for a five-cycle PCR at a final concentration of 10 mM each in a 100-μl reaction volume consisting of 60 mM Tris, 2 mM MgCl2, 15 mM ammonium sulfate, 2 mM deoxynucleoside triphosphates (0.5 mM each), and 5 U of Taq polymerase, pH 8.5. The elongated PCR product was purified and digested by SacI and SphI (sites are underlined in the primer sequences) and subcloned into the pMT/Mamu-DRB*W201-BT plasmid. The A- and B-chain constructs were cotransfected into Schneider (S2) cells by calcium phosphate precipitation together with pCoHYGRO (Invitrogen), which contains a hygromycin resistance gene. Stable cell lines were derived by culturing, under selection conditions, in complete DES Expression medium (Invitrogen) containing 300 μg of hygromycin/ml, and the resulting lines were expanded in DES serum-free medium (Invitrogen). Protein production was induced by adding 1 mM copper sulfate and incubating for 3 days. Culture supernatants were concentrated, and the histidine-tagged Mamu-DR*W201 covalently bound to p46 was purified by Ni-agarose affinity chromatography (Qiagen, Chatsworth, Calif.). Purified Mamu-DR*W201–p46 monomers were biotinylated enzymatically with the BirA enzyme (Avidity, Denver, Colo.), following the manufacturer's instructions. The efficiency of biotinylation was between 75 and 95%. A Superdex HR200 column (Amersham Pharmacia Biotech, Piscataway, N.J.) was used to remove the free biotin from the monomers. To generate Mamu-DR*W201–p46 tetramers, the biotinylated monomers were mixed with phycoerythrin (PE)-labeled streptavidin (Prozyme, San Leandro, Calif.) at a molar ratio of 4:1.
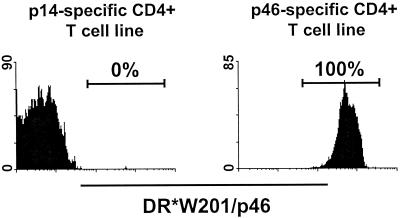
We first characterized the lymphocyte binding specificity of the tetrameric Mamu-DR*W201–p46 complex. The tetramer was evaluated for its ability to bind to two HIV-1 Env-specific CD4+ T-cell lines generated from peripheral blood mononuclear cells (PBMCs) of a Mamu-DR*W201+ rhesus monkey, one specific for p46 and the other specific for the p14 Env peptide. As shown in Fig. 2, the p46 tetramer bound to the p46-specific but not the p14-specific CD4+ T-cell line. Thus, the p46 tetramer exhibited the predicted binding specificity.
FIG. 2.
Tetrameric Mamu-DR*W201–p46 complex binds specifically to a p46-specific CD4+ T-cell line generated from PBMCs of a Mamu-DR*W201+ rhesus monkey. The p46 tetramer was used to stain two HIV-1 Env-specific T-cell lines generated from PBMCs of the same rhesus monkey, one specific for p46 and the other specific for the p14 Env peptide. Flow-cytometric analysis was performed on gated CD4+ CD3+ T cells.
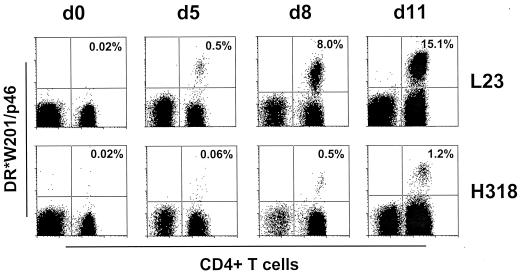
We then attempted to enumerate the p46-specific CD4+ T-lymphocyte subpopulations in PBMCs of whole-blood specimens from 10 SHIV-infected Mamu-DR*W201+ rhesus monkeys by using the tetrameric Mamu-DR*W201–p46 complex. Whole-blood specimens from Mamu-DR*W201+ SHIV-infected monkeys were stained with PE-coupled Mamu-DR*W201–p46 tetramer and evaluated by flow cytometry with gating on CD4+ CD3+ cells. More than 150,000 CD4+ T cells from each sample were analyzed. In each sample, the proportion of p46-specific cells in these CD4+ T-lymphocyte populations was less than 0.02%, the limit of detection of this assay (Fig. 3 and data not shown). However, after 5 days of in vitro stimulation with p46, the p46 tetramer-binding CD4+ T cells in the PBMCs had expanded to a level at which they could be detected. The proportion of p46 tetramer-binding CD4+ T cells in PBMC of monkey L23 expanded from a level of less than 0.02 to 15.1% of all CD4+ T cells after 11 days of p46 stimulation in vitro (Fig. 3). A similar kinetics of p46 tetramer-binding CD4+ T-cell expansion was observed in PBMCs of monkey H318, with the tetramer-binding cells reaching a level of 1.2% of the CD4+ T cells (Fig. 3). This expansion during the period of culture was not simply due to a loss of tetramer-negative cells, because the tetramer-positive cells increased in number relative to the other cells while the total numbers of CD4+ T cells did not significantly decrease (data not shown).
FIG. 3.
Kinetics of in vitro expansion of tetrameric Mamu-DR*W201–p46 complex-binding CD4+ T cells in p46-stimulated PBMCs of SHIV-infected Mamu-DR*W201+ rhesus monkeys. PBMCs of two SHIV-infected monkeys (L23 and H318) were stained at different times after beginning p46 stimulation in vitro. Flow-cytometric analysis was performed on gated CD3+ T cells stained with PE-coupled tetrameric Mamu-DR*W201–p46 complex and fluorescein isothiocyanate-coupled anti-CD4 antibody. Whole-blood staining was performed on day 0. The indicated percentages are the proportions of CD4+ T cells that bound tetramer.
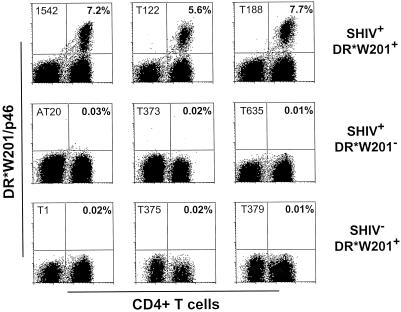
Since the T-cell receptors of antigen-specific CD4+ T cells recognize peptide bound to self-MHC class II molecules, we expected the tetrameric Mamu-DR*W201–p46 complex to bind specifically to a subpopulation of CD4+ T cells from SHIV-infected Mamu-DR*W201+ rhesus monkeys. Peptide-stimulated PBMCs from three groups of rhesus monkeys were assessed for p46 tetramer binding: SHIV-infected, Mamu-DR*W201+ monkeys; SHIV-infected, Mamu-DR*W201− monkeys; and uninfected, Mamu-DR*W201+ monkeys (Fig. 4). p46-stimulated cells from these monkeys were stained with PE-coupled tetrameric Mamu-DR*W201–p46 complex and analyzed by flow cytometry to enumerate p46-specific CD4+ T cells. Binding of the p46 tetramer was observed only on the CD4+ T cells of SHIV-infected Mamu-DR*W201+ monkeys. Therefore, this tetramer bound only to cells from antigen-primed monkeys of the appropriate MHC class II genotype.
FIG. 4.
Tetrameric Mamu-DR*W201–p46 complex binds specifically to p46-stimulated CD4+ T cells from PBMCs of SHIV-infected Mamu-DR*W201+ rhesus monkeys. p46-stimulated PBMCs of three groups of monkeys (three monkeys per group) were assessed: SHIV-positive Mamu-DR*W201+ monkeys, SHIV-positive Mamu-DR*W201-negative monkeys, and SHIV-negative Mamu-DR*W201+ monkeys. PBMCs were stimulated in vitro with p46 in IL-2-containing medium for 11 days, and flow-cytometric analysis was performed on gated CD3+ cells stained with PE-coupled tetrameric Mamu-DR*W201–p46 complex and fluorescein isothiocyanate-coupled anti-CD4 antibody. The indicated percentages are the proportions of CD4+ T cells that bound tetramer.
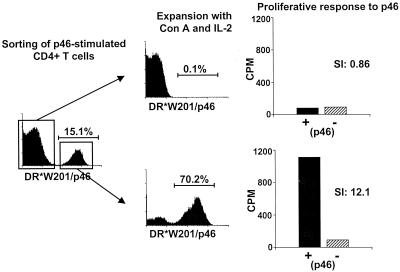
This tetrameric reagent was then used to characterize the lymphocytes that proliferated in vitro following HIV-1 Env peptide stimulation. p46-stimulated PBMCs from monkey L23 were sorted by flow cytometry into CD4+ T-cell populations that stained positively or negatively with the tetrameric Mamu-DR*W201–p46 complex (Fig. 5). Both cell populations were then expanded after concanavalin A stimulation (using irradiated PBMCs as feeder cells) in interleukin-2 (IL-2)-containing medium for 12 days, analyzed again by flow cytometry for p46 tetramer binding, and assayed for p46-specific proliferative responses. More than 70% of the sorted tetramer-positive CD4+ T cells still bound this tetramer after nonspecific in vitro expansion, and these cells showed a high p46-specific proliferative response (stimulation index, 12.1) (Fig. 5). The CD4+ T cells that did not bind the tetramers remained negative for tetramer staining after in vitro expansion and had no p46-specific proliferative response (Fig. 5). Thus, the p46-stimulated proliferative response was mediated by CD4+ T lymphocytes that bound the tetrameric Mamu-DR*W201–p46 complex.
FIG. 5.
p46-stimulated T-cell proliferation is mediated by tetrameric Mamu-DR*W201–p46 complex-binding CD4+ T lymphocytes. In vitro p46-stimulated PBMCs from the SHIV-infected Mamu-DR*W201+ rhesus monkey L23 were stained with PE-coupled tetrameric Mamu-DR*W201–p46 complex. CD4-positive, Mamu-DR*W201–p46 complex-positive cells and CD4-positive, Mamu-DR*W201–p46 complex-negative cells were sorted by flow cytometry and expanded by concanavalin A stimulation for 10 days with irradiated PBMCs in IL-2-containing medium. The cells were again stained and analyzed by flow cytometry, and the p46-specific proliferative response of each cell population was assessed. SI, stimulation index.
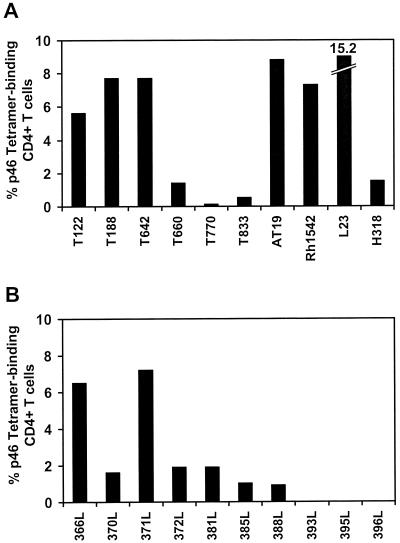
Since the numbers of tetrameric Mamu-DR*W201–p46 complex-binding CD4+ T cells in the whole blood of 10 SHIV-infected Mamu-DR*W201+ rhesus monkeys were below the limit of detection of the tetramer assay (Fig. 3 and data not shown), we sought to quantitate the magnitude of p46 tetramer-binding CD4+ T cells in PBMCs of these animals after in vitro expansion. p46-stimulated PBMCs from 10 SHIV-infected Mamu-DR*W201+ monkeys were analyzed by p46 tetramer staining to enumerate p46-specific CD4+ T cells. The clinical statuses of the SHIV-infected rhesus monkeys used in this study are shown in Table 1. Nine of the 10 infected animals had detectable levels of p46 tetramer-binding CD4+ T cells, ranging from 0.5 to 15.2% of the CD4+ T cells; only one of them had no detectable p46 tetramer-binding CD4+ T cells (Fig. 6A).
TABLE 1.
Clinical statuses of rhesus monkeys used to study p46-specific CD4+ T-lymphocyte responses
| Monkey | Virus | No. of CD4+ T cells/μl of blood
|
Viral load (no. of RNA copies/ml of plasma) | |
|---|---|---|---|---|
| Preinfection | Time of study | |||
| T122 | SHIV-SF162P | 1,959 | 1,613 | <1,500 |
| T188 | SHIV-SF162P | 1,392 | 1,296 | <1,500 |
| T642 | SHIV-SF162 | 1,332 | 1,229 | <1,500 |
| T660 | SHIV-SF162 | 1,542 | 1,722 | <1,500 |
| T770 | SHIV-SF162P | 1,247 | 574 | 104 |
| T833 | SHIV-SF162 | 2,577 | 2,080 | <1,500 |
| AT19 | SHIV-33A.2 | 1,589 | 564 | <1,500 |
| Rh1542 | SHIV-33A | 1,646 | 1,384 | <1,500 |
| L23 | SHIV-HXBc2 | 1,763 | 1,383 | <1,500 |
| H318 | SHIV-HXBc2 | 1,326 | 1,651 | <1,500 |
FIG. 6.
Peptide epitope-specific CD4+ T cells detected by tetramer binding in peptide-stimulated PBMCs of SHIV-infected rhesus monkeys and recombinant canarypox virus-vaccinated monkeys. p46-specific CD4+ T cells among in vitro p46-stimulated PBMCs from SHIV-infected Mamu-DR*W201+ monkeys (A) and recombinant canarypox virus-vaccinated Mamu-DR*W201+ rhesus monkeys (B) were identified by their binding to tetrameric Mamu-DR*W201–p46 complex. p46-stimulated PBMCs from 10 infected and 10 vaccinated rhesus monkeys were stained with PE-coupled tetrameric Mamu-DR*W201–p46 complex. Flow-cytometric analysis was performed on gated CD4+ CD3+ T cells.
This tetramer technology was then used to assess vaccine-elicited HIV-1 Env-specific CD4+ T-cell responses in rhesus monkeys. p46-stimulated PBMCs from 10 Mamu-DR*W201+ rhesus monkeys vaccinated five times, 21 months earlier, with recombinant canarypox virus–HIV-1 env were stained with tetrameric Mamu-DR*W201–p46 complex and assessed by flow cytometry. Seven of the 10 had detectable levels of p46 tetramer-binding CD4+ T cells, comprising 0.9 to 7.2% of the CD4+ T lymphocytes (Fig. 6B). Thus, p46 tetramer-binding CD4+ T cells were detectable in peptide-stimulated PBMCs of vaccinated rhesus monkeys.
The first described use of a covalently bound peptide-MHC class II construct to quantitate antigen-specific CD4+ T cells was in a murine system (1, 8). Rees and coworkers showed that immunization with a previously defined epitope peptide elicited proliferation of epitope-specific CD4+ T cells to levels comprising less than 1.3% of the CD44+ CD4+ T cells (24). Since the CD44-expressing cells represent only a subset of the CD4+ T cells, these results suggest that epitope-specific CD4+ T lymphocytes can be low-frequency cell populations. Recently, this technology was also applied to quantitate antigen-specific CD4+ T cells in human. The results of these studies also suggest that the frequency of antigen-specific CD4+ T lymphocytes is low (7, 21).
While the use of tetramers provides an exquisitely specific means of quantitating antigen-specific CD4+ T lymphocytes, it is not the only technology that has been applied for that purpose. Perhaps the most commonly applied alternate approach is the quantitation of cytokine-producing CD4+ T cells by flow cytometry after in vitro stimulation of lymphocytes with protein. Interestingly, studies using this technical approach indicate that cells that are specific for the cytomegalovirus or HIV-1 Env protein represent up to 20% and less than 0.1%, respectively, of circulating CD4+ T lymphocytes of infected individuals (23, 32). Importantly, these values are certain to be higher than those found in the present study since the tetramer-binding cells represent single-epitope-specific lymphocyte populations while the protein-specific cells likely represent multiple-epitope-specific lymphocyte populations.
In fact, the detected frequency of epitope-specific CD4+ T lymphocytes in the SHIV-infected monkeys of the present study was quite low. Interpreting studies of CD4+ T-lymphocyte responses elicited by an AIDS virus infection can, however, prove problematic. An AIDS virus infection, by virtue of the capacity of the virus to lyse and cause functional abnormalities of CD4+ T cells, is associated with a very-low-frequency, dysfunctional CD4+ T-lymphocyte response (3, 12, 20). We therefore chose to study the responses of CD4+ T lymphocytes to AIDS viruses that are nonpathogenic and other AIDS viruses that are pathogenic but do not cause profound CD4-cell depletion (5, 6, 25, 26). This strategy allows the characterization of the virus-specific CD4+ T cells in the absence of significant ongoing destruction of the cell population. We found in the present studies that despite the fact that the infected monkeys had relatively intact CD4+ T-lymphocyte numbers and function, the frequency of tetramer-binding CD4+ T lymphocytes in their peripheral blood was extremely low.
The frequency of the circulating MHC class II-peptide tetramer-binding CD4+ T cells in Mamu-DR*W201+ rhesus monkeys was substantially lower than the frequency of circulating CD8+ T cells that bind the p11C–Mamu-A*01 tetramer in infected Mamu-A*01+ and DR*W201+ monkeys (data not shown). The CD8+ T cells specific for the dominant CTL epitope p11C are consistently detected in the blood of infected monkeys by using the p11C tetramer, without resorting to in vitro stimulation of the lymphocytes to expand their numbers (9–11). Even CD8+ T-lymphocyte populations that recognize nondominant SHIV or simian immunodeficiency virus epitopes are detected in the blood of occasional infected monkeys without resorting to in vitro cultivation of the monkey PBMCs with the epitope peptide and IL-2 (2). In contrast to these findings, CD4+ T lymphocytes that bind the p46 tetramer were not detected in unstimulated whole blood of 10 SHIV-infected Mamu-DR*W201+ rhesus monkeys. This observation is consistent with the notion that CD4+ T-lymphocyte responses do not focus as intensely on a limited number of epitopes as do CD8+ T-lymphocyte responses.
Another possible explanation for our finding of a low-frequency p46-specific CD4+ T-lymphocyte response must be considered. As with epitope-specific CD8+ T-lymphocyte responses, epitope-specific CD4+ T-lymphocyte responses may be more or less dominant. It is possible that the HIV-1 Env p46-specific CD4+ T-cell response of Mamu-DR*W201+ rhesus monkeys characterized in the present study is a response to a particularly nondominant CD4+ T-cell epitope. This explanation is unlikely because p46-specific CD4+ T-cell lines were readily and repeatedly generated from HIV-1 Env protein-stimulated PBMCs of a number of SHIV-infected or HIV-1 Env-vaccinated Mamu-DR*W201+ rhesus monkeys (14, 15). Nevertheless, the frequencies of multiple other virus-epitope-specific CD4+ T-lymphocyte populations must be determined to confirm that CD4+ T-lymphocyte responses do not focus on particular predominant epitopes.
Acknowledgments
This work was supported by NIH grants AI85343 and AI20729.
We thank A. Gettie, Tulane Regional Primate Research Center, for assistance in shipping blood specimens. We also thank our collaborator James Tartaglia, Virogenetics Corporation, and our colleagues at Pasteur Merieux Connaught for contributions to these studies.
REFERENCES
- 1.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 2.Egan M A, Kuroda M J, Voss G, Schmitz J E, Charini W A, Lord C I, Forman M A, Letvin N L. Use of major histocompatibility complex class I/peptide/β2M tetramers to quantitate CD8+ cytotoxic T lymphocytes specific for dominant and nondominant viral epitopes in simian-human immunodeficiency virus-infected rhesus monkeys. J Virol. 1999;73:5466–5472. doi: 10.1128/jvi.73.7.5466-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein J S, Frederick W R, Rook A H, Jackson L, Manischewitz J F, Mayner R E, Masur H, Enterline J C, Djeu J Y, Quinnan G V., Jr Selective defects in cytomegalovirus- and mitogen-induced lymphocyte proliferation and interferon release in patients with acquired immunodeficiency syndrome. J Infect Dis. 1985;152:727–733. doi: 10.1093/infdis/152.4.727. [DOI] [PubMed] [Google Scholar]
- 4.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 5.Harouse J M, Gettie A, Tan R C, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 6.Harouse J M, Tan R C, Gettie A, Dailey P, Marx P A, Luciw P A, Cheng-Mayer C. Mucosal transmission of pathogenic CXCR4-utilizing SHIVSF33A variants in rhesus macaques. Virology. 1998;248:95–107. doi: 10.1006/viro.1998.9236. [DOI] [PubMed] [Google Scholar]
- 7.Kotzin B L, Falta M T, Crawford F, Rosloniec E F, Bill J, Marrack P, Kappler J. Use of soluble peptide-DR4 tetramers to detect synovial T cells specific for cartilage antigens in patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:291–296. doi: 10.1073/pnas.97.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozono H, White J, Clements J, Marrack P, Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 1994;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- 9.Kuroda M J, Schmitz J E, Barouch D H, Craiu A, Allen T M, Sette A, Watkins D I, Forman M A, Letvin N L. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lifton M A, Lord C I, Forman M A, Letvin N L. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- 11.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lord C I, Forman M A, Letvin N L. Comparative analysis of cytotoxic T lymphocytes in lymph nodes and peripheral blood of simian immunodeficiency virus-infected rhesus monkeys. J Virol. 1999;73:1573–1579. doi: 10.1128/jvi.73.2.1573-1579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane H C, Depper J M, Greene W C, Whalen G, Waldmann T A, Fauci A S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985;313:79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- 13.Lekutis C, Letvin N L. Biochemical and molecular characterization of rhesus monkey major histocompatibility complex class II DR. Hum Immunol. 1995;43:72–80. doi: 10.1016/0198-8859(94)00155-j. [DOI] [PubMed] [Google Scholar]
- 14.Lekutis C, Letvin N L. HIV-1 envelope-specific CD4+ T helper cells from simian/human immunodeficiency virus-infected rhesus monkeys recognize epitopes restricted by MHC class II DRB1*0406 and DRB*W201 molecules. J Immunol. 1997;159:2049–2057. [PubMed] [Google Scholar]
- 15.Lekutis C, Shiver J W, Liu M A, Letvin N L. HIV-1 env DNA vaccine administered to rhesus monkeys elicits MHC class II-restricted CD4+ T helper cells that secrete IFN-gamma and TNF-alpha. J Immunol. 1997;158:4471–4477. [PubMed] [Google Scholar]
- 16.Letvin N L. Animal models for AIDS. Immunol Today. 1990;11:322–326. doi: 10.1016/0167-5699(90)90127-u. [DOI] [PubMed] [Google Scholar]
- 17.Letvin N L, Daniel M D, Sehgal P K, Desrosiers R C, Hunt R D, Waldron L M, MacKey J J, Schmidt D K, Chalifoux L V, King N W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 18.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M E, Lekutis C, Alroy M, Freed D C, Lord C I, Handt L K, Liu M A, Shiver J W. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray H W, Rubin B Y, Masur H, Roberts R B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- 21.Novak E J, Liu A W, Nepom G T, Kwok W W. MHC class II tetramers identify peptide-specific human CD4+ T cells proliferating in response to influenza A antigen. J Clin Investig. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker D C. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 23.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 24.Rees W, Bender J, Teague T K, Kedl R M, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4+ T cell responses in vivo and in vitro. Proc Natl Acad Sci USA. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reimann K A, Li J T, Veazey R, Halloran M, Park I-W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reusser P, Riddell S R, Meyers J D, Greenberg P D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 28.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg E S, LaRosa L, Flynn T, Robbins G, Walker B D. Characterization of HIV-1-specific T-helper cells in acute and chronic infection. Immunol Lett. 1999;66:89–93. doi: 10.1016/s0165-2478(98)00165-5. [DOI] [PubMed] [Google Scholar]
- 30.Seth A, Ourmanov I, Kuroda M J, Schmitz J E, Carroll M W, Wyatt L S, Moss B, Forman M A, Hirsch V M, Letvin N L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc Natl Acad Sci USA. 1998;95:10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Herrath M G, Yokoyama M, Dockter J, Oldstone M B A, Whitton J L. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldrop S L, Pitcher C J, Peterson D M, Maino V C, Picker L J. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Investig. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]