Abstract
Recent neuroimaging and eye‐tracking studies have suggested that children with autism exhibit more variable and idiosyncratic brain responses and eye movements than typically developing (TD) children. Here, we extended this research to pupillometry recordings. We successfully acquired pupillometry recordings from 111 children (74 with autism), 4.5‐years‐old on average, who viewed three 90 s movies, twice. We extracted their pupillary time‐course for each movie, capturing their stimulus evoked pupillary responses. We then computed the correlation between the time‐course of each child and those of all others in their group as well as between each autistic child and all children in the TD group. This yielded an average inter‐subject correlation value per child, representing how similar their pupillary responses were to all others in their group or the comparison group. Children with autism exhibited significantly weaker inter‐subject correlations than TD children in all comparisons. These differences were independent of previously reported differences in gaze inter‐subject correlations and were largest in responses to a naturalistic movie containing footage of a social interaction between two TD children. The results demonstrate the utility of measuring the idiosyncrasy of pupil regulation, which can be performed with passive viewing of movies even by young children with co‐occurring intellectual disability. These findings reveal that a considerable number of children with autism have significantly less stable, idiosyncratic pupil regulation than TD children, indicative of more variable, weakly regulated, underlying neural activity.
Keywords: Autism, idiosyncrasy, inter‐subject, naturalistic, pupil, pupillometry
Lay Summary
Previous studies have suggested that children with autism exhibit more variable and idiosyncratic brain responses and eye movements than typically developing (TD) children. Here we extend these findings to measurements of pupil size in children. Our data demonstrate that children with autism exhibit more variable, weakly regulated pupil responses as they observe movies, revealing a potential child friendly technique for identifying autistic children with more variable, weakly regulated, underlying neural activity.
INTRODUCTION
Pupillometry studies in children with autism have generated considerable interest in recent years (de Vries et al., 2021). Pupil size is regulated by the pupillary light reflex (PLR) (Belliveau et al., 2023) and by norepinephrine (NE) release from the locus coeruleus (LC) (Joshi et al., 2016), which also regulates arousal, attention, and exploration/exploitation behaviors (Aston‐Jones & Cohen, 2005; Maness et al., 2022). Hence, potential differences in pupillary responses between autistic and typically developing (TD) individuals could indicate underlying physiological differences in LC‐NE and/or PLR function (Lynch, 2018).
Several pupillometry studies have reported results from comparisons of autism and TD groups. Studies measuring baseline pupil diameter during stable luminance have reported mixed results with some reporting larger pupil size in autism relative to TD (Bast et al., 2023; Kim et al., 2022), while others have reported the opposite (Müller et al., 2016) and yet others have reported no difference across groups (de Vries et al., 2021; Granovetter et al., 2020; Zhao et al., 2022). In contrast, pupil dilation responses to a target or novel stimulus with identical luminance (e.g., in oddball tasks) is consistently weaker in autism (Bast et al., 2023; Kim et al., 2022; Zhao et al., 2022), particularly in tasks with higher attentional load (e.g., one‐back memory task with added distractors) (Granovetter et al., 2020), suggestive of weaker LC‐NE activity in autism. Similarly, pupil constriction responses to increased luminance were weaker (i.e., weaker PLR) (Daluwatte et al., 2013; Fan et al., 2009) and delayed in time (de Vries et al., 2021) in children and adults with autism relative to controls. But interestingly, abnormally stronger PLR was reported in 9–10‐month‐old toddlers who developed autism at later ages, suggesting different PLR atypicalities during early versus late autism development (Nyström et al., 2018).
Taken together, the studies above suggest that pupillary responses in children and adults with autism are muted, with LC‐NE generating weaker pupil dilations during attention demanding tasks or in response to novel/surprising stimuli, and PLR generating weaker pupil constrictions in response to increased luminance. Because these differential pupillary responses generated by LC‐NE (Zhao et al., 2022) and PLR (Daluwatte et al., 2013; Fan et al., 2009) are apparent even during passive viewing of sensory stimuli in the absence of a task, they may serve a useful role in distinguishing between autistic and TD individuals in situations where task administration is not possible (e.g., non‐verbal children). Hence, there is clear motivation to develop task‐free, experimental protocols with child‐friendly, engaging stimuli for this purpose.
Previous studies have demonstrated the high ecological validity of using naturalistic movies to study brain function with fMRI (Finn & Bandettini, 2021), especially in young children (Cantlon & Li, 2013; Lerner et al., 2021; Vanderwal et al., 2021). Of particular interest are studies demonstrating that individuals with autism exhibit significantly more variable and idiosyncratic brain responses than TD individuals when observing movies (Byrge et al., 2015; Hasson et al., 2009). These studies quantified idiosyncrasy by measuring inter‐subject correlation (inter‐SC), which revealed that cortical activity was more strongly correlated across TD individuals observing the same movie than across autistic participants. Participants with autism exhibited more idiosyncratic and unique cortical responses with larger between‐subject variability. In a recent study we applied the same inter‐SC technique to recordings of gaze position during natural viewing of movies. We demonstrated that children with autism also exhibited weaker inter‐SC magnitudes, gazing at movies less consistently than TD children and exhibiting more idiosyncratic gaze patterns (Avni et al., 2020).
Here, we extend this research further by applying the inter‐SC approach to pupillometry data in children with autism using an experimental design consisting of three different movies, each presented twice, which enabled us to assess the reliability of findings across movies and presentations.
METHODS
A subset of the eye‐tracking recordings examined in the current study were analyzed previously to compare gaze patterns across autistic and TD children in a separate study (Avni et al., 2020). Here, we extracted and analyzed pupillometry data from these and additional recordings.
Participants
We initially recruited 121 children for the current study through the Azrieli National Centre for Autism and Neurodevelopment Research, between 2016 and 2019. Of these, 81 were diagnosed with autism according to DSM‐V criteria (mean age: 4.46 ± 1.91 years; age range: 1.09–10.07 years; 64 male, 17 female) and 40 were TD (mean age: 4.28 ± 2.10 years; age range: 1.03–10.03 years; 26 male, 14 female). One TD child was removed for having a Social Responsiveness Scale (SRS) score greater than the clinical cut‐off (Constantino, 2012). In addition, data from seven autistic and two TD children were excluded due to low eye‐tracking quality (see details below), yielding a final sample of 111 children (Table 1). The study was approved by the Soroka Medical Center Helsinki committee and the Ben Gurion University Internal Review Board committee. Written informed consent was obtained from all parents.
TABLE 1.
Participant characteristics.
| Variable | Mean | SD | Range |
|---|---|---|---|
| Autism group (n = 74, 59 males) | |||
| Age at eye tracking (months) | 57.49 | 22.44 | 21–127 |
| Number of included movies | 4.43 | 1.76 | 1–6 |
| ADOS‐2 Total CSS (n = 56) | 6.75 | 2.60 | 1–10 |
| ADOS‐2 SA CSS (n = 56) | 6.45 | 2.56 | 1–10 |
| ADOS‐2 RRB CSS (n = 56) | 7.46 | 2.44 | 1–10 |
| Cognitive Scores (n = 50) | 80.72 | 16.33 | 49–117 |
| TD group (n = 37, 24 males) | |||
| Age at eye tracking (months) | 53.30 | 22.43 | 15–123 |
| Number of included movies | 5.65 | 0.59 | 4–6 |
| SRS scores (n = 34) | 34.21 | 12.68 | 11–57 |
Most of the participating children with autism (56 out of 74) completed the Autism Diagnostic Observation Schedule—Second edition (ADOS‐2) (Lord et al., 2012). In addition, 50 of the 74 autistic children completed either a Wechsler Preschool and Primary Scale of Intelligence (Wechsler, 2002) or the Bayley Cognitive Scales test (Bayley, 2006). TD children did not complete ADOS‐2 or cognitive tests, but all parents of the TD children, except in three instances, completed the SRS (Constantino, 2012).
Data acquisition
Participants were seated approximately 60 cm from the display screen and the left eye pupil diameter was recorded using an EyeLink 1000+ head‐free eye‐tracking system with a sampling rate of 500 Hz (SR Research Inc., Canada). The participants' head position was tracked using a sticker placed on their forehead. An infrared camera, located below the display screen, measured pupil size. For each participant, the eye tracker was calibrated prior to data collection: the participant made saccades to each of five stimuli presented sequentially on the screen, and gaze accuracy was then validated to be <2 degrees. Additional validations of calibration accuracy were performed after each movie and re‐calibration was performed if error >2 degrees. Experiment Builder and Data Viewer (SR Research Inc., Canada) were used to construct the experiment and visualize the data.
Experimental design
Movies were presented on a 17‐inch LCD monitor with a resolution of 1280 × 1024 and a refresh rate of 60 Hz. Once calibration was successfully completed, participants were shown three different movie clips, each presented twice. Each movie was 1.5 min long and the total duration of the experiment was roughly 10 min. The first movie segment, from the Pixar animation “Jack‐Jack Attack,” showed the adventures of a babysitter taking care of an infant with supernatural powers. The second movie segment, taken from the Walt Disney animation “The Jungle Book,” contained a segment in which Mowgli meets the Monkey King who sings and dances while interacting with other monkeys. The third movie contained an un‐cut home‐video with two sisters (2 and 5 years old) interacting socially in a typical, messy room containing everyday objects.
Pre‐processing and data cleaning
The Eyelink 1000+ records the pupil area as the number of pixels within the image area identified as the pupil, which is equivalent to the angular area of the pupil (Hayes & Petrov, 2016). We identified and removed data segments where the eye tracker lost track of the children's pupil due to eye blinks and off‐screen gazes. This included segments with time‐points where the pupil diameter equaled zero, was larger than 9.8 mm, or changed faster than 0.64 mm/ms, all of which are physiologically implausible. Movies where more than 50% of the time‐points were removed, were entirely excluded from further analysis. We removed 146 of 474 (30.8%) of the movies observed by autistic children and 25 of 234 (10.7%) of the movies observed by TD children. This yielded the final sample described above of 74 children with autism and 37 TD children who contributed at least one movie to the analyses.
Of the remaining movies, we removed segments according to the criteria described above such that, on average, 19.4 ± 10.9% and 12.9 ± 10.9% of the data were removed from the autistic and TD participants, respectively (note that, below, we take the amount of removed data into account in the statistical analysis of the data). We also excluded the first second of each movie to minimize potential stimulus onset responses. Removed time‐points were set to NaN values and the remaining segments of analyzed data were smoothed using a Gaussian filter with a width of 250 samples (i.e., half a second). Finally, pupil diameter was computed from angular pupil area using the following formula:
where, d is the pupil diameter, α is a scaling factor deduced empirically by measuring an artificial pupil with known area, L is the distance of the pupil from the camera, and φ is the visual angle of the pupil, which is the square root of the angular pupil area measured by the eye tracker (see Eyelink documentation and Hayes & Petrov, 2016). In our setup, given a pupil distance of 60 cm when using a 15 mm lens, αL = 0.248.
Data analysis
All analyses were performed with custom written code in MATLAB (Mathworks Inc., USA). We extracted time‐courses of pupil diameter in mm units for each movie, separately for first and second presentations, per participant. We computed the inter‐SC per movie presentation by correlating the pupil time‐courses across all pairs of participants within each group (autism or TD). This analysis was also performed for each of the autistic children with all TD children. We then computed the mean correlation for each child and all other children, yielding a mean inter‐SC value per participant.
We performed an analogous analysis to compute a gaze pattern inter‐SC value per participant. We used the data described above (after cleaning) and computed inter‐SC in an identical manner to that described previously (Avni et al., 2020).
Statistical analyses
To determine whether there were significant differences in the percent of excluded data across groups, we performed a univariate ANOVA analysis with movie type (Jack‐Jack Attack, Jungle Book, Naturalistic) as the dependent variable and diagnostic group (autism and TD) as the between‐subjects factor. To evaluate whether there were significant differences in tonic pupil size, variance, and inter‐SC across groups, we performed univariate ANCOVAs for each movie with diagnostic group (autism and TD) as the between‐subjects factor, and percent valid data and age as covariates. Correlations between pupil diameter and behavioral measures (ADOS, cognitive scores) were assessed with Pearson's correlation coefficient. Finally, we performed receiver operating characteristic (ROC) analyses to assess classification accuracy of autistic and TD children according to their inter‐SC values in each of the movies/presentations. We used a criterion range of 0 to 1 and computed the area under the curve (AUC) (i.e., integral) for each movie/presentation to compare the accuracy of autism/TD classification across movies.
RESULTS
Initial analyses demonstrated that there were significantly more excluded/invalid data in recordings of autistic versus TD/control children across all movies (F > 4.47, p < 0.038, η 2 > 0.050) with the exception of the second Jungle Book presentation (F(1,85) = 1.24, p = 0.27, η 2 = 0.014). We, therefore, included the percent of valid data as a covariate in all further analyses. We also added age as a covariate to ensure that potential differences across groups were not attributable to this variable. The percentage of valid data did not correlate with the age (r < 0.11, p > 0.31), ADOS‐2 score (ADOS‐2 SA: r < 0.19, p > 0.079; ADOS‐2 RRB: r < 0.10, p > 0.21; ADOS‐2 Total: r < 0.20, p > 0.21) or cognitive score (r < 0.18, p > 0.16) of the autistic children. The ability to contribute valid data in this eye‐tracking study was, therefore, not significantly associated with the cognitive abilities or core autism symptoms of the autistic children.
No group difference in tonic pupil size
Tonic pupil size, estimated as the mean pupil diameter across all included time‐points of each movie, was similar across autistic and TD participants in all movies and in both presentations (Figure 1). ANCOVA analyses, per movie, demonstrated no significant differences in tonic pupil size across groups for any of the movies (F < 0.42, p > 0.52, η 2 < 0.005). Moreover, the tonic pupil size of individual children in both groups was highly reproducible and significantly correlated across the two presentations of each movie, reflecting the high within subject reliability of this measure (TD: r > 0.93, p < 0.001; autism: r > 0.94, p < 0.001; Figure 1b–d).
FIGURE 1.
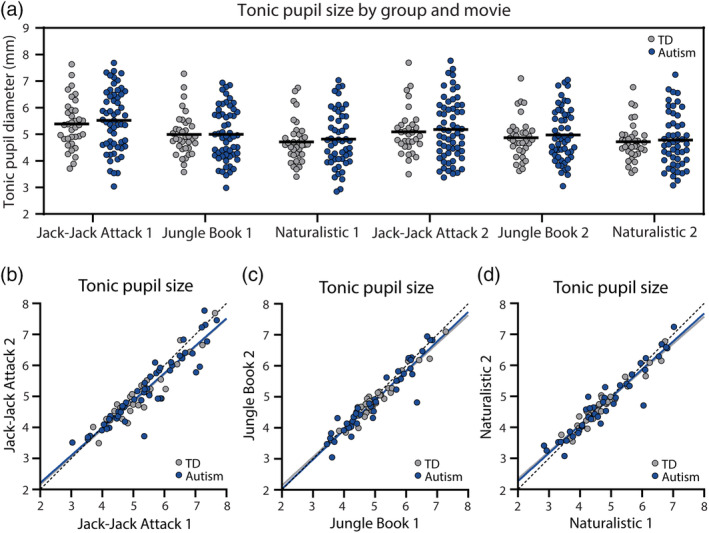
Tonic pupil diameter (mm) in each of the six movies. (a) Scatter plot demonstrating tonic pupil diameter for each individual, across both groups, per movie. Black line: mean tonic pupil diameter per group. There were no significant differences across groups. (b–d) Scatter plots demonstrating stability of tonic pupil diameter per participant across two presentations of each movie. Each point represents a single child. Solid lines: Least squares fit. Dotted line: unity line. Blue: autism, Gray: TD.
Reproducible stimulus‐evoked pupillary time‐courses
Each movie elicited a unique pupillary time‐course, generated by its unique visual content. The mean pupillary time‐courses were highly similar across autism and TD groups per movie (Figure 2) with strong correlations across groups in the Jack‐Jack Attack (first presentation: r = 0.93, p < 0.001; second presentation: r = 0.93, p < 0.001), Jungle Book (first presentation: r = 0.66, p < 0.001; second presentation: r = 0.81, p < 0.001), and Naturalistic (first presentation: r = 0.80, p < 0.001; second presentation: r = 0.81, p < 0.001) movies. Correlations were also strong across the two presentations of the Jack‐Jack Attack (autism: r = 0.90, p < 0.001; TD: r = 0.95, p < 0.001), Jungle Book (autism: r = 0.65, p < 0.001; TD: r = 0.75, p < 0.001), and Naturalistic (autism: r = 0.60, p < 0.001; TD: r = 0.56, p < 0.001) movies. In contrast, correlations across the different movies were weak and inconsistent such that, on average, the correlations in both groups were close to zero (TD: r = −0.012, p < 0.001; autism: r = 0.069, p < 0.001). While most correlation coefficients were significant due to the large number of degrees of freedom (pupillary time‐courses had 44,500 samples), within‐movie correlations were large (r = 0.74) while between‐movie correlations were negligible (r = 0.029), when computing the average across groups. Taken together, these results demonstrate movie‐evoked pupillary time‐courses were highly reproducible across presentations and unique to each movie in data from both groups of participants (Figure 2).
FIGURE 2.
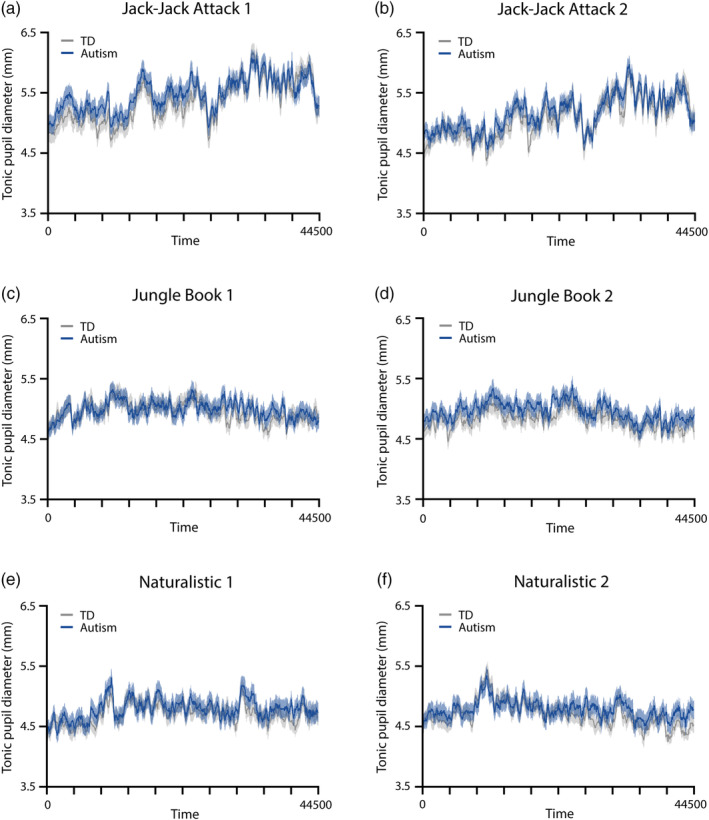
Time‐courses of pupil diameter from each presentation of the three movies. (a, c, e) First presentation. (b, d, f) Second presentation. Solid line: average pupil diameter across participants per group. Shaded area: standard error of the mean. Blue: autism. Gray: TD.
Pupillary inter‐subject correlations are consistently weaker in autism
We computed the pairwise correlation between the pupillary time‐course of a given child and each of the other children in the child's group, and then computed the mean correlation across all pairs, yielding an inter‐SC value per child for each movie presentation (Figure 3). This value represents the similarity of stimulus‐evoked pupillary changes between each child and all others in their group. We also performed this analysis an additional time by computing the pairwise inter‐SC of each autistic child and every TD child, yielding a value that represents the similarity of stimulus‐evoked pupillary changes between each autistic child and all others in the TD group.
FIGURE 3.
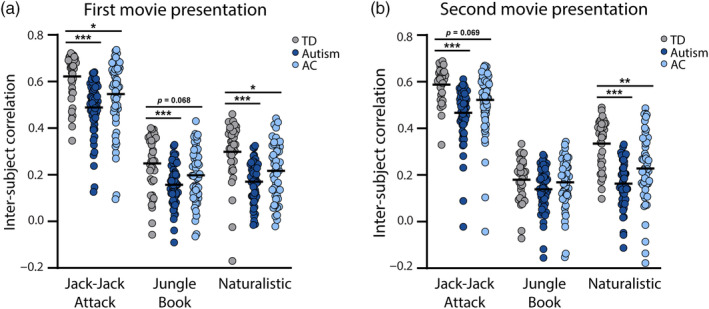
Comparison of inter‐SC values across groups for each movie and presentation. (a) First presentation. (b) Second presentation. Gray: inter‐SC within the TD group. Dark blue: inter‐SC within the autism group. Light blue: inter‐SC of children with autism relative to TD controls (i.e., AC). Asterisks: Significant differences across groups according to ANCOVA tests (*p < 0.05, **p < 0.01, ***p < 0.001).
When computing inter‐SC within each group, ANCOVA analyses revealed that inter‐SC values were significantly lower in the autism group in both presentations of Jack‐Jack Attack (first presentation: F(1,91) = 23.00, p < 0.001, η 2 = 0.21; second presentation: F(1,91) = 19.20, p < 0.001, η 2 = 0.18), both presentations of the Naturalistic movie (first presentation: F(1,85) = 23.42, p < 0.001, η 2 = 0.22; second presentation: F(1,83) = 46.66, p < 0.001, η 2 = 0.37), and the first presentation of the Jungle Book movie (F(1,90) = 15.76, p < 0.001, η 2 = 0.15). There were no significant differences in the second presentation of the Jungle Book movie (F(1,85) = 2.25, p = 0.14, η 2 = 0.026).
When computing the autism inter‐SC relative to TD children, ANCOVA analyses revealed that inter‐SC values were significantly lower in the autism group in the first presentation of Jack‐Jack Attack (F(1,91) = 3.99, p = 0.049, η 2 = 0.043) and both presentations of the Naturalistic movie (first presentation: F(1,85) = 5.31, p = 0.024, η 2 = 0.06; second presentation: F(1,83) = 8.07, p = 0.006, η 2 = 0.091). There was also a marginally significant difference in the first presentation of the Jungle Book (F(1,90) = 3.41, p = 0.068, η 2 = 0.037) and the second presentation of Jack‐Jack Attack (F(1,91) = 3.39, p = 0.069, η 2 = 0.037). No significant differences were found in the second presentation of the Jungle Book movie (F(1,85) = 0.0004, p = 0.98, η 2 < 0.001).
All further analyses were performed using the autism versus TD inter‐SC measure, which captures the uniqueness of autistic gaze behavior relative to controls.
In an additional set of analyses, we examined correlations between the inter‐SC measure and ADOS‐2 or cognitive scores of the children and did not find any significant relationships (r < 0.19, p > 0.22).
Pupillary inter‐SC are reliable across presentations
Individual inter‐SC magnitudes were correlated across presentations of the Jack‐Jack Attack (autism: r(1,49) = 0.40, p = 0.004; TD: r(1,29) = 0.60, p < 0.001), Jungle Book (autism: r(1,40) = 0.41, p = 0.008; TD: r(1,31) = 0.56, p < 0.001), and Naturalistic (autism: r(1,41) = 0.65, p < 0.001; TD: r(1,32) = 0.41, p = 0.016) movie presentations (Figure 4). This demonstrates that the inter‐SC measure exhibited relatively high test–retest reliability in both autistic and TD children.
FIGURE 4.
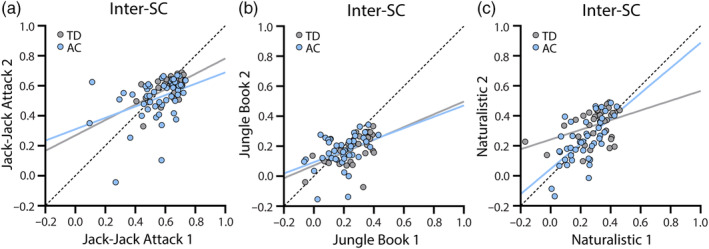
Scatter plots of inter‐SC values demonstrating their correlation across the two presentations of Jack‐Jack Attack (a), Jungle Book (b), and Naturalistic (c) movies. Gray: inter‐SC within the TD group. Light Blue: inter‐SC of children with autism relative to controls (AC). Solid lines: Least squares fit. Dotted line: unity line. Inter‐SC, inter‐subject correlation.
Classification of autistic and TD children according to their inter‐SC
Given the group differences described above, we performed a ROC analysis to quantify the ability to classify autistic and TD children according to their inter‐SC values per movie (Figure 5). For the autistic children we used the inter‐SC values that were computed relative to the TD children (Figure 3, light blue). We computed the AUC separately for each presentation of the Naturalistic (AUC first presentation = 0.71, second presentation = 0.71), Jack‐Jack Attack (AUC first presentation = 0.67, second presentation = 0.68), and Jungle Book (AUC first presentation = 0.63, second presentation = 0.53). This demonstrated that classification was highest in data from the Naturalistic movie in a consistent manner across both presentations.
FIGURE 5.
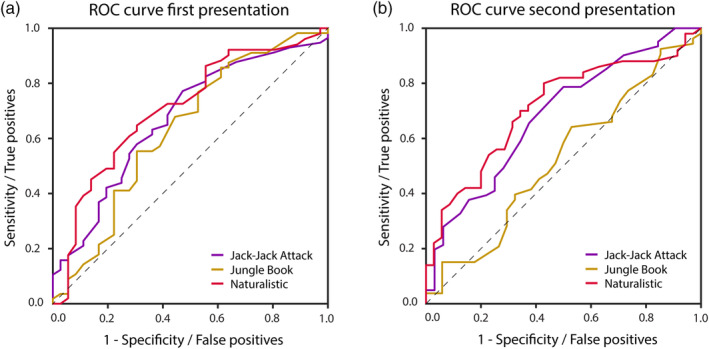
ROC analyses using inter‐SC to classify autistic and TD participants. (a) First presentation. (b) Second presentation. Purple: Jack‐Jack Attack, Yellow: Jungle Book, Red: Naturalistic movie. Black dashed line: unity line. Inter‐SC, inter‐subject correlation; ROC, receiver operating characteristic.
Control analyses
In a previous study examining gaze behavior, with a partially overlapping sample, we reported that autistic children exhibited weaker inter‐SC indicative of more variable, idiosyncratic gaze patterns compared to TD children. To determine whether the pupillary findings reported above were generated by idiosyncratic gaze patterns we assessed the correlation between the two measures. Correlations varied inconsistently across movies and presentations. A significant positive correlation was apparent for the autism group in the first presentation of Jungle Book (r(1,54) = 0.28, p = 0.033) and a significant negative correlation was apparent for the control group in the second presentation of Jack‐Jack Attack (r(1,30) = −0.42, p = 0.016). All other correlations were not significant (TD: r < 0.31, p > 0.074; autism: r < 0.23 p > 0.091), demonstrating that there was no consistent relationship across the two measures.
In a second control analysis, we extracted a time‐course of the head distance from the eye‐tracking camera as recorded by the eye tracker for each movie. We then compared the variance of these time‐courses across groups (per movie) to address the concern that larger or more frequent head movements throughout each of the movies may have generated differences in the pupillary time‐courses across groups. There were no differences across groups in this measure in any of the movies (F < 1.83, p > 0.18, η 2 < 0.022), though there was a marginally significant group difference in head distance variance during the second presentation of the Jungle Book (F(1,85) = 3.51, p = 0.065, η 2 = 0.041). This demonstrated that head distance throughout movie viewings was similarly stable across participants in both groups in movies where we report significant inter‐SC differences across groups.
In a third control analysis, we examined whether between group differences could be due to foreshortening effects. The foreshortening effect refers to the fact that pupil area is artificially reduced when a participant gazes away from the center of the camera's field of view as the pupil shape changes from a circle to an ellipse (Petersch & Dierkes, 2022). Hence, more frequent and larger gaze shifts away from the center of the screen could create larger pupil size variability/idiosyncrasy due to foreshortening. To assess this, we compared the mean and variance of gaze distances from the center of the screen across groups and found no significant difference across groups in any of the movies (mean: F < 0.31, p > 0.58, η 2 < 0.003, variance: F < 1.51, p > 0.223, η 2 < 0.018) with the exception of the second presentation of the Jungle Book (mean: F(1,85) = 4.12, p = 0.046, η 2 = 0.047; variance: F(1,85) = 5.01, p = 0.028, η 2 = 0.057). There was also a marginally significant group difference in mean gaze distance during the first presentation of Jack‐Jack Attack (F(1,91) = 3.60, p = 0.061, η 2 = 0.039). This demonstrated that gaze foreshortening did not differ significantly across groups in all but one of the movies where we report significant inter‐SC differences across groups.
DISCUSSION
Our results reveal that autistic children exhibited pupillary responses that were significantly more idiosyncratic (i.e., varied from one individual to another) than those of TD children, consistently across multiple movies and presentations (Figure 3). The group‐average pupillary time‐courses were highly correlated across autism and TD groups (Figure 2) demonstrating that each movie elicited a unique and reliable stimulus‐driven pupillary time‐course, on average. However, pupillary time‐courses of autistic children diverged from the mean (i.e., weaker inter‐SC) to a larger extent than those of TD children. This was true both when comparing autistic children to others in their group and when comparing them to the TD children (Figure 3). Individual magnitudes of inter‐SC were significantly correlated across presentations (Figure 4) demonstrating that idiosyncrasy (how different one is from the group) is a reproducible individual characteristic.
Inter‐SC differences were most pronounced in the pupillary responses to a naturalistic un‐cut home‐video of two TD children engaged in a social interaction. An ROC analysis demonstrated that classification accuracy was highest with data from both presentations of the naturalistic movie (Figure 5). These differences across groups were not explained by potential differences in gaze behavior, demonstrating that pupillometry data contains independent information regarding individual behavior and underlying neurophysiology.
These results indicate that pupil size regulation by PLR and LC‐NE mechanisms is more variable (i.e., less reliable) in children with autism than in TD children. Such an interpretation would be consistent with previous hypotheses proposing that sensory neural responses may be more variable in some autistic individuals (Dinstein et al., 2012; Dinstein et al., 2015; Heeger et al., 2017).
Finally, an important advantage of the current study was that it did not require completion of an elaborate explicit task, which is common in other eye‐tracking studies. This extends the utility and generalizability of the technique and measures described above to the broader autism population including, for example, non‐verbal children with profound autism (Lord et al., 2022).
Idiosyncrasy rather than consistent group differences
A growing body of literature demonstrates that autistic individuals exhibit idiosyncratic behavioral and physiological responses that differ from TD responses. Examples include idiosyncratic fMRI activation time‐courses in response to movies (Byrge et al., 2015; Hasson et al., 2009), idiosyncratic resting‐state fMRI functional connectivity (Benkarim et al., 2021; Hahamy et al., 2015; Nunes et al., 2019), idiosyncratic gaze patterns when observing movies (Avni et al., 2020; Keles et al., 2022), as well as other behavioral idiosyncrasies (Cavallo et al., 2018; Pegado et al., 2020). These studies suggest that rather than exhibiting consistently weaker or stronger behavioral or physiological responses, autistic individuals tend to differ from one another to larger extents and in unique ways that are often evident in the temporal structure of their responses. While TD individuals tend to respond more similarly and consistently, autistic individuals vary from one another and from TD individuals (Dinstein et al., 2015).
Pupillometry differences in autism
A variety of studies have reported that autistic participants may exhibit differences in pupil responses indicative of underlying hyper‐ or hypo‐regulation by LC‐NE and/or PLR circuits. One hypothesis is that autistic participants exhibit larger tonic pupil size indicative of stronger LC‐NE tonic activity, potentially associated with hyper‐arousal and increased behavioral flexibility (Aston‐Jones & Cohen, 2005). While some studies with relatively small samples (<32 participants in each group) have reported significantly larger tonic pupil size in autism (Bast et al., 2023; Kim et al., 2022), others have reported the opposite (Müller et al., 2016) or no differences across groups (de Vries et al., 2021; Granovetter et al., 2020; Zhao et al., 2022). Our results, with a somewhat larger sample and with multiple, repeated measurements, suggest that there is indeed no significant difference in baseline pupil size across groups (Figure 1).
Another hypothesis is that autistic individuals may exhibit weaker pupil dilations to stimuli with cognitive, attentional, or social load that may indicate weaker phasic LC‐NE modulation. Phasic increases in LC‐NE innervation are important for increasing arousal during task demanding periods and achieving optimal performance (Aston‐Jones & Cohen, 2005). Several studies have reported that pupillary responses in autistic individuals are weaker to visual stimuli in the context of a spatial attention task (Bast et al., 2023), an oddball task (Kim et al., 2022), a one‐back memory task (Granovetter et al., 2020), or when stimuli include social information (Bast et al., 2019; Polzer et al., 2022).
A third hypothesis suggests that autistic individuals may exhibit weaker pupil constriction in response to luminance increases (i.e., weaker PLR). Several studies have indeed reported weaker (Daluwatte et al., 2013; Fan et al., 2009) and delayed (de Vries et al., 2021) PLR responses in children and adults with autism. Surprisingly, there have also been reports of abnormally strong PLR responses in 9–10‐month‐old toddlers who developed autism at later ages (Nyström et al., 2018).
Taken together, the last two hypotheses suggest attenuated pupillometry time‐courses in autism with weaker PLR associated constrictions and weaker LC‐NE associated dilations. Our pupillometry results do not seem to show differences, on average, in the pupillary response time‐courses across autism and TD groups, in any of the movies (Figure 2). Pupillary time‐courses were highly correlated across groups, demonstrating that the temporal structure of pupillary responses was overall highly similar across groups. Nevertheless, our study was not designed to separate PLR and LC‐NE responses or isolate specific stimulus events that would be expected to generate a pupillary response. Hence, our results do not offer strong evidence for the existence of specific LC‐NE and PLR differences across groups or lack thereof. Further studies are necessary to test hypotheses regarding potential differences in each of these neural mechanisms in autism.
Limitations
Our study had several important limitations. Firstly, we did not design our stimuli or analyze it post‐hoc to separate pupillary responses associated with PLR versus LC‐NE mechanisms. It is likely that naturalistic, complex stimuli contain multiple transitions in both low‐level visual features (e.g., luminance and contrast) as well as high‐level content features (e.g., narrative complexity, novelty, social and emotional valence). Hence, our study measured pupillary changes that were the product of both PLR and LC‐NE regulation. Secondly, data loss was clearly an issue in the current study. While all 111 children included in the study successfully watched at least one movie, most participating children did not successfully watch all six movies. Since our experimental design included considerable redundancy with multiple movies and presentations, we believe that our results are reliable and conclusive despite partial data collection from many children. Nevertheless, this raises an important limitation of eye‐tracking studies with autistic children. While previous studies have reported success rates as high as 95% in collecting eye‐tracking data from autistic children (Shic et al., 2022), these were collected from autistic children without intellectual disability. We believe that improving stimuli and acquisition conditions to maximize data collection from young children with autism and intellectual disability should be an important goal of future studies. Finally, TD children in this study did not complete cognitive testing and we do not know if the reported findings are associated with cognitive function. Moreover, we did not match participants by sex and the analyzed data included 80% males in the autism group and 65% males in the TD group.
Conclusions
Our results suggest that children with autism exhibit pupillometry time‐courses that differ significantly from those of TD children. This suggests that pupil regulation differs in autism in idiosyncratic ways, which can be quantified using inter‐SC. Beyond the potential clinical value of this measure for identifying individuals with autism, it suggests that PLR and LC‐NE circuits do not operate in a reliable manner across autistic individuals. Rather than attenuated or excessive circuit responses, we speculate that these circuits may exhibit larger variability in autistic individuals, thereby generating unique, idiosyncratic pupillary time‐courses that differ from one individual to the next. Further studies delineating PLR and LC‐NE responses while using naturalistic stimuli that can be used with large samples of autistic children, including those with intellectual disability, are highly warranted.
CONFLICT OF INTEREST STATEMENT
Behrmann is a founder of Precision Neuroscopics, a company developing medical technologies with a focus on equity and inclusion in healthcare. All other authors declare no competing financial interests.
ETHICS STATEMENT
The study was approved by the Soroka Medical Center Helsinki committee and the Ben Gurion University Internal Review Board committee. Written informed consent was obtained from all parents.
ACKNOWLEDGMENTS
This research was supported by Fulbright U.S. Student Program to I.H.B., Israeli Science Foundation (1150/20), Israeli Ministry of Science and Technology, and Azrieli Foundation grants to I.D., National Institute of General Medical Sciences grants T32GM008208 to I.H.B. and M.C.G., T32GM081760 to M.C.G., and a Simons Foundation Autism Research Initiative grant to M.B. M.B. acknowledges support from P30 CORE award EY08098 from the National Eye Institute, NIH, and unrestricted supporting funds from The Research to Prevent Blindness Inc., NY, and the Eye and Ear Foundation of Pittsburgh. The content is solely the responsibility of the authors and does not necessarily represent the official view of the Fulbright Program, the National Institute of General Medical Sciences, or the National Institutes of Health. Finally, the authors thank the participants and their families for making this research possible.
Bleimeister, I. H. , Avni, I. , Granovetter, M. C. , Meiri, G. , Ilan, M. , Michaelovski, A. , Menashe, I. , Behrmann, M. , & Dinstein, I. (2024). Idiosyncratic pupil regulation in autistic children. Autism Research, 17(12), 2503–2513. 10.1002/aur.3234
Isabel H. Bleimeister and Inbar Avni contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Aston‐Jones, G. , & Cohen, J. D. (2005). An integrative theory of locus coeruleus‐norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403–450. 10.1146/ANNUREV.NEURO.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- Avni, I. , Meiri, G. , Bar‐Sinai, A. , Reboh, D. , Manelis, L. , Flusser, H. , Michaelovski, A. , Menashe, I. , & Dinstein, I. (2020). Children with autism observe social interactions in an idiosyncratic manner. Autism Research, 13(6), 935–946. 10.1002/AUR.2234 [DOI] [PubMed] [Google Scholar]
- Bast, N. , Banaschewski, T. , Dziobek, I. , Brandeis, D. , Poustka, L. , & Freitag, C. M. (2019). Pupil dilation progression modulates aberrant social cognition in autism spectrum disorder. Autism Research, 12(11), 1680–1692. 10.1002/AUR.2178 [DOI] [PubMed] [Google Scholar]
- Bast, N. , Boxhoorn, S. , Supér, H. , Helfer, B. , Polzer, L. , Klein, C. , Cholemkery, H. , & Freitag, C. M. (2023). Atypical arousal regulation in children with autism but not with attention‐deficit/hyperactivity disorder as indicated by pupillometric measures of locus coeruleus activity. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 8(1), 11–20. 10.1016/J.BPSC.2021.04.010 [DOI] [PubMed] [Google Scholar]
- Bayley, N. (2006). Bayley scales of infant and toddler development (3rd ed.). Harcourt Assessment. 10.1177/0734282906297199 [DOI] [Google Scholar]
- Belliveau, A. P. , Somani, A. N. , & Dossani, R. H. (2023). Pupillary light reflex. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK537180/ [PubMed] [Google Scholar]
- Benkarim, O. , Paquola, C. , Park, B. Y. , Hong, S. J. , Royer, J. , Vos De Wael, R. , Lariviere, S. , Valk, S. , Bzdok, D. , Mottron, L. C. , & Bernhardt, B. (2021). Connectivity alterations in autism reflect functional idiosyncrasy. Communications Biology, 4(1), 1078. 10.1038/S42003-021-02572-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrge, L. , Dubois, J. , Tyszka, J. M. , Adolphs, R. , & Kennedy, D. P. (2015). Idiosyncratic brain activation patterns are associated with poor social comprehension in autism. The Journal of Neuroscience, 35(14), 5837–5850. 10.1523/JNEUROSCI.5182-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon, J. F. , & Li, R. (2013). Neural activity during natural viewing of Sesame Street statistically predicts test scores in early childhood. PLoS Biology, 11(1), e1001462. 10.1371/JOURNAL.PBIO.1001462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo, A. , Romeo, L. , Ansuini, C. , Podda, J. , Battaglia, F. , Veneselli, E. , Pontil, M. , & Becchio, C. (2018). Prospective motor control obeys to idiosyncratic strategies in autism. Scientific Reports, 8(1), 13717. 10.1038/S41598-018-31479-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino, J. (2012). Social responsiveness scale (SRS‐2). Western Psychological Services. [Google Scholar]
- Daluwatte, C. , Miles, J. H. , Christ, S. E. , Beversdorf, D. Q. , Takahashi, T. N. , & Yao, G. (2013). Atypical pupillary light reflex and heart rate variability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 43(8), 1910–1925. 10.1007/S10803-012-1741-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, L. , Fouquaet, I. , Boets, B. , Naulaers, G. , & Steyaert, J. (2021). Autism spectrum disorder and pupillometry: A systematic review and meta‐analysis. Neuroscience and Biobehavioral Reviews, 120, 479–508. 10.1016/J.NEUBIOREV.2020.09.032 [DOI] [PubMed] [Google Scholar]
- Dinstein, I. , Heeger, D. J. , & Behrmann, M. (2015). Neural variability: Friend or foe? Trends in Cognitive Sciences, 19(6), 322–328. 10.1016/J.TICS.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Dinstein, I. , Heeger, D. J. , Lorenzi, L. , Minshew, N. J. , Malach, R. , & Behrmann, M. (2012). Unreliable evoked responses in autism. Neuron, 75(6), 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, X. , Miles, J. H. , Takahashi, N. , & Yao, G. (2009). Abnormal transient pupillary light reflex in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(11), 1499–1508. 10.1007/S10803-009-0767-7 [DOI] [PubMed] [Google Scholar]
- Finn, E. S. , & Bandettini, P. A. (2021). Movie‐watching outperforms rest for functional connectivity‐based prediction of behavior. NeuroImage, 235, 117963. 10.1016/J.NEUROIMAGE.2021.117963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granovetter, M. C. , Burlingham, C. S. , Blauch, N. M. , Minshew, N. J. , Heeger, D. J. , & Behrmann, M. (2020). Uncharacteristic task‐evoked pupillary responses implicate atypical locus ceruleus activity in autism. The Journal of Neuroscience, 40(19), 3815–3826. 10.1523/JNEUROSCI.2680-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy, A. , Behrmann, M. , & Malach, R. (2015). The idiosyncratic brain: Distortion of spontaneous connectivity patterns in autism spectrum disorder. Nature Neuroscience, 18(2), 302–309. 10.1038/NN.3919 [DOI] [PubMed] [Google Scholar]
- Hasson, U. , Avidan, G. , Gelbard, H. , Vallines, I. , Harel, M. , Minshew, N. , & Behrmann, M. (2009). Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real‐life viewing conditions. Autism Research, 2(4), 220–231. 10.1002/AUR.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, T. R. , & Petrov, A. A. (2016). Mapping and correcting the influence of gaze position on pupil size measurements. Behavior Research Methods, 48(2), 510–527. 10.3758/S13428-015-0588-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger, D. J. , Behrmann, M. , & Dinstein, I. (2017). Vision as a beachhead. Biological Psychiatry, 81(10), 832–837. 10.1016/j.biopsych.2016.09.019 [DOI] [PubMed] [Google Scholar]
- Joshi, S. , Li, Y. , Kalwani, R. M. , & Gold, J. I. (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate Cortex. Neuron, 89(1), 221–234. 10.1016/J.NEURON.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keles, U. , Kliemann, D. , Byrge, L. , Saarimäki, H. , Paul, L. K. , Kennedy, D. P. , & Adolphs, R. (2022). Atypical gaze patterns in autistic adults are heterogeneous across but reliable within individuals. Molecular Autism, 13(1), 39. 10.1186/S13229-022-00517-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. , Kadlaskar, G. , Keehn, R. M. N. , & Keehn, B. (2022). Measures of tonic and phasic activity of the locus coeruleus‐norepinephrine system in children with autism spectrum disorder: An event‐related potential and pupillometry study. Autism Research, 15(12), 2250–2264. 10.1002/AUR.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner, Y. , Scherf, K. S. , Katkov, M. , Hasson, U. , & Behrmann, M. (2021). Changes in cortical coherence supporting complex visual and social processing in adolescence. Journal of Cognitive Neuroscience, 33(11), 2215–2230. 10.1162/JOCN_A_01756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C. , Charman, T. , Havdahl, A. , Carbone, P. , Anagnostou, E. , Boyd, B. , Carr, T. , De Vries, P. J. , Dissanayake, C. , Divan, G. , & McCauley, J. B. (2022). The Lancet Commission on the future of care and clinical research in autism. The Lancet, 399(10321), 271–334. 10.1016/S0140-6736(21)01541-5 [DOI] [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , DiLavore, P. , Risi, S. , Gotham, K. , & Bishop, S. (2012). Autism diagnostic observation schedule–Second edition (ADOS‐2). Western Psychological Corporation. [Google Scholar]
- Lynch, G. (2018). Using pupillometry to assess the atypical pupillary light reflex and LC‐NE system in ASD. Behavioral Sciences, 8(11), 108. 10.3390/BS8110108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness, E. B. , Burk, J. A. , McKenna, J. T. , Schiffino, F. L. , Strecker, R. E. , & McCoy, J. G. (2022). Role of the locus coeruleus and basal forebrain in arousal and attention. Brain Research Bulletin, 188, 47–58. 10.1016/J.BRAINRESBULL.2022.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, N. , Baumeister, S. , Dziobek, I. , Banaschewski, T. , & Poustka, L. (2016). Validation of the movie for the assessment of social cognition in adolescents with ASD: Fixation duration and pupil dilation as predictors of performance. Journal of Autism and Developmental Disorders, 46(9), 2831–2844. 10.1007/S10803-016-2828-Z [DOI] [PubMed] [Google Scholar]
- Nunes, A. S. , Peatfield, N. , Vakorin, V. , & Doesburg, S. M. (2019). Idiosyncratic organization of cortical networks in autism spectrum disorder. NeuroImage, 190, 182–190. 10.1016/J.NEUROIMAGE.2018.01.022 [DOI] [PubMed] [Google Scholar]
- Nyström, P. , Gliga, T. , Jobs, E. N. , Gredebäck, G. , Charman, T. , Johnson, M. H. , Bölte, S. , & Falck‐Ytter, T. (2018). Enhanced pupillary light reflex in infancy is associated with autism diagnosis in toddlerhood. Nature Communications, 9(1), 1678. 10.1038/S41467-018-03985-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegado, F. , Hendriks, M. H. A. , Amelynck, S. , Daniels, N. , Steyaert, J. , Boets, B. , & Op de Beeck, H. (2020). Adults with high functioning autism display idiosyncratic behavioral patterns, neural representations and connectivity of the “Voice Area” while judging the appropriateness of emotional vocal reactions. Cortex, 125, 90–108. 10.1016/J.CORTEX.2019.11.008 [DOI] [PubMed] [Google Scholar]
- Petersch, B. , & Dierkes, K. (2022). Gaze‐angle dependency of pupil‐size measurements in head‐mounted eye tracking. Behavior Research Methods, 54(2), 763–779. 10.3758/S13428-021-01657-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzer, L. , Freitag, C. M. , & Bast, N. (2022). Pupillometric measures of altered stimulus‐evoked locus coeruleus‐norepinephrine activity explain attenuated social attention in preschoolers with autism spectrum disorder. Autism Research, 15(11), 2167–2180. 10.1002/AUR.2818 [DOI] [PubMed] [Google Scholar]
- Shic, F. , Naples, A. J. , Barney, E. C. , Chang, S. A. , Li, B. , McAllister, T. , Kim, M. , Dommer, K. J. , Hasselmo, S. , Atyabi, A. , Wang, Q. , Helleman, G. , Levin, A. R. , Seow, H. , Bernier, R. , Charwaska, K. , Dawson, G. , Dziura, J. , Faja, S. , … McPartland, J. C. (2022). The autism biomarkers consortium for clinical trials: Evaluation of a battery of candidate eye‐tracking biomarkers for use in autism clinical trials. Molecular Autism, 13(1), 1–17. 10.1186/S13229-021-00482-2/FIGURES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal, T. , Eilbott, J. , Kelly, C. , Frew, S. R. , Woodward, T. S. , Milham, M. P. , & Castellanos, F. X. (2021). Stability and similarity of the pediatric connectome as developmental measures. NeuroImage, 226, 117537. 10.1016/J.NEUROIMAGE.2020.117537 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (2002). Wechsler preschool and primary scale of intelligence—Third edition. The Psychological Corporation. [Google Scholar]
- Zhao, S. , Liu, Y. , & Wei, K. (2022). Pupil‐linked arousal response reveals aberrant attention regulation among children with autism spectrum disorder. The Journal of Neuroscience, 42(27), 5427–5437. 10.1523/JNEUROSCI.0223-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


