Abstract
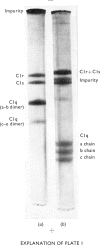
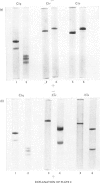
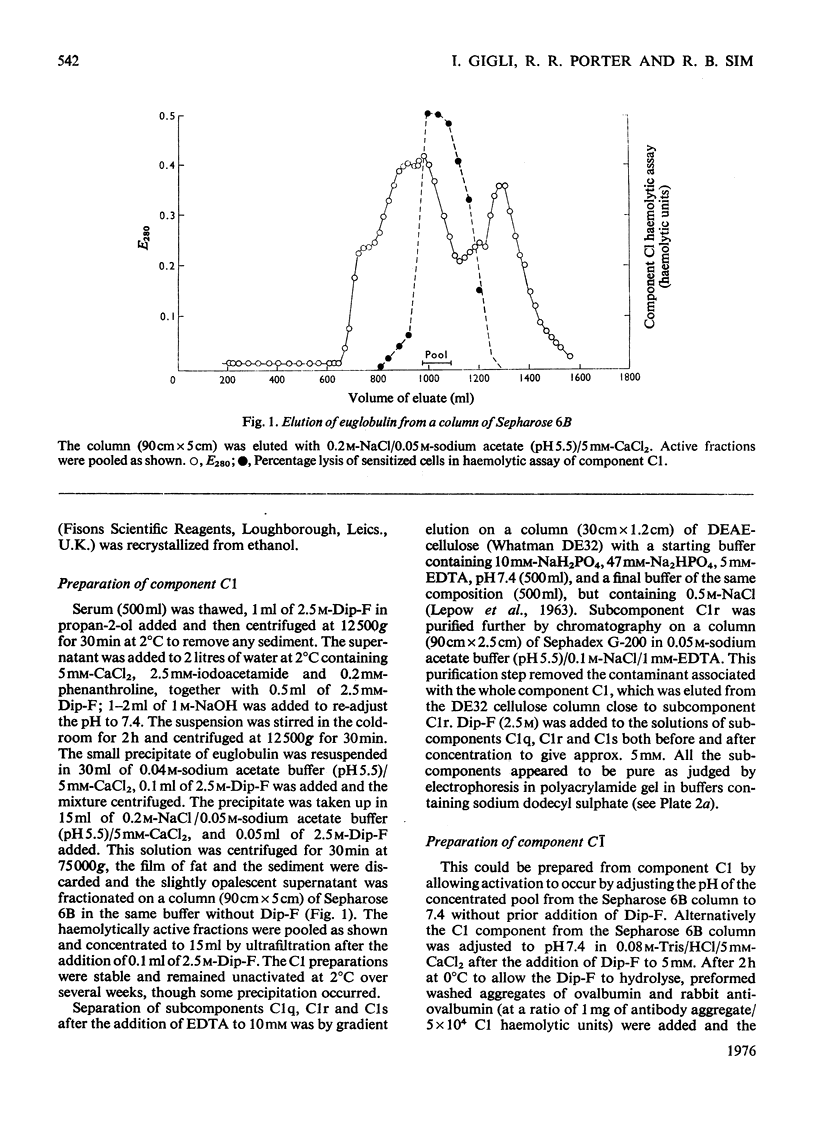
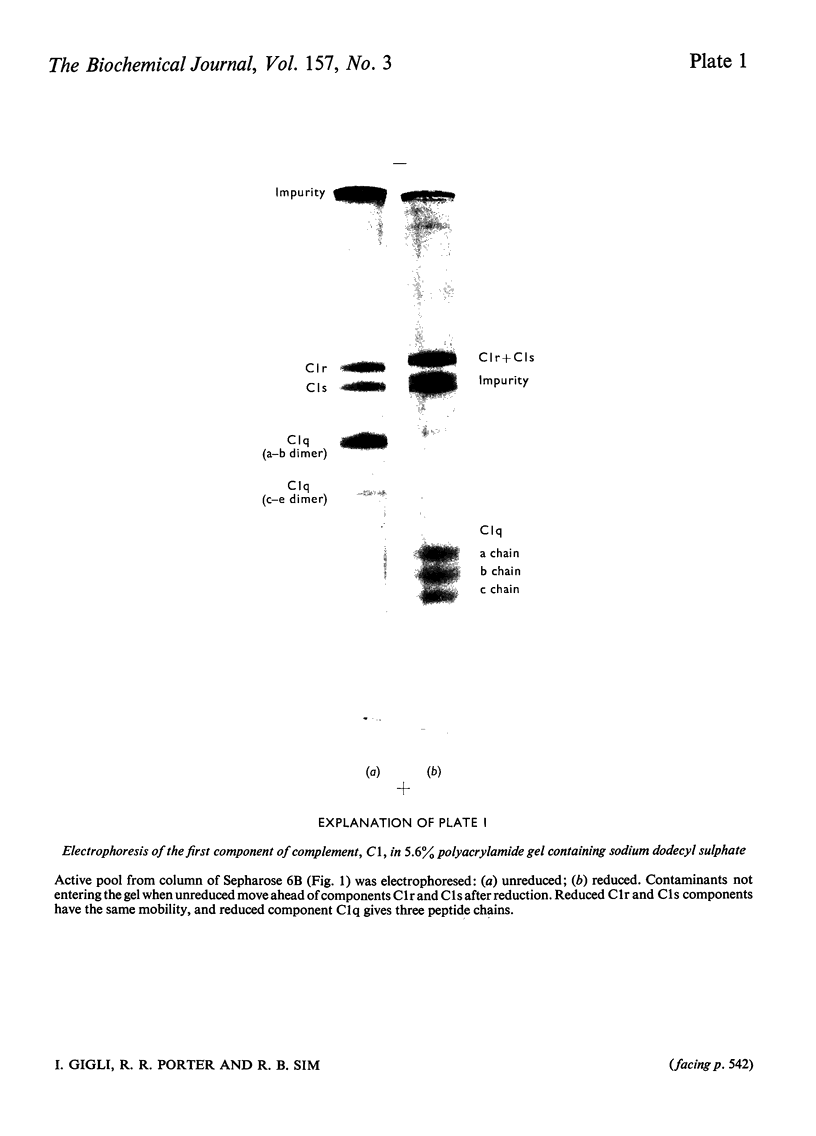
The first component of complement, C1, was isolated unactivated from human serum by repeated additions of di-isopropyl phosphorofluoridate during isolation. The unactivated subcomponents were also isolated, and evidence is given that the three subcomponents C1q, C1r and C1s account wholly for the activity of component C1 in serum. No evidence could be found for a fourth subcomponent, C1t. The approximate molar proportions of the subcomponents in serum are C1q/C1r/C1s = 1:2:2. Optimum activity by haemolytic assay was found at approximate molar proportions C1q/C1r/C1s of 1:4:4. No activity was found when subcomponents were assayed singly or in pairs, except for subcomponents C1q and C1s, which in molar ratio 1:4 gave 15-20% of the activity of the mixture C1q + C1r + C1s. The proteolytic activity of the isolated subcomponent C1s varied according to the method of activation used. Subcomponents C1q + C1r + C1s and C1q + C1s in the presence of antibody-antigen aggregates were activated and inactivated simultaneously, showing a peak of activity and subsequent loss of activity. Both reactions are probably due to proteolysis, and analysis of the peptide bonds split will be necessary to distinguish these two phenomena.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimeh S. N., Painter R. H. The identification of a previously unrecognized subcomponent of the first component of complement. J Immunol. 1975 Aug;115(2):482–487. [PubMed] [Google Scholar]
- BORSOS T., RAPP H. J. CHROMATOGRAPHIC SEPARATION OF THE FIRST COMPONENT OF COMPLEMENT AND ITS ASSAY ON A MOLECULAR BASIS. J Immunol. 1963 Dec;91:851–858. [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Immune hemolysis: a simplified method for the preparation of EAC'4 with guinea pig or with human complement. J Immunol. 1967 Aug;99(2):263–268. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gigli I., Austen K. F. Fluid phase destruction of C2hu by C1hu. I. Its enhancement and inhibition by homologous and heterologous C4. J Exp Med. 1969 Apr 1;129(4):679–696. doi: 10.1084/jem.129.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPOW I. H., NAFF G. B., TODD E. W., PENSKY J., HINZ C. F. Chromatographic resolution of the first component of human complement into three activities. J Exp Med. 1963 Jun 1;117:983–1008. doi: 10.1084/jem.117.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPOW I. H., RATNOFF O. D., ROSEN F. S., PILLEMER L. Observations on a pro-esterase associated with partially purified first component of human complement (C'1). Proc Soc Exp Biol Med. 1956 May;92(1):32–37. doi: 10.3181/00379727-92-22376. [DOI] [PubMed] [Google Scholar]
- Morgan P. H., Nair I. G. Autocatalytic activation of the proenzyme form of the C1s subunit of the first component of complement. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1037–1041. doi: 10.1016/0006-291x(75)90744-5. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Müller-Eberhard H. J. Fourth component of human complement: description of a three polypeptide chain structure. J Exp Med. 1974 Nov 1;140(5):1324–1335. doi: 10.1084/jem.140.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Porter R. R. Isolation and comparison of the proenzyme and activated forms of the human serum complement subcomponents C1r and C1s. Biochem Soc Trans. 1976;4(1):127–129. doi: 10.1042/bst0040127. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]