Abstract
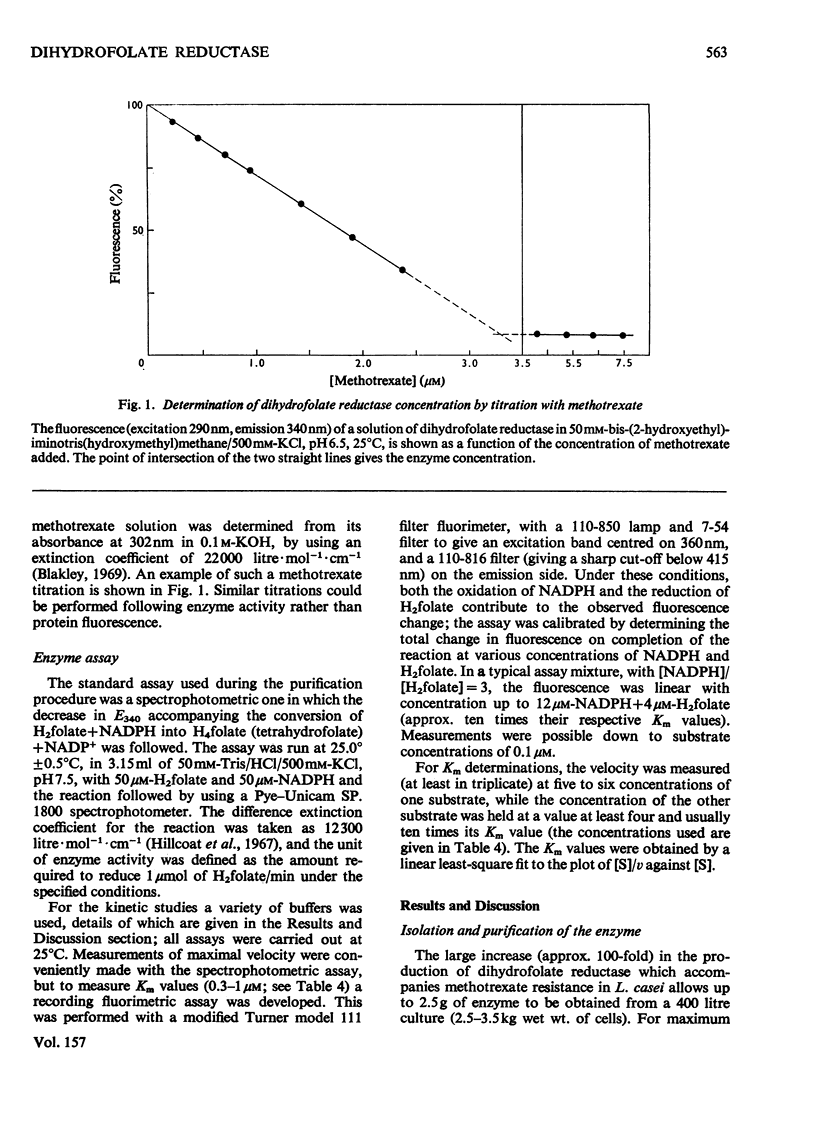
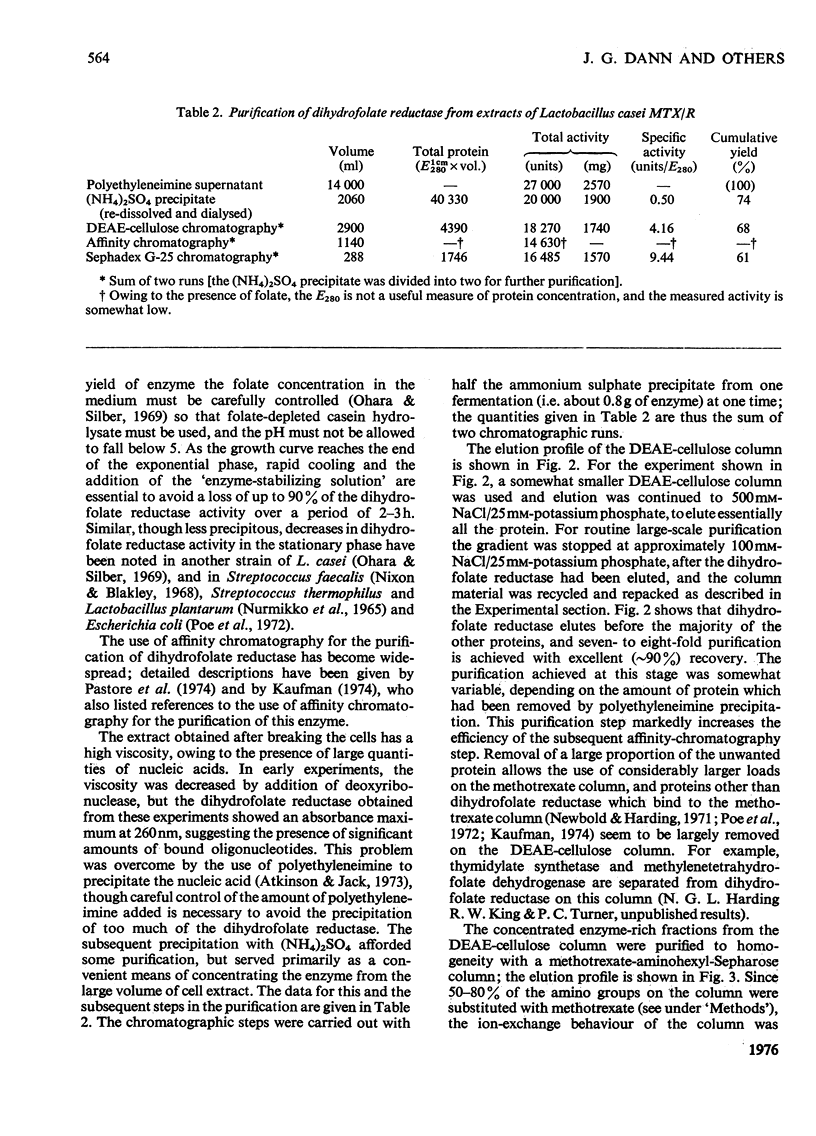
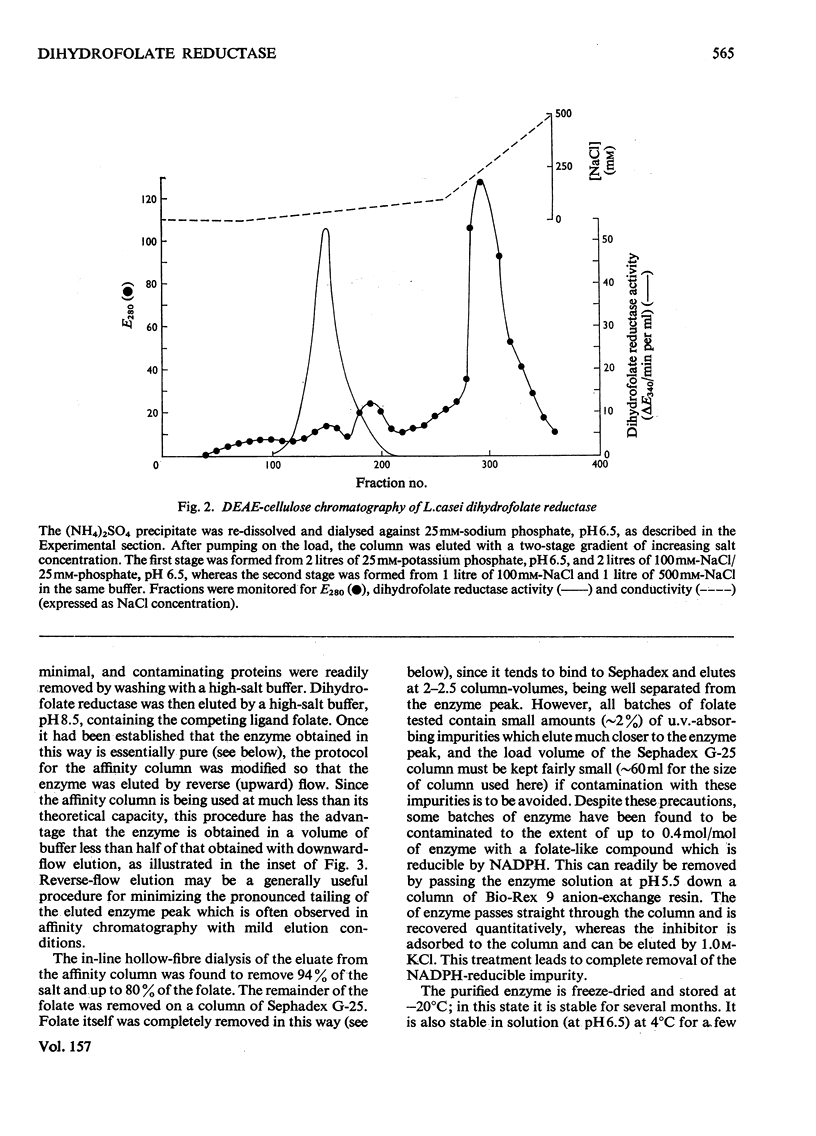
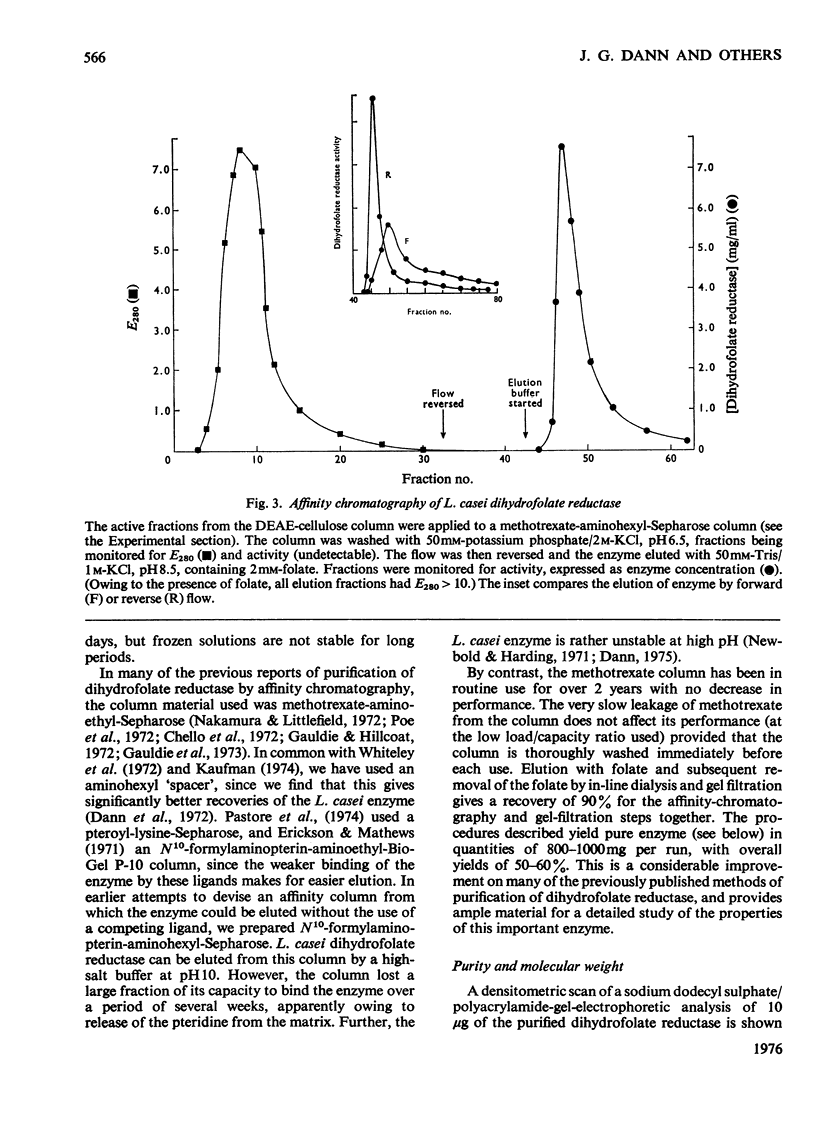
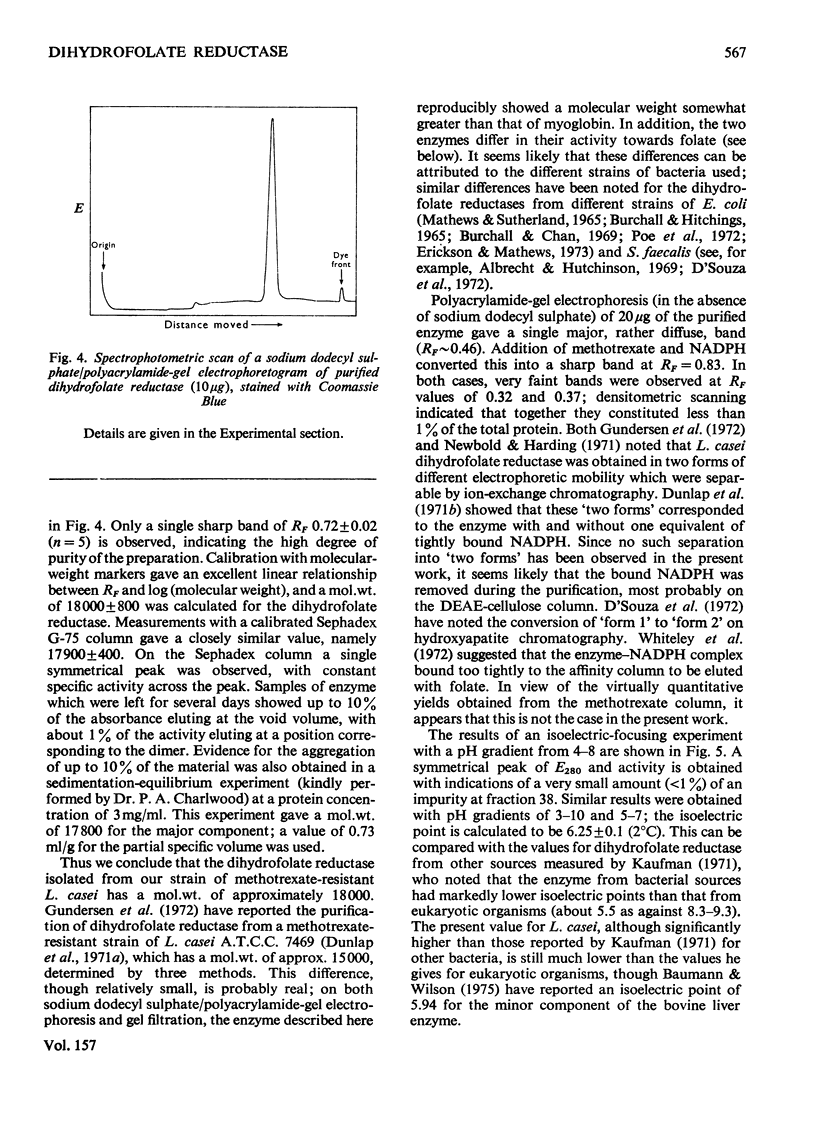
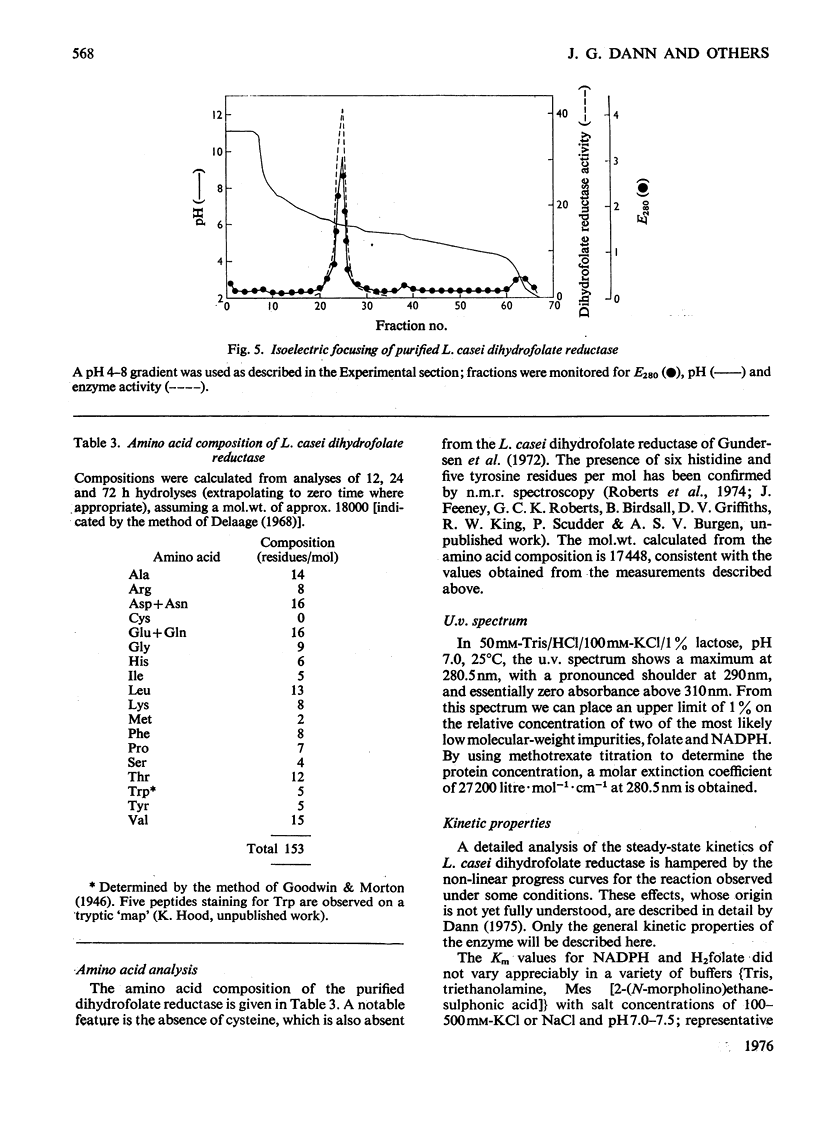
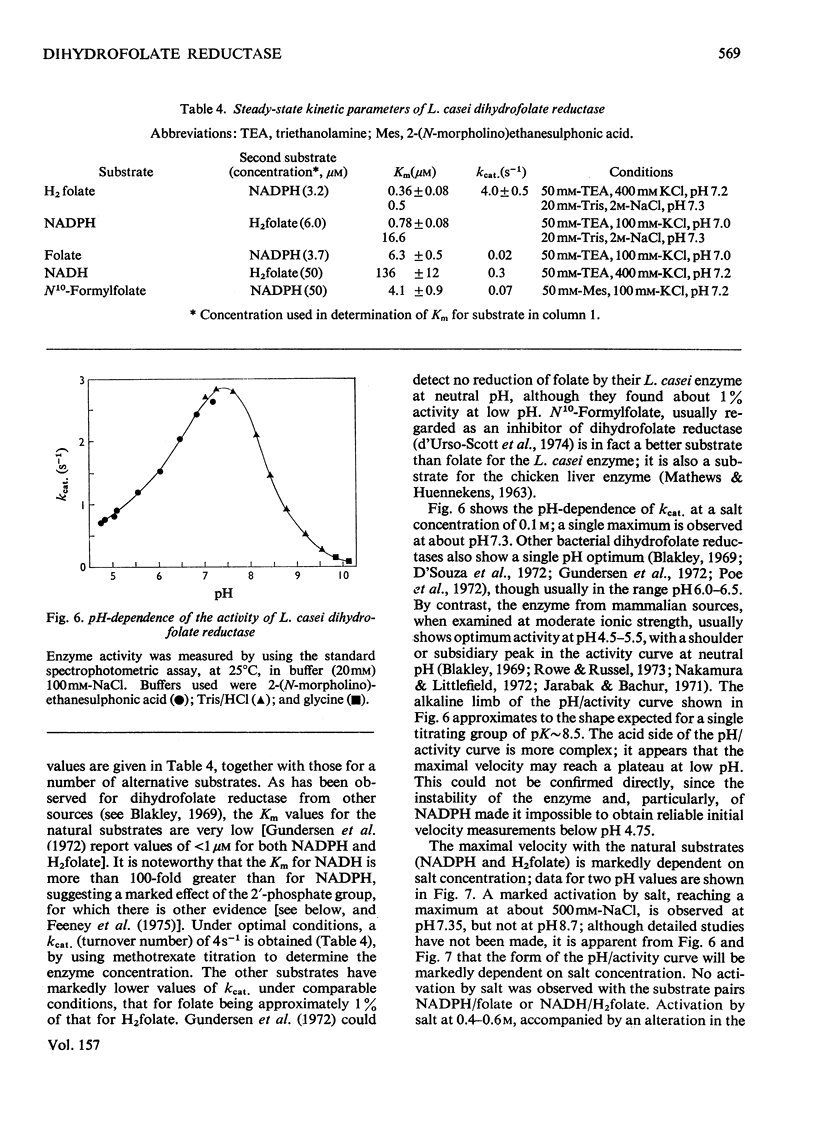
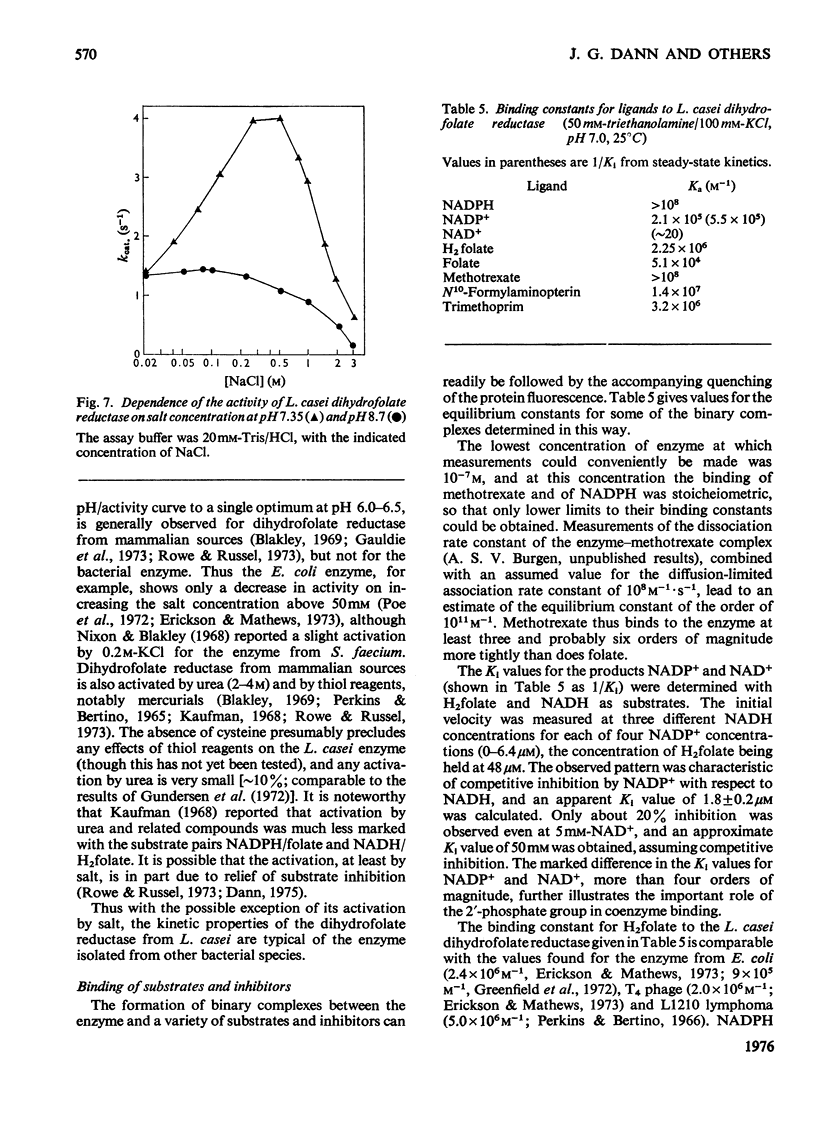
Dihydrofolate reductase has been purified from a methotrexate-resistant strain of Lactobacillus casei NCB 6375. By careful attention to growth conditions, up to 2.5 g of enzyme is obtained from a 400 litre culture. The purification procedure, involving poly-ethyleneimine treatment, DEAE-cellulose chromatography and affinity chromatography on methotrexate-aminohexyl-Sepharose, operates on the gram scale, with overall yields of 50-60%. Elution of the affinity column by reverse (upward) flow was used, as it led to recovery of the enzyme in a much smaller volume. The enzyme obtained appears to be more than 98% pure, as judged by gel electrophoresis, isoelectric focusing, and gel filtration. It has a mol.wt. of approx. 17900 and a turnover number of 4s-1 (50mM-triethanolamine/400mM-KCl, pH 7.2, 25 degrees C) with dihydrofolate and NADPH as substrates. The turnover number for folate is 0.02s-1. Michaelis constants for a variety of substrates have been measured by using a new fluorimetric assay (0.36 muM-dihydrofolate; 0.78 muM-NADPH), and binding constants determined by using the quenching of protein fluorescence (dihydrofolate, 2.25 X 10(6)M-1; NADPH, greater than 10(8)M-1). The pH/activity profile shows a single maximum at pH 7.3; at this pH, marked activation by 0.5M-NaCl is observed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht A. M., Hutchison D. J. Folate reductase and specific dihydrofolate reductase activities of the amethopterin-sensitive Streptococcus faecium var. durans. J Bacteriol. 1969 Oct;100(1):533–534. doi: 10.1128/jb.100.1.533-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson A., Jack G. W. Precipitation of nucleic acids with polyethyleneimine and the chromatography of nucleic acids and proteins on immobilised polyethyleneimine. Biochim Biophys Acta. 1973 Apr 21;308(7):41–52. doi: 10.1016/0005-2787(73)90120-2. [DOI] [PubMed] [Google Scholar]
- BLAKLEY R. L. The reaction of tetrahydropteroylglutamic acid and related hydropteridines with formaldehyde. Biochem J. 1959 Aug;72:707–715. doi: 10.1042/bj0720707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Wilson K. J. Dihydrofolate reductase from bovine liver. Enzymatic and structural properties. Eur J Biochem. 1975 Dec 1;60(1):9–15. doi: 10.1111/j.1432-1033.1975.tb20969.x. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Chello P. L., Cashmore A. R., Jacobs S. A., Bertino J. R. Improved purification of tetrahydrofolate dehydrogenase from L1210 leukemia by affinity chromatography. Biochim Biophys Acta. 1972 Apr 7;268(1):30–34. doi: 10.1016/0005-2744(72)90193-3. [DOI] [PubMed] [Google Scholar]
- D'Souza L., Warwick P. E., Freisheim J. H. Purification and properties of dihydrofolate reductase from an amethopterin-resistant strain of Streptococcus faecium. Biochemistry. 1972 Apr 11;11(8):1528–1534. doi: 10.1021/bi00758a030. [DOI] [PubMed] [Google Scholar]
- D'Urso-Scott M., Uhoch J., Bertino J. R. Formation of 10-formylfolic acid, a potent inhibitor of dihydrofolate reductase, in rat liver slices incubated with folic acid. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2736–2739. doi: 10.1073/pnas.71.7.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaage M. Sur la recherche du poids moléculaire le plus cohérent avec l'analyse des acides aminés d'une protéine. Biochim Biophys Acta. 1968 Dec 3;168(3):573–575. [PubMed] [Google Scholar]
- Dunlap R. B., Gundersen L. E., Huennekens F. M. Interconversion of the multiple forms of dihydrofolate reductase from amethopterin-resistant Lactobacillus casei. Biochem Biophys Res Commun. 1971 Mar 5;42(5):772–777. doi: 10.1016/0006-291x(71)90495-5. [DOI] [PubMed] [Google Scholar]
- Dunlap R. B., Harding N. G., Huennekens F. M. Thymidylate synthetase from amethopterin-resistant Lactobacillus casei. Biochemistry. 1971 Jan 5;10(1):88–97. doi: 10.1021/bi00777a014. [DOI] [PubMed] [Google Scholar]
- Erickson J. S., Mathews C. K. Dihydrofolate reductases of Escherichia coli and bacteriophage Tr. A spectrofluorometric study. Biochemistry. 1973 Jan 30;12(3):372–380. doi: 10.1021/bi00727a002. [DOI] [PubMed] [Google Scholar]
- Erickson J. S., Mathews C. K. T4 bacteriophage-specific dihydrofolate reductase: purification to homogeneity by affinity chromatography. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1164–1170. doi: 10.1016/0006-291x(71)90585-7. [DOI] [PubMed] [Google Scholar]
- Feeney J., Birdsall B., Roberts G. C., Burgen A. S. 31P NMR studies of NADPH and NADP+ binding to L. casei dihydrofolate reductase. Nature. 1975 Oct 16;257(5527):564–566. doi: 10.1038/257564a0. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Hillcoat B. L. Purification of tetrahydrofolate dehydrogenase by affinity chromatography. Biochim Biophys Acta. 1972 Apr 7;268(1):35–40. doi: 10.1016/0005-2744(72)90194-5. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Marshall L., Hillcoat B. L. Purification and properties of dihydrofolate reductase from cultured mammalian cells. Biochem J. 1973 Jun;133(2):349–356. doi: 10.1042/bj1330349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield N. J., Williams M. N., Poe M., Hoogsteen K. Circular dichroism studies of dihydrofolate reductase from a methotrexate-resistant strain of Escherichia coli. Biochemistry. 1972 Dec 5;11(25):4706–4711. doi: 10.1021/bi00775a011. [DOI] [PubMed] [Google Scholar]
- Gundersen L. E., Dunlap R. B., Harding N. G., Freisheim J. H., Otting F., Huennekens F. M. Dihydrofolate reductase from amethopterin-resistant Lactobacillus casei. Biochemistry. 1972 Mar 14;11(6):1018–1023. doi: 10.1021/bi00756a011. [DOI] [PubMed] [Google Scholar]
- Hillcoat B. L., Nixon P. F., Blakley R. L. Effect of substrate decomposition on the spectrophotometric assay of dihydrofolate reductase. Anal Biochem. 1967 Nov;21(2):178–189. doi: 10.1016/0003-2697(67)90179-0. [DOI] [PubMed] [Google Scholar]
- Jarabak J., Bachur N. R. A soluble dihydrofolate reductase from human placenta: purification and properties. Arch Biochem Biophys. 1971 Feb;142(2):417–425. doi: 10.1016/0003-9861(71)90505-4. [DOI] [PubMed] [Google Scholar]
- Kaufman B. T. Isoelectric focusing studies on dihydrofolic reductase. Ann N Y Acad Sci. 1971 Nov 30;186:100–106. doi: 10.1111/j.1749-6632.1971.tb46959.x. [DOI] [PubMed] [Google Scholar]
- Kaufman B. T. Methotrexate-agarose in the purification of dihydrofolate reductase. Methods Enzymol. 1974;34:272–281. doi: 10.1016/s0076-6879(74)34025-6. [DOI] [PubMed] [Google Scholar]
- Kaufman B. T. Studies on dihydrofolic reductase. 3. Activation of the chicken liver enzyme by urea and thiourea. J Biol Chem. 1968 Nov 25;243(22):6001–6008. [PubMed] [Google Scholar]
- MATHEWS C. K., HUENNEKENS F. M. FURTHER STUDIES ON DIHYDROFOLIC REDUCTASE. J Biol Chem. 1963 Oct;238:3436–3442. [PubMed] [Google Scholar]
- MATHEWS C. K., SUTHERLAND K. E. COMPARATIVE BIOCHEMISTRY OF BACTERIAL AND PHAGE-INDUCED DIHYDROFOLATE REDUCTASES. J Biol Chem. 1965 May;240:2142–2147. [PubMed] [Google Scholar]
- NURMIKKO V., SOINI J., AAERIMAA O. FORMATION OF FOLATE ENZYMES DURING THE GROWTH CYCLE OF BACTERIA. 3. CHANGES IN TETRAHYDROFOLATE DEHYDROGENASE ACTIVITY DURING THE ACTIVE GROWTH PHASES OF STREPTOCOCCUS THERMOPHILUS AND LACTOBACILLUS ARABINOSUS. Acta Chem Scand. 1965;19:129–134. doi: 10.3891/acta.chem.scand.19-0129. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Littlefield J. W. Purification, properties, and synthesis of dihydrofolate reductase from wild type and methotrexate-resistant hamster cells. J Biol Chem. 1972 Jan 10;247(1):179–187. [PubMed] [Google Scholar]
- Newbold P. C., Harding N. G. Affinity chromatography of dihydrofolate reductase. Biochem J. 1971 Aug;124(1):1–12. doi: 10.1042/bj1240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon P. F., Blakley R. L. Dihydrofolate reductase of Streptococcus faicium. II. Purification and some properties of two dihydrofolate reductases from the amethopterin-resistant mutant Streptococcus faecium var. Durans strain A. J Biol Chem. 1968 Sep 25;243(18):4722–4731. [PubMed] [Google Scholar]
- Ohara O., Silber R. Studies on the regulation of one-carbon metabolism. The effects of folate concentration in the growth medium on the activity of three folate-dependent enzymes in Lactobacillus casei. J Biol Chem. 1969 Apr 25;244(8):1988–1993. [PubMed] [Google Scholar]
- PERKINS J. P., BERTINO J. R. INTERACTION OF ORGANIC MERCURIAL COMPOUNDS WITH DIHYDROFOLATE REDUCTASE FROM EHRLICH ASCITES CARCINOMA CELLS. Biochemistry. 1965 May;4:847–853. doi: 10.1021/bi00881a008. [DOI] [PubMed] [Google Scholar]
- Pastore E. J., Plante L. T., Kisliuk R. L. Pteroyllysine-agarose in the purification of dihydrofolate reductase. Methods Enzymol. 1974;34:281–288. doi: 10.1016/s0076-6879(74)34026-8. [DOI] [PubMed] [Google Scholar]
- Perkins J. P., Bertino J. R. Dihydrofolate reductase from the L1210R murine lymphoma. Fluorometric measurements of the interaction of the enzyme with coenzymes, substrates, and inhibitors. Biochemistry. 1966 Mar;5(3):1005–1012. doi: 10.1021/bi00867a028. [DOI] [PubMed] [Google Scholar]
- Poe M., Greenfield N. J., Hirshfield J. M., Williams M. N., Hoogsteen K. Dihydrofolate reductase. Purification and characterization of the enzyme from an amethopterin-resistant mutant of Escherichia coli. Biochemistry. 1972 Mar 14;11(6):1023–1030. doi: 10.1021/bi00756a012. [DOI] [PubMed] [Google Scholar]
- Poe M., Greenfield N. J., Williams M. N. Dihydrofolate reductase from a methotrexate-resistant Escherichia coli. J Biol Chem. 1974 May 10;249(9):2710–2716. [PubMed] [Google Scholar]
- Roberts G. C., Feeney J., Burgen A. S., Yuferov V., Dann J. G., Bjur R. Nuclear magnetic resonance studies of the binding of substrate analogs and coenzyme to dihydrofolate reductase from Lactobacillus casei. Biochemistry. 1974 Dec 17;13(26):5351–5357. doi: 10.1021/bi00723a015. [DOI] [PubMed] [Google Scholar]
- Roberts G. C. The binding of substrates and inhibitors to dihydrofolate reductase. Biochem Soc Trans. 1975;3(5):630–631. doi: 10.1042/bst0030630. [DOI] [PubMed] [Google Scholar]
- Rowe P. B., Russel P. J. Dihydrofolate reductase. Studies on the activation of the bovine liver enzyme. J Biol Chem. 1973 Feb 10;248(3):984–991. [PubMed] [Google Scholar]
- Way J. L., Birdsall B., Feeney J., Roberts G. C., Burgen A. S. A nuclear magnetic resonance study of nicotinamide adenine dinucleotide phosphate binding to Lactobacillus casei dihydrofolate reductase. Biochemistry. 1975 Jul 29;14(15):3470–3475. doi: 10.1021/bi00686a028. [DOI] [PubMed] [Google Scholar]
- Whiteley J. M., Jackson R. C., Mell G. P., Drais J. H., Huennekens F. M. Folate antagonists covalently linked to carbohydrates: synthesis, properties, and use in the purification of dihydrofolate reductases. Arch Biochem Biophys. 1972 May;150(1):15–22. doi: 10.1016/0003-9861(72)90004-5. [DOI] [PubMed] [Google Scholar]
- Williams M. N., Greenfield N. J., Hoogsteen K. Evidence for two reduced triphosphopyridine nucleotide binding sites on dihydrofolate reductase from a methotrexate-resistant strain of Escherichia coli. J Biol Chem. 1973 Sep 25;248(18):6380–6386. [PubMed] [Google Scholar]


