Abstract
Induction of virus-specific T-cell responses in mucosal as well as systemic compartments of the immune system is likely to be a critical feature of an effective AIDS vaccine. We investigated whether virus-specific CD8+ lymphocytes induced in rhesus macaques by immunization with attenuated simian immunodeficiency virus (SIV), an approach that is highly effective in eliciting protection against mucosal challenge, express the mucosa-homing receptor α4β7 and traffic to the intestinal mucosa. SIV-specific CD8+ T cells expressing α4β7 were detected in peripheral blood and intestine of macaques infected with attenuated SIV. In contrast, virus-specific T cells in blood of animals immunized cutaneously by a combined DNA-modified vaccinia virus Ankara regimen did not express α4β7. These results demonstrate the selective induction of SIV-specific CD8+ T lymphocytes expressing α4β7 by a vaccine approach that replicates in mucosal tissue and suggest that induction of virus-specific lymphocytes that are able to home to mucosal sites may be an important characteristic of a successful AIDS vaccine.
Transmission of human immunodeficiency virus (HIV) infection occurs predominantly across genital or rectal mucosal surfaces. Following viral dissemination, the large pool of activated CD4+ T cells that reside in gut-associated lymphoid tissue (GALT) serves as a predominant site of HIV or simian immunodeficiency virus (SIV) replication (25, 26, 28). Thus, the ability of virus-specific immune responses to prevent or contain HIV or SIV replication in mucosal sites is likely to play a critical role in the ability of the host to defend itself against lentiviral infection.
The mucosal immune system is functionally and phenotypically distinct from the peripheral immune system (3, 20). Induction of immune responses by peripheral immunization at cutaneous or intramuscular sites results in antigen-specific lymphocytes that do not efficiently traffic to mucosal sites (3, 20). Homing of lymphocytes to cutaneous or mucosal sites is determined in part by expression of cell surface adhesion molecules (5). The intestinal homing receptor α4β7 plays a central role in directing migration of lymphocytes into intestinal mucosal tissue (30). Lymphocytes expressing α4β7 bind to the mucosal addressin MAdCAM-1, which is selectively expressed on capillary endothelium in gastrointestinal immune inductive and effector sites (4, 12).
Despite evidence for the compartmentalization of peripheral and mucosal immune systems, little information is available on the ability of different AIDS vaccine strategies to induce cytotoxic T lymphocytes (CTL) that are able to home to mucosal sites. HIV-1 and SIV-specific CTL have been detected in genital and gastrointestinal mucosal tissues following infection with pathogenic viruses (8, 19, 21, 22). However, there has been little or no information on the ability of candidate AIDS vaccines to induce cellular immune responses at mucosal sites. In this study, we asked whether CD8+ T cells induced in macaques by immunization with attenuated SIV express the intestinal homing receptor α4β7 and home to gastrointestinal lymphoid tissue. Induction of α4β7+ virus-specific CD8+ T cells was also investigated in monkeys that were immunized cutaneously with a combined DNA-modified vaccinia virus Ankara strain vaccine expressing an immunodominant SIV CTL epitope.
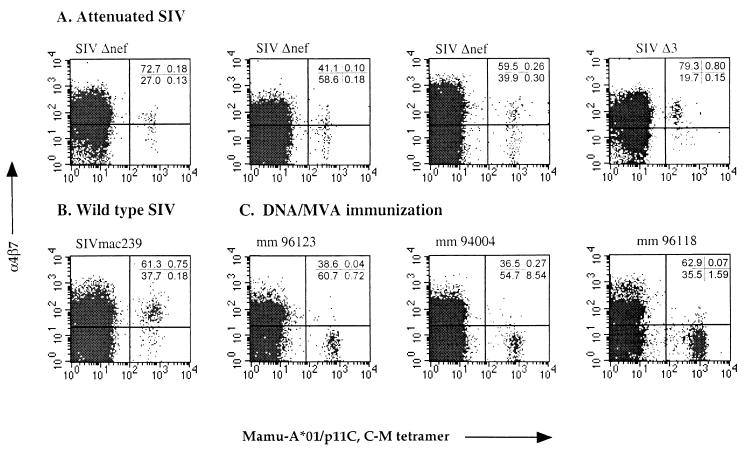
In general, induction of memory lymphocytes expressing α4β7 requires viral infection at gastrointestinal sites and is not observed with infection at extraintestinal sites (24). However, because replication of attenuated SIV occurs preferentially in the pool of activated CD4+ T lymphocytes found in GALT, even following intravenous infection (28), we reasoned that macaques intravenously infected with attenuated SIV would have SIV-specific CD8+ T cells that express α4β7. To address this hypothesis, we studied adult rhesus macaques infected intravenously with either SIVmac239Δnef, containing a 182-bp deletion in nef (17), or SIVmac293Δ3, which contains deletions in nef, vpr, and upstream short sequences of the long terminal repeat (31) 5 to 10 years after infection. Two of these animals, 353.88 and 71.88, had been challenged intravenously with pathogenic SIVmac239 or SIVmac251 3 to 7 years earlier; none of the monkeys exhibited evidence of infection with pathogenic virus at the time of this study (10, 31). SIV-specific CD8+ T cells were identified by multiparameter flow cytometry using tetramers of the rhesus major histocompatibility complex (MHC) class I molecule Mamu-A*01 complexed with the immunodominant Gag peptide p11C,C→M (CTPYDINQM) (1, 13). Tetramers were prepared from purified monomers by the gradual addition over 24 h of a allophycocyanin (APC)–streptavidin (Molecular Probes) to a 4:1 final molar ratio. Four monkeys were identified as expressing Mamu-A*01 using the PCR SSP as previously described (18). Immunofluorescent staining was performed on Ficoll-Hypaque-isolated peripheral blood mononuclear cells (PBMC) or whole blood using Mamu-A*01–peptide tetramers and anti-α4β7 antibody (a gift from LeukoSite, Inc.) on a FACSCalibur flow cytometer (Becton Dickinson). Four-color analysis of CD3+ CD8+ gated lymphocytes revealed between 0.28 and 0.95% tetramer-binding cells in peripheral blood of live attenuated SIV-immunized monkeys (Fig. 1A and Table 1). Two monkeys infected with wild-type SIV (SIVmac239) had 0.93 and 0.32% tetramer-binding CD8+ T cells (Fig. 1B and Table 1). Simultaneous analysis of tetramer binding and expression of α4β7 revealed that between 36 and 84% of SIV-specific CD8+ T cells in animals vaccinated with live attenuated SIV expressed α4β7, a frequency generally similar to the level of expression on all CD3+ CD8+ T cells. A comparable percentage of tetramer binding cells in wild-type SIV-infected animals expressed α4β7. These findings support the conclusion that replication of attenuated SIV in intestinal lymphoid tissue results in a virus-specific CD8+ T cell response that is capable of intestinal homing. These results are also consistent with a previous report describing expression of α4β7 by circulating T cells recognizing rotavirus, a pathogen for which replication is generally restricted to the small intestine (24).
FIG. 1.
The mucosal homing receptor α4β7 is expressed on SIV-specific CD8+ T cells in peripheral blood of attenuated SIV-infected but not DNA-MVA-immunized macaques. PBMC from macaques infected with attenuated SIV (A) or pathogenic SIVmac (B) were analyzed by flow cytometry for tetramer binding and α4β7 expression after gating on CD3+ and CD8+ lymphocytes. The percentage of cells in each quadrant is indicated for each plot. (C) PBMC from macaques immunized cutaneously with DNA and MVA. PBMC were analyzed by flow cytometry for tetramer binding and α4β7 expression on CD3+ CD8+ lymphocytes 1 week after the last boost.
TABLE 1.
Expression of the mucosal homing receptor α4β7 on SIV-specific CD8+ T cells in peripheral blood of vaccinated monkeys
| Animal | SIV strain or immunizationa | % Tetramer-binding cellsb | % α4β7+ of:
|
|
|---|---|---|---|---|
| Tetramer+ cellsc | CD3+ CD8+ T cellsd | |||
| 118.87 | Δnef | 0.28 | 36 | 41 |
| 353.88 | Δnef | 0.31 | 58 | 73 |
| 71.88 | Δnef | 0.56 | 46 | 60 |
| 313.91 | Δ3 | 0.95 | 84 | 79 |
| 96118 | DNA i.d./MVA i.d. | 1.69 | 5 | 67 |
| 96123 | DNA i.d./MVA i.d. | 0.77 | 5 | 42 |
| 94004 | DNA i.d./MVA i.d. | 9.10 | 6 | 41 |
| 19777 | Wild-type SIVmac | 0.93 | 81 | 62 |
| 95114 | Wild-type SIVmac | 0.32 | 22 | 36 |
| 158.87 | Uninfected | 0.03 | 0 | 78 |
i.d., intradermal.
Percentage of Mamu-A*01/p11C,C→M tetramer-binding cells in PBMC determined after gating on CD3+ CD8+ lymphocytes.
Percentage of CD3+ CD8+ tetramer-binding cells that also express α4β7 integrin.
Percentage of all CD3+ CD8+ lymphocytes that express α4β7.
In order to examine whether induction of α4β7+ SIV-specific CD8+ T cells necessarily requires antigen presentation in gastrointestinal tissue, we examined the phenotype of SIV-specific CD8+ T cells induced by cutaneous immunization with DNA and modified vaccinia virus Ankara (MVA). Three Mamu-A*01-positive macaques were immunized with a multiepitope gene using a DNA prime-MVA boost regimen as described previously, which has been shown to induce p11C,C→M-specific CTL in peripheral blood of immunized macaques (2, 14). The DNA vaccine was administered to the skin on five occasions using the Dermal PowderJect XR “gene gun” (PowderJect Vaccines, Madison, Wis.), followed by two boosts with MVA delivered intradermally. Cutaneous immunization with MVA results in localized expression of viral antigens, since MVA infects but does not replicate in mammalian cells (27), and thus tetramer-positive CD8+ T cells induced by immunization with DNA-MVA would be expected to be negative for α4β7. We therefore examined blood from DNA-MVA-immunized monkeys for tetramer binding and α4β7 expression. In contrast to CD8+ T cells from monkeys infected with attenuated SIV, tetramer-binding cells induced by the DNA-MVA vaccine regimen 1 week after a cutaneous MVA boost did not express α4β7 (Fig. 1C). Similar results were observed at 2 and 3 weeks after MVA boosting (data not shown). These results suggest that antigen-specific T cells induced by cutaneous immunization with vaccines that do not disseminate to GALT are unlikely to express α4β7, a conclusion supported by the prior observation that memory T cells induced by peripheral immunization with an attenuated mumps vaccine did not express α4β7 (24). However, the DNA-MVA- and attenuated SIV-vaccinated animals may differ in several respects, including the degree of T-cell activation and the presence of T-cell help, and further studies with expanded numbers of vaccinated animals will be necessary to document whether expression of α4β7 on antigen-specific T cells is necessarily induced only by antigen presentation in GALT.
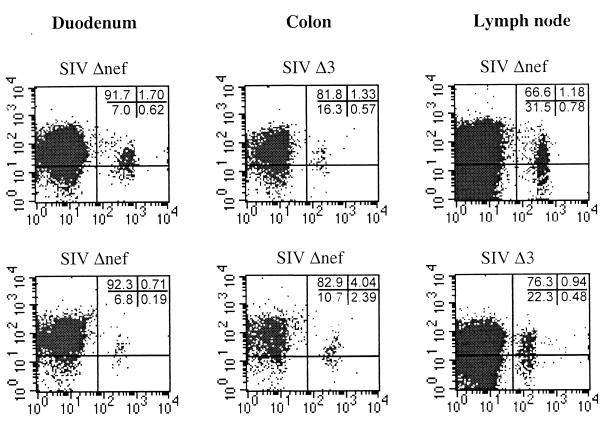
Having established that attenuated SIV immunization elicits α4β7+ SIV-Gag-specific CTL in peripheral blood, we investigated whether cells with this phenotype were able to home to gastrointestinal mucosal sites. Lymphocytes were isolated from endoscope-guided pinch biopsies of duodenum and colon and excisional biopsies of peripheral lymph node from four macaques immunized with attenuated SIV, and the cells were analyzed for expression of α4β7 and tetramer binding. Cells were isolated from intestinal tissues using a combination of mechanical and enzymatic dissociation procedures described elsewhere (29). Lymphocyte populations isolated using this procedure include a mixture of intraepithelial and lamina propria lymphocytes. SIV-specific CD8+ T cells were detected in peripheral lymph nodes and in both intestinal sites of all four animals. Three of four monkeys exhibited increases of 2- to 20-fold in tetramer-binding cells in one or both intestinal sites compared to levels in peripheral blood (Table 2 and Fig. 2). Higher percentages of tetramer-binding cells were also found in peripheral lymph nodes relative to levels in blood in two out of three animals. At least 50% of tetramer-binding cells in duodenum, colon, and lymph node expressed α4β7, a similar proportion to that observed in peripheral blood. As expected, the total CD3+ CD8+ lymphocyte population in duodenum and colon were enriched for α4β7+ cells compared to these populations in peripheral blood and lymph node (Table 2).
TABLE 2.
Expression of the mucosal homing receptor α4β7 on SIV-specific CD8+ T cells in blood, lymph node, and intestine of monkeys vaccinated with live attenuated SIV
| PBMC
|
Lymph node cells
|
Duodenum
|
Colon
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | % Tetramer-binding cellsa | % α4β7+ of:
|
% Tetramer-binding cells | % α4β7+ of:
|
% Tetramer-binding cells | % α4β7+ of:
|
% Tetramer-binding cells | % α4β7+ of:
|
||||
| Tetramer+b | CD3+ CD8+c | Tetramer+ | CD3+ CD8+ | Tetramer+ | CD3+ CD8+ | Tetramer+ | CD3+ CD8+ | |||||
| 118.87 | 0.28 | 36 | 41 | 1.96 | 60 | 68 | 0.24 | 54 | 78 | 0.47 | 40 | 72 |
| 353.88 | 0.31 | 58 | 73 | NTd | NT | NT | 0.90 | 79 | 87 | 6.4 | 63 | 87 |
| 71.88 | 0.56 | 46 | 60 | 0.80 | 38 | 67 | 2.32 | 73 | 94 | 0.32 | 81 | 90 |
| 313.91 | 0.95 | 84 | 79 | 1.31 | 63 | 75 | 0.38 | 84 | 87 | 1.9 | 70 | 83 |
Percentage of Mamu-A*01/p11C-M tetramer-binding cells, determined after gating on CD3+ CD8+ lymphocytes.
Percentage of tetramer-binding cells expressing α4β7 integrin, determined after gating on CD3+ CD8+ lymphocytes.
Percentage of all CD3+ CD8+ lymphocytes expressing α4β7.
NT, not tested.
FIG. 2.
SIV-specific CD8+ T cells in attenuated SIV-immunized macaques home to intestinal mucosa and express α4β7. Cells were isolated from fresh biopsy specimens, stained and analyzed by flow cytometry for tetramer binding and α4β7 expression on CD3+ CD8+ lymphocytes. A minimum of 100,000 events were collected for each analysis.
The ability of virus-specific T cells to home to mucosal sites of viral replication plays a critical role in their ability to control viral replication. Evidence for the importance of mucosal homing can be found in pulmonary influenza virus infection, where the ability of adoptively transferred CD8+ T cells to migrate to the site of infection is critical for protection (6). This also appears to be the case in intestinal rotavirus infection, where viral clearance has been correlated with induction of virus-specific CD8+ T cells expressing α4β7 (23). Similarly, subcutaneous immunization of mice with an HIV peptide immunogen induced systemic but not mucosal CTL and failed to protect mice against rectal challenge with a recombinant vaccinia virus expressing the HIV-1 envelope, whereas rectal immunization induced both mucosal and systemic CTL and protection against mucosal challenge (3). It is probable that mucosal homing will prove to also be important for protection against HIV and SIV infection. While the DNA prime-MVA boost vaccination regimen (14), as well as several other HIV vaccine approaches (7, 11), are effective at inducing virus-specific CD8+ T cells, no information is presently available regarding the mucosal homing ability of cells induced by these vaccines, and little is known about their protective effects. Our recent results suggest that virus-specific CD8+ T cells induced by the DNA-MVA vaccine may not be able to traffic to the intestine, possibly rendering this approach less effective in inducing protection against SIV or HIV infection. Consistent with this hypothesis, an initial report examining the ability of a cutaneous DNA-MVA vaccination regimen to protect against rectal SIV challenge failed to observe protection in two of three animals studied, despite the fact that levels of tetramer-binding cells at the time of challenge ranged from 1 to 5% of CD8+ T cells in peripheral blood (14), a level approximately two- to fivefold greater than we observed in animals vaccinated with attenuated SIV. In contrast, attenuated SIV immunization has previously been shown to protect against rectal and vaginal SIV challenge (9, 16).
In summary, our results indicate that virus-specific CD8+ T cells are induced by immunization with attenuated SIV express α4β7 and home to mucosal sites, whereas those induced by a DNA-MVA vaccine lack expression of the intestinal homing receptor. Since induction of virus-specific immune responses in mucosal sites is likely to be a critical component of an effective AIDS vaccine, these findings may have important implications for future vaccine design.
All animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (15) and institutional animal use committee guidelines.
Acknowledgments
This work was supported by Public Health Service grants RR00168, AI43044, AI43045, and DK50550. R.P.J., A.A.L., and D.I.W. are Elizabeth Glaser Scientists and are supported by the Elizabeth Glaser Pediatric AIDS Foundation.
We thank Ron Desrosiers, Kelledy Manson, and Michael Wyand for providing samples from SIV-infected animals, Michael Briskin and Meryl Forman for the conjugated α4β7 antibody, and Ron Desrosiers for helpful discussions and review of the manuscript.
REFERENCES
- 1.Allen T M, Sidney J, delGuercio M-F, Glickman R L, Lensmeyer G L, Wiebe D A, Pauza C D, Johnson R P, Sette A, Watkins D I. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from SIV. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 2.Allen T M, Vogel T U, Fuller D H, Mothe B R, Steffen S, Boyson J E, Shipley T, Fuller J, Hanke T, Sette A, Altman J D, Moss B, McMichael A J, Watkins D I. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J Immunol. 2000;164:4968–4978. doi: 10.4049/jimmunol.164.9.4968. [DOI] [PubMed] [Google Scholar]
- 3.Belyakov I M, Ahlers J D, Brandwein B Y, Earl P, Kelsall B L, Moss B, Strober W, Berzofsky J A. The imporance of local mucosal HIV-specific CD8+ cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J Clin Investig. 1998;102:2072–2081. doi: 10.1172/JCI5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlin C, Berg E L, Briskin M J, Andrew D A, Kilshaw P J, Holzmann B, Weissman I L, Hamann A, Butcher E C. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 5.Butcher E C. Lymphocyte homing and intestinal immunity. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunity. San Diego, Calif: Academic Press; 1999. pp. 507–522. [Google Scholar]
- 6.Cerwenka A, Morgan T M, Harmsen A G, Dutton R W. Migration kinetics and final destination of type 1 and type 2 CD8+ effector cells predict protection against pulmonary virus infection. J Exp Med. 1999;189:423–434. doi: 10.1084/jem.189.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corey L, McElrath M J, Weinhold K, Matthews T, Stablein D, Graham B, Keefer M, Schwartz D, Gorse G. Cytotoxic T cell and neutralizing antibody responses to human immunodeficiency virus type 1 envelope with a combination vaccine regimen. J Infect Dis. 1998;177:301–309. doi: 10.1086/514202. [DOI] [PubMed] [Google Scholar]
- 8.Couedel-Courteille A, Le Grand R, Tulliez M, Guillet J G, Venet A. Direct ex vivo simian immunodeficiency virus (SIV)-specific cytotoxic activity detected from small intestine intraepithelial lymphocytes of SIV-infected macaques at an advanced stage of infection. J Virol. 1997;71:1052–1057. doi: 10.1128/jvi.71.2.1052-1057.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cranage M P, Whatmore A M, Sharpe S A, Cook N, Polyanskaya N, Leech S, Smith J D, Rud E W, Dennis M J, Hall G A. Macaques infected with live attenuated SIVmac are protected against superinfection via the rectal mucosa. Virology. 1997;229:143–154. doi: 10.1006/viro.1996.8419. [DOI] [PubMed] [Google Scholar]
- 10.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 11.El-Daher N, Keefer M C, Reichman R C, Dolin R, Roberts N J J. Persisting human immunodeficiency virus type 1 gp160-specific human T lymphocyte responses including CD8+ cytotoxic activity after receipt of envelope vaccines. J Infect Dis. 1993;168:306–313. doi: 10.1093/infdis/168.2.306. [DOI] [PubMed] [Google Scholar]
- 12.Hamman A, Andrew D P, Jablonski-Westrich D, Holzmann B, Butcher E C. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 13.Hanke T, Graham F L, Rosenthal K L, Johnson D C. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol. 1991;65:1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanke T, Samuel R V, Blanchard T J, Neumann V C, Allen T M, Boyson J E, Sharpe S A, Cook N, Smith G L, Watkins D I, Cranage M P, McMichael A J. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J Virol. 1999;73:7524–7532. doi: 10.1128/jvi.73.9.7524-7532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. U.S. Department of Health and Human Services publication no. NIH 85-23. Washington, D.C.: National Research Council, National Institutes of Health; 1996. [Google Scholar]
- 16.Johnson R P, Lifson J D, Czajak S C, Cole K S, Manson K H, Glickman R, Yang J, Montefiori D C, Montelaro R, Wyand M S, Desrosiers R C. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J Virol. 1999;73:4952–4961. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 18.Knapp L A, Lehmann E, Piekarczyk M S, Urvater J A, Watkins D I. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens. 1997;50:657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 19.Lohman B L, Miller C J, McChesney M B. Antiviral cytotoxic T lymphocytes in vaginal mucosa of simian immunodeficiency virus-infected rhesus macaques. J Immunol. 1995;155:5855–5860. [PMC free article] [PubMed] [Google Scholar]
- 20.McGhee J R, Lamm M E, Strober W. Mucosal immune responses. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. San Diego, Calif: Academic Press; 1999. pp. 485–506. [Google Scholar]
- 21.Murphey-Corb M, Wilson L A, Trichel A M, Roberts D E, Xu K, Ohkawa S, Woodson B, Bohm R, Blanchard J. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J Immunol. 1999;162:540–549. [PubMed] [Google Scholar]
- 22.Musey L, Hu Y, Eckert L, Christensen M, Karchmer T, McElrath M J. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J Exp Med. 1997;185:293–303. doi: 10.1084/jem.185.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosé J R, Williams M B, Rott L S, Butcher E C, Greenberg H B. Expression of the mucosal homing receptor α4β7 correlates with the ability of CD8+ memory T cells to clear rotavirus infection. J Virol. 1998;72:726–730. doi: 10.1128/jvi.72.1.726-730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rott L S, Rose J R, Bass D, Williams M B, Greenberg H B, Butcher E C. Expression of mucosal homing receptor α4β7 by circulating CD4+ cells with memory for intestinal rotavirus. J Clin Investig. 1997;100:1204–1208. doi: 10.1172/JCI119633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider T, Jahn H U, Schmidt W, Riecken E O, Zeitz M, Ullrich R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut. 1995;37:524–529. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider T, Ullrich R, Zeitz M. The immunological aspects of human immunodeficiency virus infection in the gastrointestinal tract. Semin Gastrointest Dis. 1996;7:19–29. [PubMed] [Google Scholar]
- 27.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D, Pauley D, Knight H L, Rosenzweig M, Johnson R P, Desrosiers R C, Lackner A A. The gastrointestinal tract is the major site of CD4+ lymphocyte depletion and viral replication in primary SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 29.Veazey R S, Tham I C, Mansfield K G, DeMaria M, Forand A E, Shvetz D E, Chalifoux L, Sehgal P K, Lackner A A. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams M B, Butcher E C. Homing of naive and memory T lymphocyte subsets to Peyer's patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
- 31.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]