Abstract
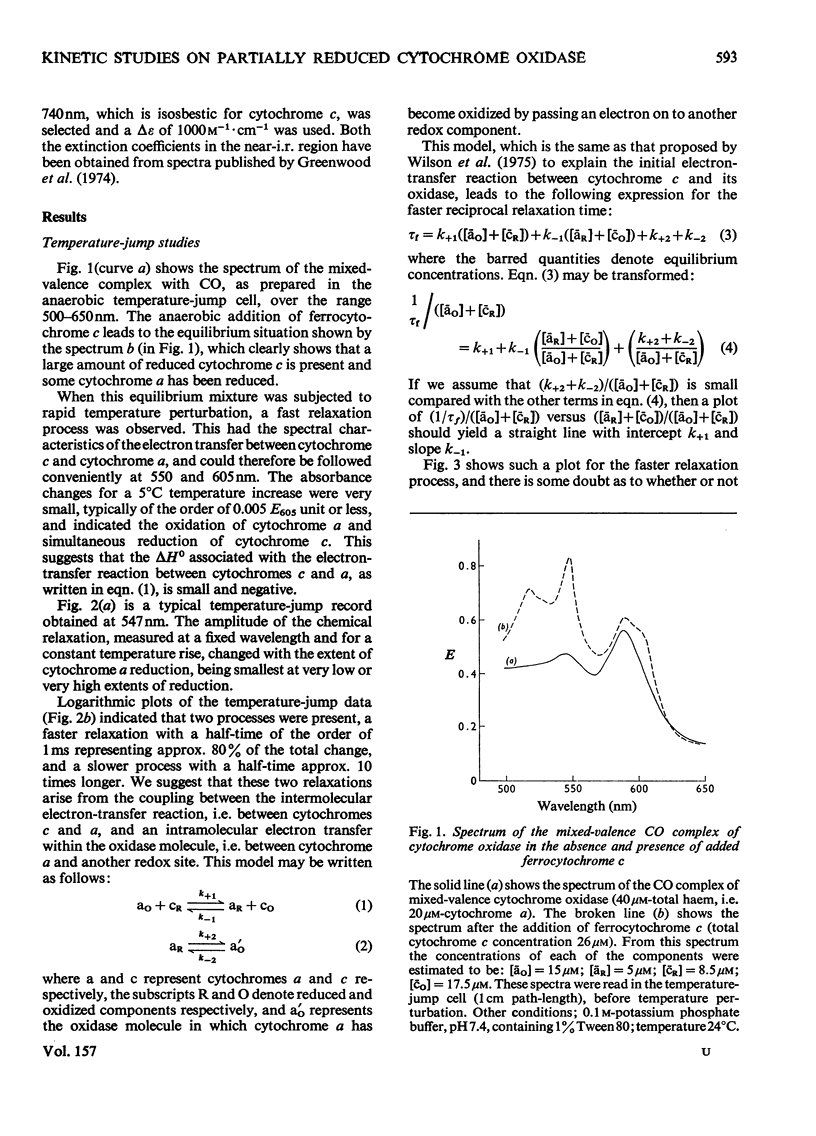
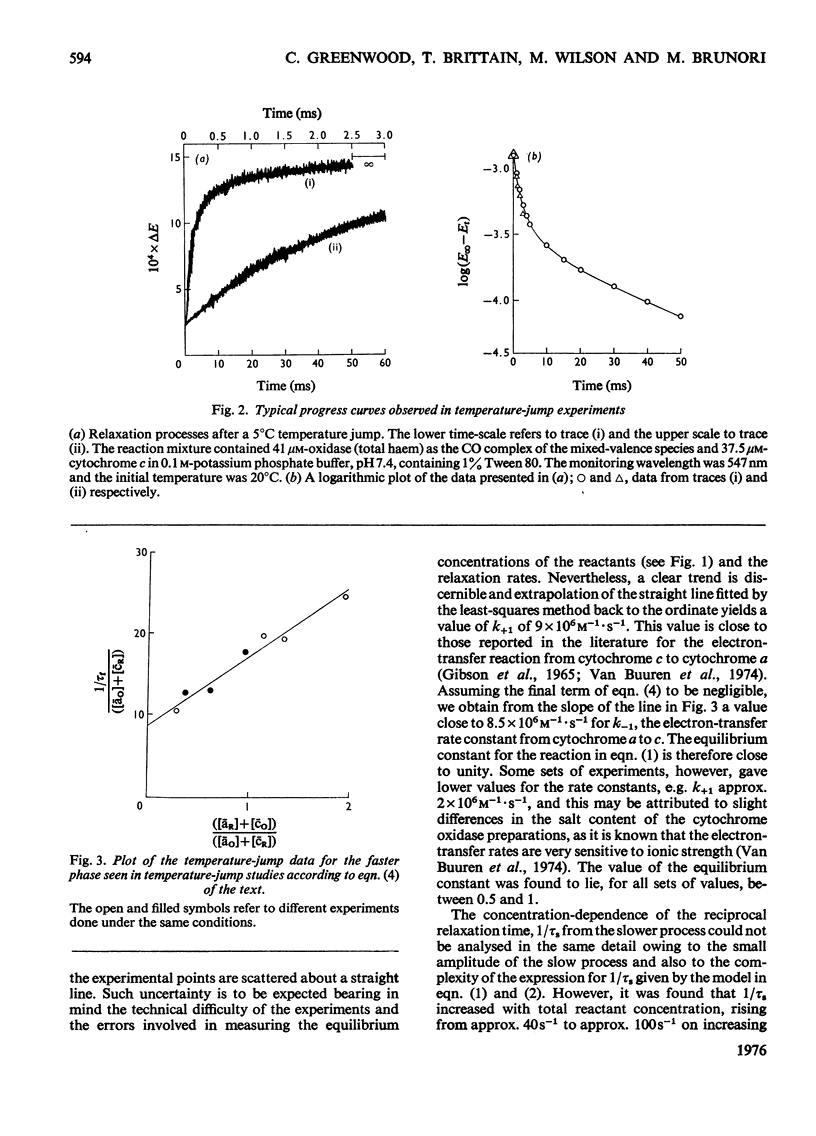
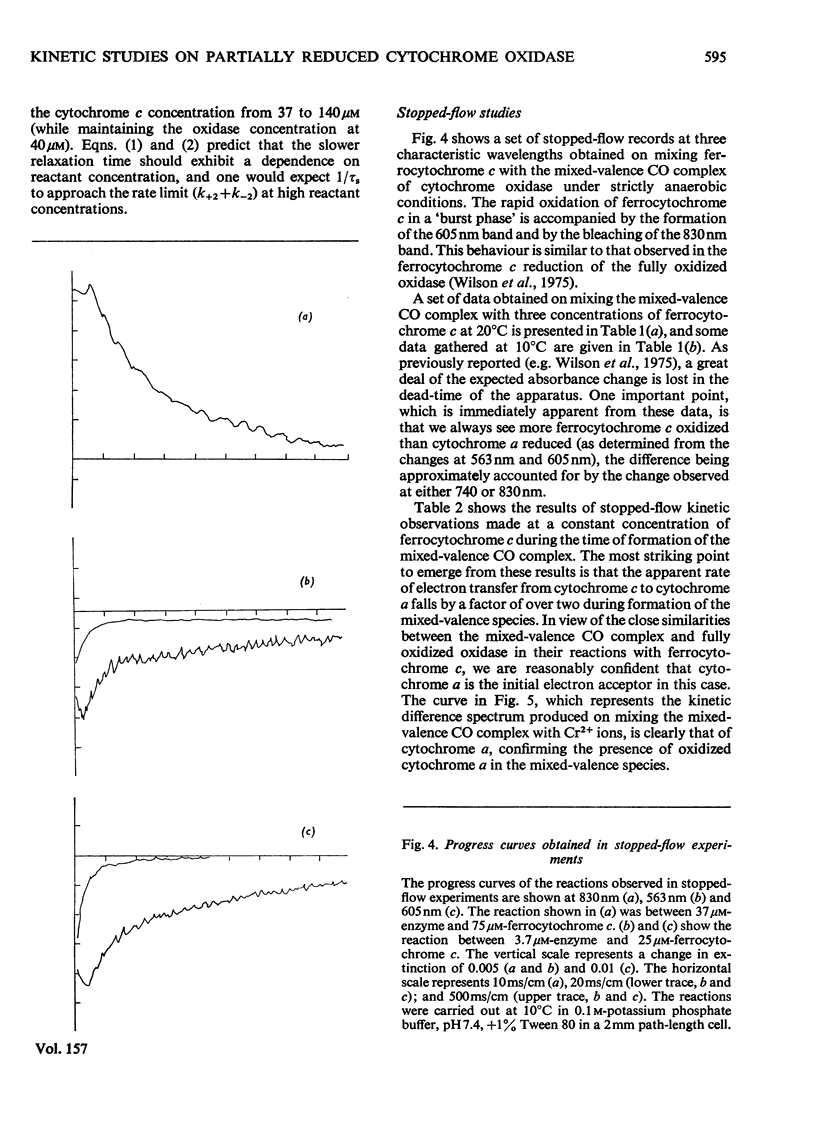
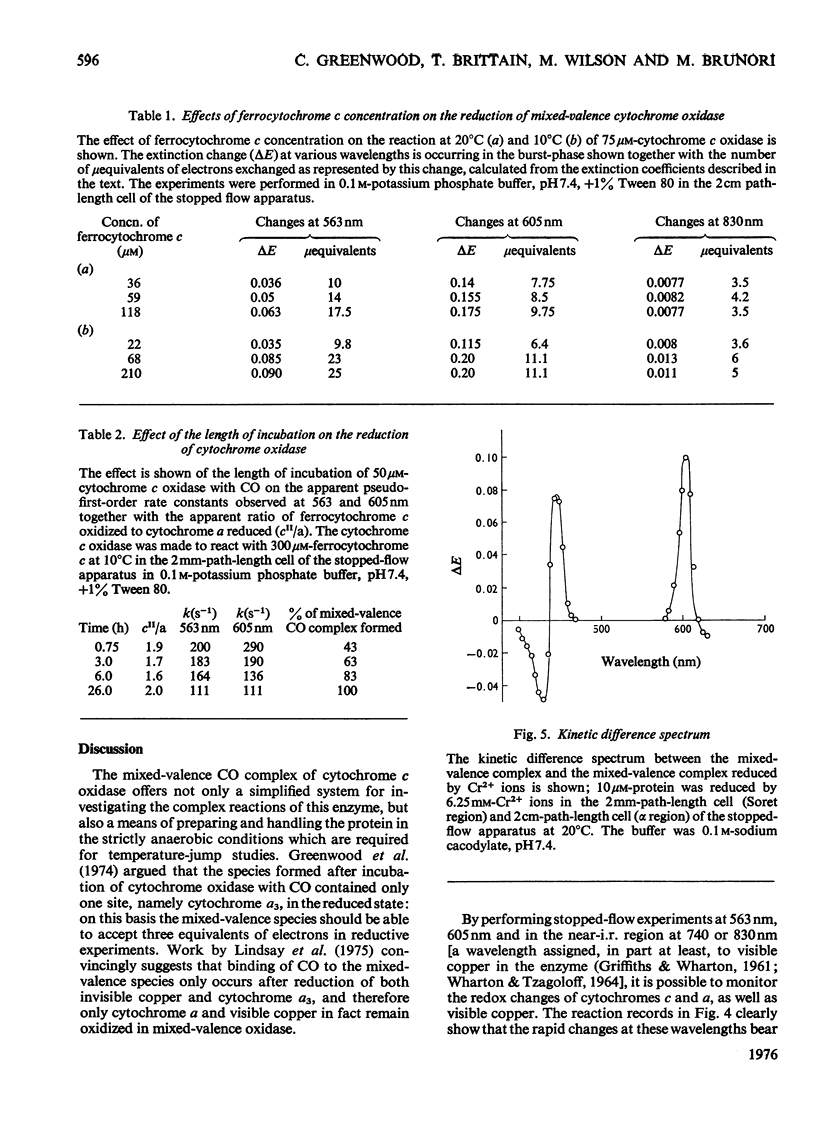
The kinetics of the electron-transfer process which occurs between ferrocytochrome c and partially reduced mammalian cytochrome oxidase were studied by the rapid spectrophotometric techniques of stopped flow and temperature jump. Stopped-flow experiments showed initial very fast extinction changes at 605 nm and at 563 nm, indicating the simultaneous reduction of cytochrome a and oxidation of ferrocytochrome c. During this 'burst' phase, say the first 50 ms after mixing, it was invariably found that more cytochrome c had been oxidized than cytochrome a had been reduced. This discrepancy in electron equivalents may be accounted for by the rapid reduction of another redox site in the enzyme, possibly that associated with the extinction changes observed at 830 nm. During the incubation period in which the partially reduced oxidase was prepared, the rate of reduction of cytochrome a by ferrocytochrome c, at constant reactant concentrations, decreased with time. Temperature-jump experiments showed the presence of two relaxation processes. The faster of the two phases was assigned to the electron-transfer reaction between cytochrome c and cytochrome a. A study of the concentration-dependence of the reciprocal relaxation time for this phase yielded a rate constant of 9 X 10(6)M-1-s-1 for the electron transfer from cytochrome c to cytochrome a, and a value of 8.5 X 10(6)M-1-s-1 for the reverse reaction. The equilibrium constant for the electron-transfer reaction is therefore close to unity. The slower phase has been interpreted as signalling the transfer of electrons between cytochrome a and another redox site within the oxidase molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson L. -E., Malmström B. G., Strömberg C., Vänngård T. The reaction of ferrocytochrome c with cytochrome oxidase: A new look. FEBS Lett. 1972 Dec 15;28(3):297–301. doi: 10.1016/0014-5793(72)80735-x. [DOI] [PubMed] [Google Scholar]
- Andréasson L. E. Characterization of the reaction between ferrocytochrome c and cytochrome c oxidase. Eur J Biochem. 1975 May 6;53(2):591–597. doi: 10.1111/j.1432-1033.1975.tb04102.x. [DOI] [PubMed] [Google Scholar]
- BEINERT H., GRIFFITHS D. E., WHARTON D. C., SANDS R. H. Properties of the copper associated with cytochrome oxidase as studied by paramagnetic resonance spectroscopy. J Biol Chem. 1962 Jul;237:2337–2346. [PubMed] [Google Scholar]
- BEINERT H., PALMER G. OXIDATION-REDUCTION OF THE COPPER COMPONENT OF CYTOCHROME OXIDASE. KINETIC STUDIES WITH A RAPID FREEZING TECHNIQUE. J Biol Chem. 1964 Apr;239:1221–1227. [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- GIBSON Q. H., PALMER G., WHARTON D. C. THE BINDING OF CARBON MONOXIDE BY CYTOCHROME C OXIDASE AND THE RATIO OF THE CYTOCHROMES A AND A3. J Biol Chem. 1965 Feb;240:915–920. [PubMed] [Google Scholar]
- GRIFFITHS D. E., WHARTON D. C. Studies of the electron transport system. XXXVI. Properties of copper in cytochroma oxidase. J Biol Chem. 1961 Jun;236:1857–1862. [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg M. R. Cytochrome oxidase. Physiol Rev. 1969 Jan;49(1):48–121. doi: 10.1152/physrev.1969.49.1.48. [DOI] [PubMed] [Google Scholar]
- Lindsay J. G., Owen C. S., Wilson D. F. The invisible copper of cytochrome c oxidase. pH and ATP dependence of its midpoint potential and its role in the oxygen reaction. Arch Biochem Biophys. 1975 Aug;169(2):492–505. doi: 10.1016/0003-9861(75)90192-7. [DOI] [PubMed] [Google Scholar]
- Muijsers A. O., Tiesjema R. H., Henderson R. W., Van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VII. The effect of cytochrome c on the oxidation-reduction potential of isolated cytochrome aa 3 . Biochim Biophys Acta. 1972 Apr 20;267(1):216–221. doi: 10.1016/0005-2728(72)90154-5. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Petersen L. C. Haem-haem interactions in cytochrome aa3 during the anaerobic-aerobic transition. Biochim Biophys Acta. 1974 Sep 20;357(3):462–467. doi: 10.1016/0005-2728(74)90038-3. [DOI] [PubMed] [Google Scholar]
- SCHEJTER A., GLAUSER S. C., GEORGE P., MARGOLIASH E. SPECTRA OF CYTOCHROME C MONOMER AND POLYMERS. Biochim Biophys Acta. 1963 Aug 6;73:641–643. doi: 10.1016/0006-3002(63)90334-2. [DOI] [PubMed] [Google Scholar]
- Tiesjema R. H., Muijsers A. O., van Gelder B. F. Biochemical and biophysical studies on cytochrome c oxidase. X. Spectral and potentiometric properties of the hemes and coppers. Biochim Biophys Acta. 1973 Apr 27;305(1):19–28. doi: 10.1016/0005-2728(73)90227-2. [DOI] [PubMed] [Google Scholar]
- Van Buuren J. H., Van Gelder B. F., Wilting J., Braams R. Biochemical and biophysical studies on cytochrome C oxidase. XIV. The reaction with cytochrome as studied by pulse radiolysis. Biochim Biophys Acta. 1974 Mar 26;333(3):421–429. doi: 10.1016/0005-2728(74)90126-1. [DOI] [PubMed] [Google Scholar]
- WHARTON D. C., TZAGOLOFF A. STUDIES ON THE ELECTRON TRANSFER SYSTEM. LVII. THE NEAR INFRARED ABSORPTION BAND OF CYTOCHROME OXIDASE. J Biol Chem. 1964 Jun;239:2036–2041. [PubMed] [Google Scholar]
- Wilson M. T., Greenwood C., Brunori M., Antonini E. Kinetic studies on the reaction between cytochrome c oxidase and ferrocytochrome c. Biochem J. 1975 Apr;147(1):145–153. doi: 10.1042/bj1470145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960 Mar;235:845–852. [PubMed] [Google Scholar]


