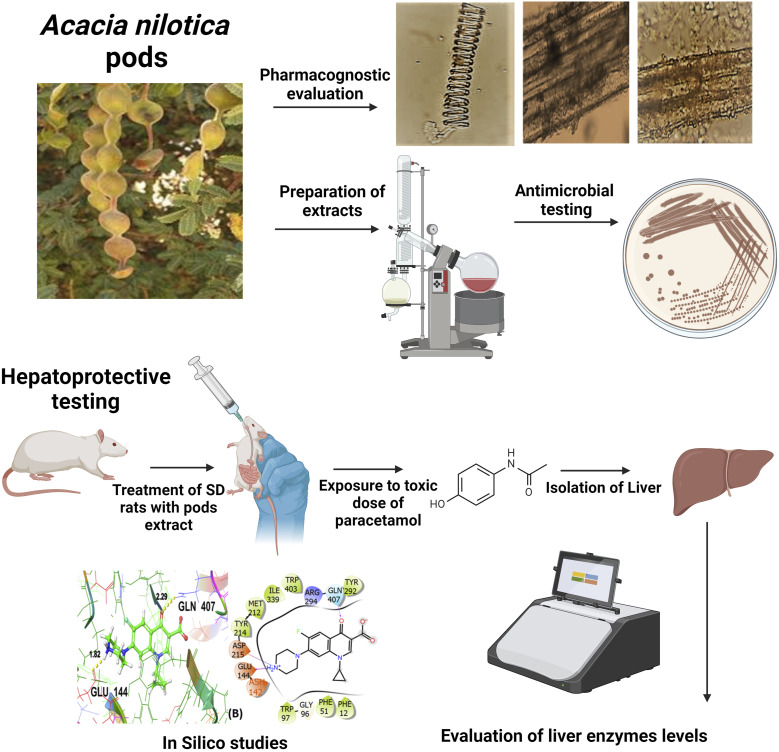
Abstract
Background
Acacia nilotica is a multipurpose plant known for its remedial properties but the antimicrobial and hepatoprotective activity of its pods remained unexplored.
Objective
This study aimed to evaluate the antimicrobial and hepatoprotective activity of n-hexane (ANPH) and methanol (ANPM) extracts of pods to scientifically validate their medicinal claims.
Methods
After the pharmacognostic evaluation of pods, in vitro tests were carried out to estimate phenolic and flavonoid content and antimicrobial potential. In vivo experiments involved testing of both extracts (250 and 500 mg/kg) paracetamol (PCM)-induced hepatotoxicity model in rats. The molecular docking studies explored insights into the potential binding capabilities of the ligands with the specific target proteins.
Results
ANPH and ANPM were enriched with phenols and flavonoids and showed antimicrobial effects. In the hepatoprotective test, the rats chronically treated with extracts had a dose-dependent hepatoprotection as markers of liver functionality were notably reduced (P < 0.05). The in silico studies revealed strong binding interactions of ergost-5-en-3-ol and oxiranyl methyl ester 9-octadecenoic acid with target proteins for antibacterial activity and hepatoprotective activity, respectively.
Conclusion
The antimicrobial and hepatoprotective potential of pods might be due to their phenols and flavonoids. The Pyrogallol, Ergost-5-en-3-and 9-octadecenoic acid might be bringing these remedial benefits through antioxidant and anti-inflammatory effects.
Keywords: Acacia nilotica, hepatoprotective, paracetamol, liver injury, antimicrobial, in silico
Graphical Abstract.
Introduction
Plant-based remedial strategies have been popular practices since ancient eras and are still trusted due to easy approachability, cost-effectivity and safe therapeutic profiles. 1 Though synthetic drugs are in extensive use in developed nations, people residing in developing countries still prefer plant-based remedies due to socio-economic factors. 2 Moreover, the WHO is supporting the scientifically validated traditional medication, particularly in developing countries, which has been validated scientifically thus emphasizing the research on herbal remedies. Despite incredible advancements in modern medicine, there are very limited drugs to provide hepatoprotection or induce regeneration of damaged liver cells. 3 That’s why the folkloric claims of remedial benefits of plants for hepatic ailments have been tested scientifically which has led to the development of hundreds of hepatoprotective herbal formulations. 4
The liver is involved in the regulation of physiological metabolic homeostasis and crucially participates in detoxification, biotransformation and elimination of endogenous and exogenous origin ie, drugs and their metabolites. 5 Liver disorders are considered one of the major causes of morbidity and mortality globally. Among these liver problems, drug-induced liver injury is the commonest etiology precipitating its exaggerated global prevalence. 6 The clinical manifestations of drug-induced liver toxicity range from elevated liver enzymes to fulminant hepatic failure. Overall, the annual incidence of drug-induced liver injury is 14-24 in every 100,000 global inhabitants emphasizing the need for reliable hepatoprotective medications in medical practice. 7
Molecular docking is a well-established in silico structure-based tool, widely employed in the drug discovery process because it has the potential to identify novel compounds of therapeutic interest and to predict ligand-target interactions at a molecular level without necessarily knowing the chemical structure of target proteins. 8 Glide molecular docking is widely used to explore various therapeutic activities of investigated ligands such as antibacterial, 9 antioxidant and cytotoxicity 10 as well as hepatoprotective activity. 11 In this study, glide molecular docking studies have been executed to screen out the potential therapeutic agents of interest.
Acacia nilotica (L.) Willd. ex Delile from the “Mimosacae” family is commonly known as Babul or Babul acacia. The literature reports the medicinal potential of different parts of A. nilotica as its leaves attenuated hyperglycemia and insulin resistance in mice 12 and its aerial parts protected rats from hepatocellular damage. 13 In a previous study by Gilani et al, methanol extract of A. nilotica pods showed caused vasorelaxant and antispasmodic effects. 14 Another study by Sadiq et al reported the antioxidant and antimalarial effects of bark, leaves and pods of A. nilotica. 15 Moreover, A. nilotica pod extract protected the rats from streptozotocin-induced diabetes and associated nephropathy 16 while antiulcer activity was noted in the NSAID-induced ulcer model in rats. 17 Though various medicinal benefits of A. nilotica pods have been explored, the hepatoprotective potential of pods remained unidentified. Hence, the current study aimed to examine the hepatoprotective effects of n-hexane and methanol extracts of A. nilotica pods by using a paracetamol (PCM)-induced hepatotoxicity model in rats. In addition, the pods were studied for pharmacognostic characteristics while in vitro testing was carried out to estimate the phenolic and flavonoid content as well as the antimicrobial potential of test extracts. The outcomes of in vitro and in vivo testing were coupled with in-silico studies to provide mechanistic insights into observed pharmacological effects to validate the observed benefits of A. nilotica.
Material and Methods
Drugs and Chemicals
All drugs and chemicals of research grade were used in the current study. The n-hexane and methanol (Duksan chemicals), nutrient agar and DMSO (Sigma Aldrich) and silymarin (Mallard Pharmaceuticals) were purchased.
Preparation of Plant Extracts
The pods of A. nilotica were gathered fresh in April from the premises of Multan, Pakistan. After authentication from an expert taxonomist, voucher 518 was deposited in the herbarium. The freshly collected pods were shade-dried and subjected to coarse grinding. The coarsely ground powder was macerated separately in n-hexane and methanol. These soakings were stored at 25°C in amber-color bottles for a week with intermittent shaking. Subsequently, the mixtures were filtered and the whole process of maceration and filtration was repeated twice. The filtrates were evaporated at reduced pressure on a rotary evaporator (Butchi, Switzerland) and extracts were obtained with 5% and 6% yield with n-hexane and methanol, respectively.
Animals
The 4-6 weeks-old Sprague Dawley rats (150-250 g) of both sexes were used in this study. The rats were bred and housed in the animal house facility situated at the Faculty of Pharmacy, Bahauddin Zakariya University, Multan. The hygienic housing of animals was maintained at 25°C with 12 hrs of light and dark cycle. The rats were provided with rodent chow comprising 21% protein and 60% carbohydrate and water ad libitum. All animal studies were conducted after permission from the university’s ethical committee.
Organoleptic Evaluation of A. nilotica Pods
The evaluation of fresh or dried specimens of pods of A. nilotica was made for organoleptic parameters ie, shape, surface texture, size, taste and smell depending on visual, touch, and smell senses. 18
Microscopic Evaluation of A. nilotica Pods
The powdered samples of pods of A. nilotica were microscopically examined with a light microscope under 4X, 10X, and 40X lenses with full-scale amplification of 40x, 100x and 400x independently. For powder microscopy, the shade-dried pods were powdered and strained through sieve 10. The test powder was examined using chloral hydrate, phloroglucinol and glycerine. In detail, the needle tip made wet with water was immediately dipped into a powered sample and stuck particles were shifted to the glass slide. A few drops of chloral hydrate, phloroglucinol, or glycerine were added to the slide. After covering with a coverslip, the slides were examined under the microscope for affirmation of cell structures. 19
UV Fluorescence Analysis of A. nilotica Pods
To 0.5 g of A. nilotica pod powder sample, 5 mL of different organic solvents were added in separate test tubes. After shaking the mixtures, the samples were retained for 20-25 min and subsequently observed under the visible daylight and UV light of short (254 nm) and long (365 nm) wavelengths for their characteristic color. 20
Evaluation of Phenols and Flavonoid Content
The n-hexane (ANPH) and methanol (ANPM) extracts of A. nilotica pods were evaluated for phenolic content using a previously reported method. 21 The 2 mg of extract was mixed thoroughly with 10% Folin- Ciocalteu followed by the addition of Na2CO3. After allowing the mixture to incubate at room temperature for 2 hrs, absorbance was noted at 765 nm and results were expressed as mg gallic acid equivalent per gram extract (mg GAE/g extract).
The flavonoid content was estimated by the AlCl₃ colorimetric method as reported previously. 22 The 2 mg of ANPH and ANPM extracts were mixed with 10% of the aluminum chloride. Subsequently, 200 μl of sodium acetate and 25 μl of methanol were added. After adding 200 μl of distilled water, the mixtures were kept in a dark place and allowed to incubate at room temperature for 40-50 minutes. The absorbance was noted at 415 nm and outcomes were expressed as mg Quercetin Equivalents per gram of extract (mg QE/g extract).
Antimicrobial Activity of n-hexane and Methanol Extracts of A. nilotica Pods
A broadly used agar disc-diffusion method was used to evaluate the antibacterial susceptibility of A. nilotica pod extracts. After preparing the agar plate, it was autoclaved at 120°C and allowed to cool and solidify with subsequent inoculation of Stenotrophomonas maltophilia, Micrococcus luteus and Serratia marcescens. The discs impregnated with different concentrations (200, 400 and 600 mg) of ANPH and ANPM were placed on agar plates and incubated for 24 h. After 24 h, the plates were examined to measure the zone of inhibition to be expressed in millimeters. The experiment was repeated in triplicates using ciprofloxacin as standard antibacterial drugs and outcomes were compared with negative control disc impregnated with DMSO only. 23
Hepatoprotective Activity of n-hexane and Methanol Extracts of A. nilotica Pods
A total of 56 rats were used in this study which were categorized into 7 equal groups (n = 8 animals per group) after calculation of sample size by using the previously described method. 24
• Group I: Healthy control, received distilled water (0.2 mL/kg) once daily for 14 days.
• Group II: Disease control rats received distilled water (0.2 mL/kg) once daily for 14 days followed by administration of the toxic dose of PCM (2 g/kg).
• Group III: The rats received hepatoprotective drug silymarin (200 mg/kg) once daily for 14 days followed by administration of the toxic dose of PCM (2 g/kg).
• Group IV and Group V: The rats were treated with 250 and 500 mg/kg of ANPH, respectively, for 14 days followed by administration of the toxic dose of PCM (2 g/kg).
• Group VI and Group VII: Test groups, received 250 and 500 mg/kg of ANPM, respectively, for 14 days followed by administration of the toxic dose of PCM (2 g/kg).
The animals of all groups received their group-wise designated treatments once daily for consecutive 14 days through oral gavage. 25 On the 15th day, the animals of Groups II-VII received PCM (2 g/kg) to induce acute hepatotoxicity in rats. 26
Biochemical Analysis of Liver Enzymes
After 24 hours of PCM administration, the blood samples were taken via retro-orbital puncture from chloroform-anesthetized animals (n = 6 from each group) and permitted to coagulate for 0.5 h at 37°C. After centrifugation at 2500 r/min, the serum was separated and stored at −20°C for the evaluation of aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine transaminase (ALT) and total bilirubin according to the previously described methods. 27
Glide Molecular Docking Methodology
Selection of Target Proteins
The three-dimensional crystal structure of the antibacterial target protein (PDB ID:3WD1), 28 and macromolecular target protein for hepatoprotective activity (PDB ID: 5HYK), 29 were fetched from the RCSB protein data bank (https://www.rcsb.org/) 30 in PDB format. PDB ID:3WD1 is S. marcescens Chitinase B complexed with syn-triazole inhibitor, and PDB ID:5HYK is the crystal structure of the complex PPARalpha/AL26-29 having the resolution of 2.30 Å and 1.83 Å respectively.
Pre-processing of Target Protein Structures
To prepare the protein structures, the Protein Preparation Wizard module of the Maestro platform was used. The module was assigned jobs to prepare bond orders, add hydrogen atoms and make zero-order bonds to metals, as well as create di-sulphate bonds followed by changing the seleno-methionines into methionine, and filling up the missing side chains. The structures were then optimized by energy minimization followed by hydrogen bond optimization under the OPLS4 force field. 31
Selection of Ligands
The ligands were selected based on previously performed chemical characterization through GC-MS analysis reporting 19 phytoconstituents in methanolic extract of the fruit of A. nilotica (pods). 32 Moreover, the standard drugs used in the antibacterial and hepatoprotective activities performed in the current study were also used as the ligands and are presented with their PubChem ID in Table 1. The 3D coordinate files of all the tabulated phytoconstituents (ligands) were downloaded from the PubChem database. 33 However, the co-crystallized ligands of target proteins were prepared by BIOVIA Discovery Studio. 34
Table 1.
List of Phytoconstituents Previously Reported to be Isolated From Fruit (Pods) of A. nilotica Methanolic Extract by GC-MS Analysis as Well as the Standard Drugs Used in the Current Study Along With Their PubChem ID.
| S. No. | Compound Name | Mol. Wt. | Mol. Formula | PubChem ID |
|---|---|---|---|---|
| 1 | N,N-Dimethylglycine | 103 | C4H9NO2 | 673 |
| 2 | 4-methylbenzenethiol | 124.21 | C7H8S | 7811 |
| 3 | Pyrogallol | 126.11 | C6H6O3 | 1057 |
| 4 | 1,8,11-Heptadecatriene, (Z,Z)- | 234.5 | C17H30 | 5352709 |
| 5 | 4-O methylmannose | 194.18 | C7H14O6 | 345716 |
| 6 | Hexadecanoic acid, methyl ester | 270.5 | C17H34O2 | 8181 |
| 7 | 14,17-Octadecadienoic acid, methyl ester | 294.5 | C19H34O2 | 5365751 |
| 8 | 9,12-Octadecadienoic acid (Z,Z)- | 280.4 | C18H32O2 | 5280450 |
| 9 | Methyl oleate | 296.5 | C19H36O2 | 5364509 |
| 10 | Methyl linoleate | 294.5 | C19H34O2 | 5284421 |
| 11 | Methyl 9-cis,11-trans- octadecadienoate | 294.5 | C19H34O2 | 11748436 |
| 12 | Methyl stearate | 298.5 | C19H38O2 | 8201 |
| 13 | 15-Hydroxypentadecanoic acid | 258.4 | C15H30O3 | 78360 |
| 14 | Glycedyl palmitate (methyl ricinoleate) | 312.5 | C19H36O3 | 5354133 |
| 15 | Oxiranyl methyl ester 9-octadecenoic acid | 338.5 | C21H38O3 | 5354568 |
| 16 | 9-Octadecenamide | 281.5 | C18H35NO | 1930 |
| 17 | Phthalic acid, bis(2-ethylhexyl) ester | 390.6 | C24H38O4 | 8343 |
| 18 | Ergost-5,22-dien-3-ol (3-beta.22E) | 398.7 | C28H46O | 5281327 |
| 19 | Ergost-5-en-3-ol | 400.7 | C28H48O | 18660356 |
| For standard drugs | ||||
| Ciprofloxacin | 331.34 | C17H18FN3O3 | 2764 | |
| Silymarin | 482.4 | C25H22O10 | 5213 | |
Pre-processing of Ligand Structures
The LigPrep module of the Maestro platform was allocated the job of preparing the co-crystallized ligand of targeted proteins 35 and the phytoconstituents isolated from the A. nilotica methanolic extract.
Receptor Grid Generation
The receptor grid generation module within the Schrödinger suite was incorporated to create a grid box, the dimensions of which were adjusted to encompass the co-crystallized ligand within the binding pocket of the chosen protein. 36
Glide Docking
Glide docking of the target proteins and ligand molecules was conducted by the Maestro platform’s Glide docking module. 37 The docking procedure was conducted by using the Glide tool in standard precision (SP) mode. 38 The glide and Emodel scores were documented for each ligand’s optimal conformational pose and were subsequently compared with the scores of the corresponding co-crystallized ligands and the standard drugs incorporated for the said investigated activities. The ligand interaction module visualized a 2D interaction diagram of the ligand-protein complex molecule which was then visualized to understand the interaction between the ligand molecules and the target proteins during the binding process through the resulting SP posture.
Physicochemical Evaluation
SwissADME 39 was used to estimate the physicochemical properties and to compute the behavior of hit ligands concerning Lipinski’s rule of 5. 40
Statistical Analysis
The data were statistically analyzed by using GraphPad Prism (8.0). After the evaluation of data for normality through the Shapiro-Wilk test, statistical evaluation was carried out using one-way ANOVA followed by Tukey’s test for inter-group comparison of means. The P < 0.05 was considered significant.
Results
Organoleptic Evaluation
The organoleptic examination showed the 7.5-15 cm long and 1.3-1.6 cm wide moniliform-shaped pods had a dark brown to greyish external appearance and were black from the inside. The taste was bitter and each pod comprised 6-16 seeds of black color.
Microscopic Evaluation
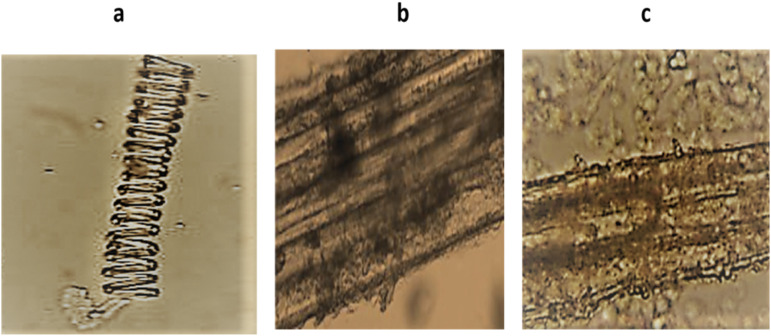
Powder microscopy of A. nilotica pods showed scleroids, phloem fiber and parenchyma cells with starch grains as depicted in Figure 1.
Figure 1.
The microscopic examination of A. nilotica pods showed (A) scleroids (B) phloem fiber (C) parenchyma cells with starch grains.
Fluorescence Evaluation
The outcomes of fluorescence evaluation of the powder of A. nilotica pods are given in Table 2.
Table 2.
UV Fluorescence Evaluation of the Powder of A. nilotica Pods.
| Protocol | Daylight | UV Light Short-Wavelength (254 nm) | UV Light Long-Wavelength (365 nm) |
|---|---|---|---|
| Powder + acetic acid | Brown | Brown | Black |
| Powder + 10% HCl | Yellow | Brown | Green |
| Powder + 10% FeCl3 | Green | Brown | Black |
| Powder + methanol | Brown | Brown | Green |
| Powder + acetone | Orange | Red | Black |
| Powder + 50% H2SO4 | Black | Yellow | Brown |
| Powder + 50% HNO3 | Brown | Purple | Brown |
| Powder + 10% ethanol | Brown | Green | Green |
| Powder + 99% ethanol | Yellow | Green | Brown |
| Powder + NaOH | Orange | Green | Yellow |
| Powder + chloroform | Brown | Green | Yellow |
| Powder + benzene | Yellow | Brown | Black |
| Powder + acetonitrile | Brown | Brown | Green |
| Powder + picric acid | Yellow | Brown | Yellow |
| Powder + diethyl ether | Brown | Brown | Black |
| Powder + n-Butanol | White | Green | Yellow |
Phenols and Flavonoid Content
In the estimation of total phenolic content, the outcomes showed that 1 gram of ANPH comprised 18.19 ± 7.1 mg of gallic acid and 1 gram of ANPM comprised 21.28 ± 9.6 mg of gallic acid. Moreover, 1 gram of ANPH comprised 10.22 ± 6.4 mg of quercetin and 1 gram of ANPM comprised 14.38 ± 2.6 mg of quercetin in the estimation of the total flavonoids.
Antibacterial Activity
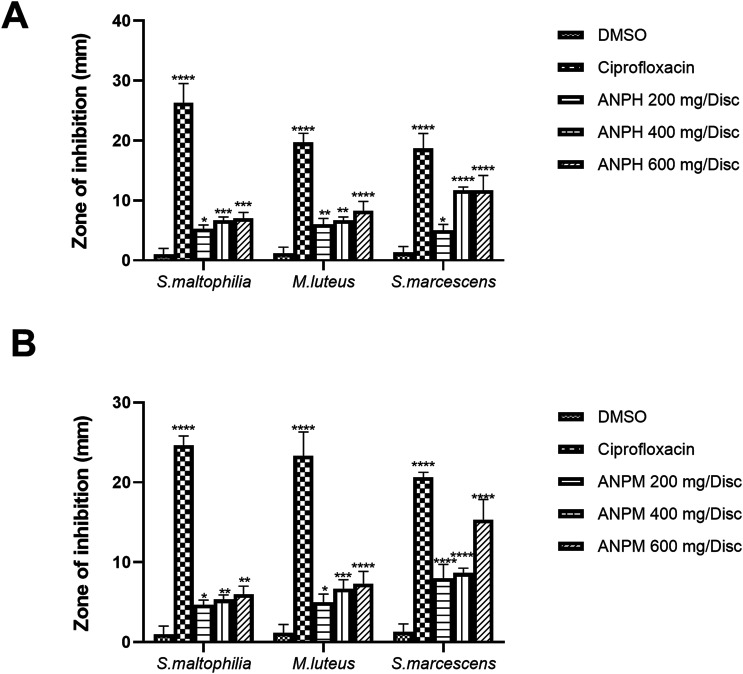
The agar disc diffusion method was used to evaluate the potential of ANPH and ANPM against S. maltophilia, M. luteus and S. marcescens. The zone of inhibition against all microbial strains was concentration-dependently increased by both extracts. In detail, the maximum zone of inhibition by ANPH at 600 mg/disc was reduced against S. maltophilia (P < 0.001), M. luteus (P < 0.0001) and S. marcescens (P < 0.0001) in comparison with DMSO-comprising negative control disc as shown in Figure 2A.
Figure 2.
The different concentrations (200, 400 and 600 mg/disc) of (A) n-hexane extract of A. nilotica pods (ANPH) and (B) methanol extract of A. nilotica (ANPM) were tested and zones of inhibition (mm) were noted against for S. maltophilia, M. luteus and S. marcescens to evaluate the antibacterial potential of extracts by agar disc diffusion method using ciprofloxacin as a positive control. The whole experiment was carried out in triplicates and outcomes were evaluated by one-way ANOVA followed by Tukey’s test. The *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 were considered statistically significant in comparison with the DMSO-treated control disc while ns shows non-significant outcomes. DMSO: Dimethyl sulfoxide; ANPH: n-hexane extract of A. nilotica pods; ANPM: methanol extract of A. nilotica.
Likewise, the ANPM at 600 mg/disc caused the maximum reduction in zone of inhibition against S. maltophilia (P < 0.01), M. luteus (P < 0.0001) and S. marcescens (P < 0.0001) in comparison with DMSO-loaded negative control disc and outcomes were comparable with ciprofloxacin as depicted in Figure 2B.
Hepatoprotective Activity
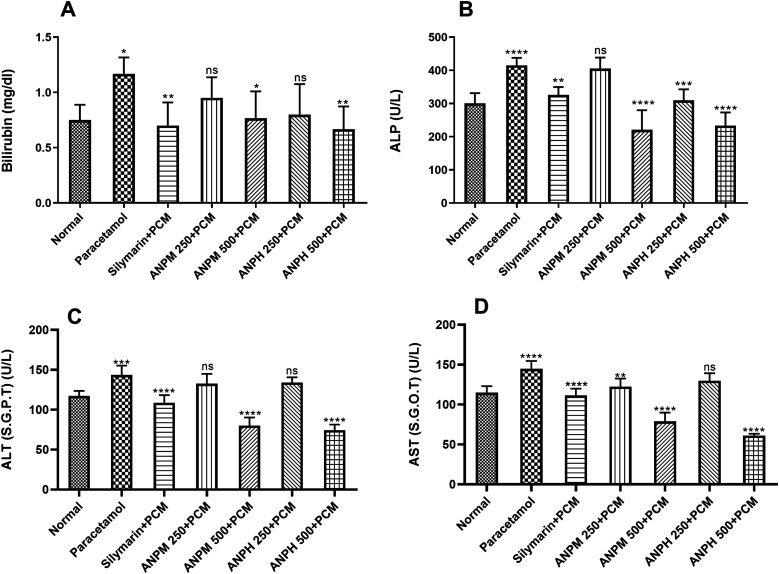
The rats administered with a hepatotoxic dose of PCM (2 g/kg) demonstrated increased levels of bilirubin levels (P < 0.05) in comparison to control animals. The animals chronically pre-treated with ANPM and ANPH showed dose-dependent protection for hepatic damage as the bilirubin levels were significantly less with P < 0.05 and P < 0.01 in comparison to the PCM-treated group, respectively (Figure 3A).
Figure 3.
The rats (n = 6) were treated with 250 and 500 mg/kg of n-hexane extract of A. nilotica pods (ANPH) and methanol extract of A. nilotica (ANPM) for 2 weeks followed by administration of a toxic dose of paracetamol (PCM). The hepatoprotective potential of both extracts was assessed by monitoring the (A) bilirubin, (B) ALP, (C) ALT and (D) AST levels. The data are presented as mean ± SD and evaluated by one-way ANOVA followed by Tukey’s test. The *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 were considered statistically significant in comparison with the PCM-treated rats while ns shows non-significant outcomes. ALP: alkaline phosphatase; ALT: alanine transaminase also known as SGPT: serum glutamate pyruvate transaminase; AST: aspartate aminotransferase also known as SGOT: glutamic-oxaloacetic transaminase.
The levels of ALP were also monitored to observe the hepatoprotective potency of both extracts. The levels were increased on PCM administration (P < 0.0001 vs control animals) revealing the severity of hepatic damage. However, the ANPM protected the rats from this deterioration with P < 0.0001 at the dose of 500 mg/kg. Likewise, ANPH was found potent at 250 and 500 mg/kg as the ALP levels were notably lesser with P < 0.001 and P < 0.0001, respectively (Figure 3B).
Similarly, the ALT and AST levels were elevated in animals exposed to a hepatotoxic dose of PCM as compared to healthy control animals with P < 0.001 and P < 0.0001, respectively (Figure 3(C) and (D)). The liver was protected by these damages ANPM and ANPH dose-dependently with P < 0.0001 at a dose of 500 mg/kg of both extracts and outcomes were comparable with the impact of silymarin.
Molecular Docking
The supplementary information provided in Table S1 and Table S2 represent the glide molecular docking data involving the co-crystallized ligand and already reported phytoconstituents from A. nilotica against the target proteins of interest ie, anti-bacterial and hepatoprotective activities respectively. These tables reflect the docking results including the essential parameters such as DScore, GScore and Glide Emodel whereas Table 3 shows the additional data of polar interactions, hydrogen bonding with relative distance measured in angstroms (Å), and hydrophobic interactions of these ligands with the target proteins of the co-crystallized ligand, the standard drug and the hit compound.
Table 3.
Glide Docking Data, Polar Interactions, Hydrogen Bonding With Relative Distance Measured in Angstroms (Å), and Hydrophobic Interactions of These Ligands (Co-crystallized Ligand, the Standard Drugs, and the Hit Compound) With the Target Proteins.
| Ligands With Target Protein | GScore (kcal/mol) | Emodel (kcal/mol) | Polar Residues | H-Bond With Distance in Å | Hydrophobic Interacting Amino Acid Residues |
|---|---|---|---|---|---|
| Ligand interaction with bacterial target protein (3WD1) | |||||
| (A) co-crystallized ligand | −12.233 | −156.222 | Not found | TYR98 (2.77) GLU144 (1.84, 2.25) ASP215 (1.66, 2.12) ASP316 (1.83, 2.31) |
PHE12, PRO14, PHE51, TRP97, TYR98, TYR145, PHE191, MET212, TYR214, TRP220, TYR292, PRO317, TRP403 |
| (B) ciprofloxacin | −6.399 | −56.826 | GLN407 | GLU144 (1.82) GLN407 (2.29) |
PHE12, PHE51, TRP97, MET212, TYR214, TYR292, ILE339, TRP403 |
| (C) ergost-5-en-3-ol | −6.223 | −46.164 | THR15 ASN16 |
Not found | PHE12, PRO14, PHE51 TRP97, TYR98, TYR99, MET212, TYR214, TYR292, TRP403 |
| Ligand interaction for hepatoprotective activity (5HYK) | |||||
| (a) co-crystallized ligand | −9.023 | −59.095 | HIS274 GLN277 SER280 |
SER280 (2.17) TYR314 (1.85, 1.97) |
VAL270, PHE273, CYS276, TYR314, PHE318, PHE351, ILE354, MET355, VAL444, ILE447, ALA454, ALA455, LEU456, LEU460, TYR464 |
| (b) silymarin | −5.682 | −42.161 | GLN277 SER452 GLN461 |
SER452 (1.84 Å) LEU456 (2.34 Å) | VAL270, PHE273, CYS276, PHE351, ILE354, VAL444, ILE447, ALA454, ALA455, LEU456, PRO458, LEU460. |
| (c) oxiranyl methyl ester 9-octadecenoic acid | −6.882 | −51.783 | GLN277 SER280 SER452 GLN461 |
LEU456 (1.99) LYS448 (1.86, 1.95) |
PHE273, CYS276, TYR314, ILE317, PHE318, LEU321, PHE351, ILE354, MET355, VAL444, ILE447, ALA454, ALA455, LEU456, LEU460, TYR464 |
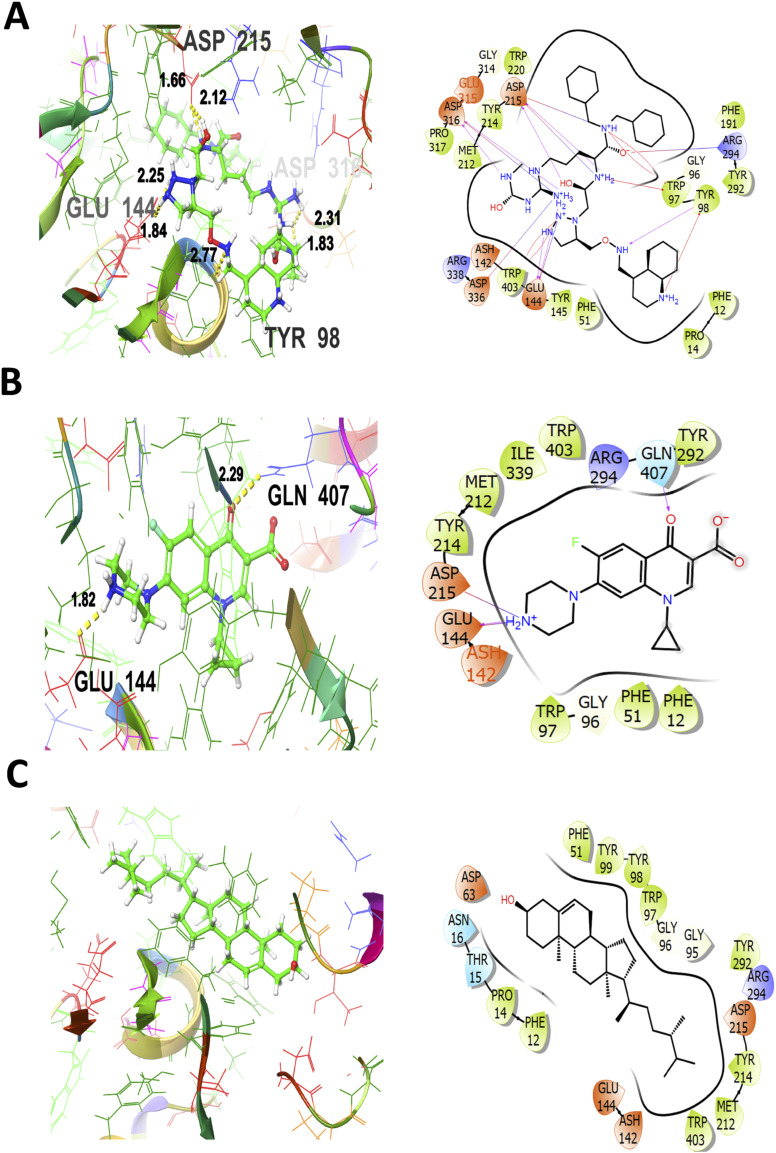
For the 3WD1 receptor (for anti-bacterial activity) as shown in Figure 4A, the co-crystallized ligand is showing a notable binding score as GScore and Emodel −12.233 kcal/mol and −156.222 kcal/mol respectively. It exhibited no polar interactions, forming hydrogen bonding interactions with amino acids TYR98 (2.77 Å), GLU144 (1.84, 2.25 Å), ASP215 (1.66, 2.12 Å), ASP316 (1.83 Å, 2.31 Å) at their corresponding distances expressed in angstroms, respectively. Additionally, hydrophobic interactions were observed with PHE12, PRO14, PHE51, TRP97, TYR98, TYR145, PHE191, MET212, TYR214, TRP220, TYR292, PRO317, and TRP403. The standard drug (ciprofloxacin) interaction with the target receptor is depicted in Figure 4B. The ciprofloxacin is engaged in hydrogen bonding interactions with GLU144 (1.82 Å), and GLN407 (2.29 Å). Hydrophobic interactions are noted with PHE12, PHE51, TRP97, MET212, TYR214, TYR292, ILE339, and TRP403. The polar interactions are evident by amino acid residues GLN407. The relative GScore and Emodel are found to be −6.399 kcal/mol and −56.826 kcal/mol respectively. The ergost-5-en-3-ol yielded a GScore and Emodel of −6.223 kcal/mol and −46.164 kcal/mol. The hydrophobic interactions are evident with PHE12, PRO14, PHE51, TRP97, TYR98, TYR99, MET212, TYR214, TYR292, and TRP403. The amino acids designated as THR15 and ASN16 are engaged in the polar interactions without any evidence of hydrogen bonding as shown in Figure 4C.
Figure 4.
3D and 2D interactive view of co-crystallized ligand (A), antibacterial standard drug ciprofloxacin (B), and Ergost-5-en-3-ol (C) with bacterial target protein (PDB ID: 3WD1).
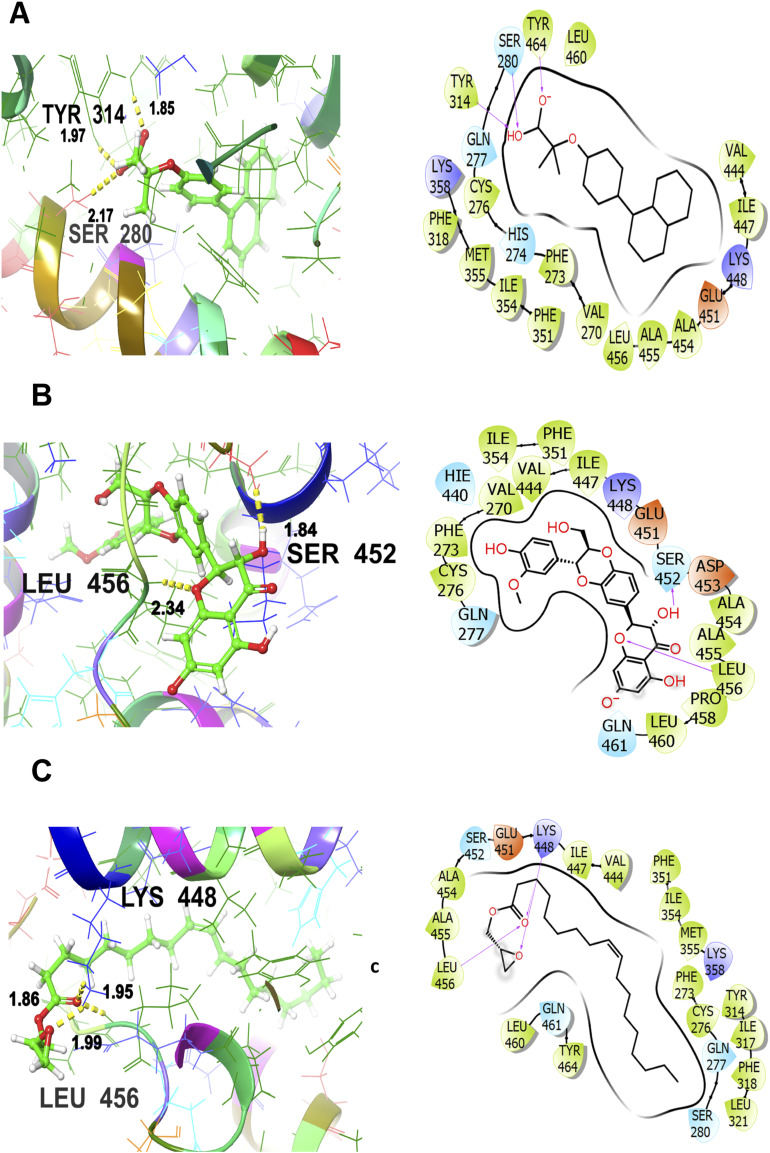
To visualize the interaction with macromolecular target protein for hepatoprotective activity (5HYK), the co-crystallized ligand shows a significant binding score as GScore and Emodel −9.023 and −59.095 kcal/mol respectively. It exhibits polar interactions with HIS274, GLN277, and SER280. The hydrogen bonding interactions are noticed with amino acids SER280 (2.17 Å) and TYR314 (1.85, 1.97 Å). In addition, hydrophobic interactions are observed with VAL270, PHE273, CYS276, TYR314, PHE318, PHE351, ILE354, MET355, VAL444, ILE447, ALA454, ALA455, LEU456, LEU460, TYR464 as shown in Figure 5A The standard drug (silymarin) interacts with the 5HYK receptor which is depicted in Figure 5B It is engaged in hydrogen bonding interactions with SER452 (1.84 Å) and LEU456 (2.34 Å). Hydrophobic interactions are noted with VAL270, PHE273, CYS276, PHE351, ILE354, VAL444, ILE447, ALA454, ALA455, LEU456, PRO458, and LEU460. The polar interactions are evident by amino acid residues GLN277, SER452 and GLN461. The corresponding GScore and Emodel values are found to be −5.682 and −42.161 kcal/mol respectively. The oxiranyl methyl ester 9-octadecenoic acid (the hit compound) gives a GScore and Emodel of −6.882 and −51.783 kcal/mol without the formation of hydrogen bonding. The hydrophobic interactions are evident with PHE273, CYS276, TYR314, ILE317, PHE318, LEU321, PHE351, ILE354, MET355, VAL444, ILE447, ALA454, ALA455, LEU456, LEU460, TYR464 and GLN277, SER280, SER452 and GLN461 are engaged in polar interactions as shown in Figure 5C LEU456 (1.99 Å), and LYS448 (1.86 Å, 1.95 Å) are the hydrogen bonding involved residues.
Figure 5.
3D and 2D interactive view of co-crystallized ligand (A), standard drug-silymarin (B), and oxiranyl methyl ester 9-octadecenoic acid (C) with PDB ID: 5HYK target protein.
The lower the value of GScore, the more potent would be the ligand to bind to the target protein, convincing the probability of establishment of potential drug candidates. The order of antibacterial activity is co-crystallized ligand > ciprofloxacin (standard drug) > ergost-5-en-3-ol with corresponding GScore values as −12.233 > −6.399 > −6.223 respectively. Hence, it assists in predicting that the hit compound (ergost-5-en-3-ol) is very close in GScore values to the standard drug (ciprofloxacin). In addition, the order of hepatoprotective activity is co-crystallized ligand > oxiranyl methyl ester 9-octadecenoic acid > silymarin (standard drug) with corresponding GScore values as −9.023 > −6.882 > −5.682 respectively. Hence, the hit compound (oxiranyl methyl ester 9-octadecenoic acid) is showing much lower GScore values as compared to the standard drug, forecasting that it is even far better in hepatoprotective activity than the standard drug.
Physicochemical Properties Evaluation (Lipinkski’s Rule of Five)
The estimated physicochemical properties of hit ligands of interest, presented in Table 4, indicate their potential as effective drug candidates. Both of these compounds have molecular weights that fall within the acceptable threshold of less than 500 Da, signifying their suitability for drug development. The partition coefficient (LogP) values for these compounds range from 6.89 to 5.73, slightly deviating from the acceptable threshold of less than 5, emphasizing on their moderate lipophilicity for efficient intestinal absorption. The solubility values (LogS) fall between −4.31 and −6.32, aligning with the acceptable range of 0 to −6, which indicates good solubility profiles. The topological polar surface area (TPSA) values of these compounds are 20.23 and 38.83 Å2 respectively, which are within the standard range of ≤140 Å2, facilitating cellular permeability. As far as hydrogen bonding is concerned, the hydrogen bond acceptors (HBA) range from 1 to 3, and the hydrogen bond donors (HBD) range from 1 to 0, both within the acceptable limits of ≤10 and ≤5, respectively. The number of rotatable bonds (RotBs) for these compounds varies between 5 and 18, and the acceptable threshold is less than 10, indicating potential flexibility and favorable conformational adaptability in the case of ergost-5-en-3-ol. However, the ligand named oxiranyl methyl ester 9-octadecenoic acid deviates from this rule regarding RotBs.
Table 4.
Estimated Physicochemical Properties of the Hit Compounds of Interest.
| Physicochemical Properties | Anti-bacterial Hit Compound | Hepatoprotective Hit Compound | Acceptable Threshold Rule of Five |
|---|---|---|---|
| Ergost-5-en-3-ol | Oxiranyl methyl ester 9-octadecenoic acid | ||
| Molecular weight (g/mol) | 400.68 | 338.52 | <500 Da |
| LogP | 6.89 | 5.73 | <5 |
| LogS | −5.79 | −6.15 | 0 → −6 |
| TPSA (A2) | 20.23 | 38.83 | ≤140 |
| HBA | 1 | 3 | ≤10 |
| HBD | 1 | 0 | ≤5 |
| RotBs | 5 | 18 | ≤10 |
LogP: Partition Coefficient; LogS: Solubility in mol/L; TPSA: Topological Polar Surface Area; HBA Hydrogen Bond Acceptors; HBD: Hydrogen Bond Donors; RotBs Rotatable Bonds.
Discussion
The standardization of plant-based medications is very important to ensure drug quality as well as to avoid counterfeit herbal substitutes. 41 The initial phase of the current study included the assessment of morphological and microscopic parameters of A. nilotica pods to predict their quality. As extractive value has been used as a tool to predict the nature of phytoconstituents owned by plants, the extraction of A. nilotica pods was carried out using n-hexane and methanol and % yield predicted that phytoconstituents owned by A. nilotica pods were slightly more soluble in methanol than n-hexane.
The in vitro testing of ANPH and ANPM showed that pods were enriched with phenols and flavonoid content. Moreover, both extracts demonstrated excellent antimicrobial effects against S. maltophilia, M. luteus and S. marcescens. The outcomes of antibacterial testing showed maximum inhibition for S. marcescens by extracts as compared to M. luteus and S. maltophilia. S. marcescens, is a multi-drug resistant gram-negative bacillus causing respiratory tract and urinary tract infections, septicemia, osteomyelitis and meningitis. 42 Antibiotic resistance is a challenge worldwide, especially in developing and underdeveloped nations. The emergence of antibacterial-resistant strains is becoming a threatening task for health professionals, 43 thus motivating researchers to struggle for novel phytocompounds possessing antibacterial potential. The noted antibacterial properties of pods might be attributed to the presence of flavonoids as they are reported to exert an antimicrobial role by suppressing energy metabolism, functionality of cytoplasmic membranes and synthesis of nucleic acid synthesis. 44
The impact of liver disorders on global health is a challenge as the incidence and prevalence of liver diseases are increasing. The dilemma is the limited therapeutic options available to cure liver diseases causing the increased dependence on herbal drugs for the management of liver ailments. Plants have been relied on as the source of natural products of ameliorative potential since ancient times and are still in regular use. 45 In the present study, A. nilotica pods have been investigated for hepatoprotective potential in the PCM-induced hepatotoxicity rodent model. The administration of PCM caused elevation in bilirubin, ALP, ALT and AST levels in serum samples of diseased rats revealing hepatocellular damage. These outcomes are supported by previous studies in which the administration of high-dose PCM precipitated liver damage.46,47 PCM is an NSAID and its overdose induces hepatocellular damage through its highly reactive metabolite, N-acetyl-para-benzoquinonimine which binds to the sulfhydryl group of protein resulting in hepatocellular necrosis and leakage of the plasma. 48 The pre-treatment of rats with ANPH and ANPM protected the rats from the toxic effects of PCM and these findings are supported by Kannan et al who reported hepatoprotective potential in methanolic extract of aerial parts of A. nilotica. 13
Oxidative stress and inflammation play critical roles in the development and pathogenesis of liver diseases. The elevated free radicals and oxidative stress cause dysregulated homeostasis and irreversible alterations in proteins and DNA leading to liver damage. 49 A hepatotoxic dose of PCM causes the activation of Kupffer cells which are further linked with the release of proinflammatory cytokines. These cytokines induce various pathophysiological responses leading to hepatocellular damage. 50 Phenolic compounds exert anti-inflammatory effects by regulating cytokines and holding the activation of T cells, key instigators of inflammation. 51 The hepatoprotective effects exerted by A. nilotica pods might be due to flavonoids and phenolics as they are known to scavenge toxins and reduce the incidence of various hepatic ailments due to antioxidant activity. 52 Pyrogallol, present in pods, is a polyphenol its radical scavenging and reducing abilities are well-established. 53 Ergost-5-en-3-ol present in pods is a sterol belonging to terpenoids and has been known and reported to possess antioxidant potential. 54 9-octadecenoic acid methyl ester, present in A. nilotica pods, has been reported to possess antioxidant activity by previous research. 55
The study lacks an understanding of the mechanism behind the hepatoprotective benefits of A. nilotica pods as phytochemicals may bring these benefits through multiple mechanisms. One might be the regulatory impact of A. nilotica pods on the gastrointestinal system as the risk of ascites is reduced when constipation is improved and absorption of harmful substances is reduced. 56 Moreover, recent studies have highlighted the involvement of gut microbiota in the therapeutic efficacy and bioavailability of phytochemicals, so the bi-directional relationship of phytoconstituents owned by pods and gut microbiota might be undertaken in future studies to understand the 1 of possible mechanisms behind the medicinal properties of plant.57,58
Conclusion
The results of the present study revealed that A. nilotica pods are highly loaded with phenols and flavonoids ascribing antimicrobial and hepatoprotective benefits. The administration of A. nilotica pods protected the rats from PCM-induced hepatotoxicity as Pyrogallol, Ergost-5-en-3-ol and oxiranyl methyl ester 9-octadecenoic acid owned by pods might be ameliorating the hepatocellular damage by combating the PCM-induced oxidative stress and inflammation. However, it is a preliminary study and further experimentation must be carried out in the future to understand the molecular mechanisms behind these medicinal activities and the impact of phytoconstituents on the expression of specific genes involved in hepatoprotection.
Supplemental Material
Supplemental Material for Antimicrobial and Hepatoprotective Properties of Pods of Acacia nilotica (L.) Willd. ex Delile: In Vivo and In Silico Approaches by Mehak Idrees, Sana Javaid, Sumaira Nadeem, Faria Khurshid, Abida Parveen, Abdul Malik, Azmat Ali Khan, Suhail Akhtar, and Sabiha Fatima in Dose-Response.
Acknowledgments
The authors are thankful to Dr Khizar Abbas, Chairperson at the Department of Pharmacognosy, Faculty of Pharmacy, Bahauddin Zakariya University Multan for providing the research facilities. The authors also extend their appreciation to King Saud University for funding this work through a research-supporting project (RSPD2024R966), Riyadh, Saudi Arabia.
Author Contributions: M.I. conceptualized the experiment and performed the experiments; analyzed the data and wrote the paper. S.J. interpreted the data and wrote the paper. S.N. contributed analysis tools and performed in silico studies. F.K reviewed and proofread the manuscript. A.P. interpreted the data and wrote the paper. A.M. reviewed and proofread the manuscript. A.A.K. interpreted the data and reviewed the manuscript. S.A. reviewed the manuscript. S.F. interpreted the data and revised the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by King Saud University through research research-supporting project (RSPD2024R966), Riyadh, Saudi Arabia.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Sana Javaid https://orcid.org/0000-0001-5826-4429
References
- 1.Patel S, Rauf A, Khan H. The relevance of folkloric usage of plant galls as medicines: finding the scientific rationale. Biomed Pharmacother. 2018;97:240-247. doi: 10.1016/j.biopha.2017.10.111 [DOI] [PubMed] [Google Scholar]
- 2.Ahmad R, Ahmad Z, Khan AU, Mastoi NR, Aslam M, Kim J. Photocatalytic systems as an advanced environmental remediation: recent developments, limitations and new avenues for applications. J Environ Chem Eng. 2016;4:4143-4164. doi: 10.1016/J.JECE.2016.09.009 [DOI] [Google Scholar]
- 3.Chattopadhyay RR. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. J Ethnopharmacol. 2003;89:217-219. doi: 10.1016/J.JEP.2003.08.006 [DOI] [PubMed] [Google Scholar]
- 4.Ram VJ. Herbal preparations as a source of hepatoprotective agents. Drug News Perspect. 2001;14:353-363. https://pubmed.ncbi.nlm.nih.gov/12813598/ [PubMed] [Google Scholar]
- 5.Lahon K, Das S. Hepatoprotective activity of Ocimum sanctum alcoholic leaf extract against paracetamol-induced liver damage in Albino rats. Pharmacogn Res. 2011;3:13-18. doi: 10.4103/0974-8490.79110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chitturi S, Farrell GC. Drug-induced liver disease. Schiff’s Diseases of the Liver 2011;11th Edition:703-783. doi: 10.1002/9781119950509.CH27 [DOI] [Google Scholar]
- 7.Chughlay MF, Kramer N, Werfalli M, Spearman W, Engel ME, Cohen K. N-acetylcysteine for non-paracetamol drug-induced liver injury: a systematic review protocol. Syst Rev. 2015;4:84-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Ruyck J, Brysbaert G, Blossey R, Lensink MF. Molecular docking as a popular tool in drug design, an in silico travel. Adv Appl Bioinform Chem. 2016;9:1-11. doi: 10.2147/AABC.S105289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Mernissi R, El Menyiy N, Moubachir R, et al. Cannabis sativa L. essential oil: chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: in vitro, in vivo, and in silico study. Open Chem. 2024;22:20230214. doi: 10.1515/Chem-2023-0214 [DOI] [Google Scholar]
- 10.tul Muntaha S, Shahzadi I, Khan I, et al. Biochemical, molecular characterization, antioxidant, and cytotoxicity of Dicliptera bupleuroides with potential drug discovery: in vitro and in silico analysis. ChemistrySelect. 2024;9:e202304385. doi: 10.1002/SLCT.202304385 [DOI] [Google Scholar]
- 11.Bouothmany K, Amrati F, Chebaibi M, et al. Hepatoprotective effects of a chemically-characterized extract from cistus ladanifer: in vivo and in silico investigations. Nat Prod Commun. 2024;19:1-14. doi: 10.1177/1934578X241228944 [DOI] [Google Scholar]
- 12.Saha MR, Dey P, Sarkar I, et al. Acacia nilotica leaf improves insulin resistance and hyperglycemia associated acute hepatic injury and nephrotoxicity by improving systemic antioxidant status in diabetic mice. J Ethnopharmacol. 2018;210:275-286. doi: 10.1016/J.JEP.2017.08.036 [DOI] [PubMed] [Google Scholar]
- 13.Kannan N, Sakthivel KM, Guruvayoorappan C. Protective effect of Acacia nilotica (L.) against acetaminophen-induced hepatocellular damage in wistar rats. Adv Pharmacol Sci. 2013;2013:987692. doi: 10.1155/2013/987692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilani AH, Jabeen Q, Ghayur MN, Janbaz KH, Akhtar MS. Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum copticum seed extract. J Ethnopharmacol. 2005;98:127-135. doi: 10.1016/j.jep.2005.01.017 [DOI] [PubMed] [Google Scholar]
- 15.Sadiq MB, Tharaphan P, Chotivanich K, Tarning J, Anal AK. In vitro antioxidant and antimalarial activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. BMC Compl Alternative Med. 2017;17:372. doi: 10.1186/S12906-017-1878-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omara EA, Nada SA, Farrag ARH, Sharaf WM, El-Toumy SA. Therapeutic effect of Acacia nilotica pods extract on streptozotocin induced diabetic nephropathy in rat. Phytomedicine. 2012;19:1059-1067. doi: 10.1016/J.PHYMED.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 17.Bansal VK, Goel RK. Gastroprotective effect of Acacia nilotica young seedless pod extract: role of polyphenolic constituents. Asian Pac J Tropical Med. 2012;5:523-528. doi: 10.1016/S1995-7645(12)60092-3 [DOI] [PubMed] [Google Scholar]
- 18.Xu D, Lin Y, Bauer R, et al. Organoleptic evaluation of amomi fructus and its further background verified via morphological measurement and GC coupled with E-nose. Evid Based Complement Alternat Med. 2018;2018:4689767. doi: 10.1155/2018/4689767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurav S, Tilloo S, Burade K. Histological and histochemical staining techniques. In Gurav SS, Gurav NS. (Eds.), Indian Herbal Drug Microscopy. 2014;9-14. doi: 10.1007/978-1-4614-9515-4_3 [DOI] [Google Scholar]
- 20.Chase CR, Pratt R. Fluorescence of powdered vegetable drugs with particular reference to development of a system of identification. J Am Pharm Assoc Am Pharm Assoc. 1949;38:324-331. doi: 10.1002/JPS.3030380612 [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc. 2007;2:875-877. doi: 10.1038/nprot.2007.102 [DOI] [PubMed] [Google Scholar]
- 22.Sembiring EN, Elya B, Sauriasari R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Phcog J. 2018;10:123-127. doi: 10.5530/pj.2018.1.22 [DOI] [Google Scholar]
- 23.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71-79. doi: 10.1016/J.JPHA.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charan J, Kantharia N. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4:303-306. doi: 10.4103/0976-500X.119726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain Z, Khan JA, Arshad A, Asif P, Rashid H, Arshad MI. Protective effects of Cinnamomum zeylanicum L. (Darchini) in acetaminophen-induced oxidative stress, hepatotoxicity and nephrotoxicity in mouse model. Biomed Pharmacother. 2019;109:2285-2292. doi: 10.1016/J.BIOPHA.2018.11.123 [DOI] [PubMed] [Google Scholar]
- 26.Okokon JE, Simeon JO, Umoh EE. Hepatoprotective activity of the extract of Homalium letestui stem against paracetamol-induced liver injury. Avicenna J Phytomed. 2017;7:27-36. [PMC free article] [PubMed] [Google Scholar]
- 27.Abirami A, Nagarani G, Siddhuraju P. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci Hum Wellness. 2015;4:35-41. doi: 10.1016/j.fshw.2015.02.002 [DOI] [Google Scholar]
- 28.de Oliveira Viana J, Silva e Souza E, Sbaraini N, et al. Scaffold repositioning of spiro-acridine derivatives as fungi chitinase inhibitor by target fishing and in vitro studies. Sci Rep. 2023;13:7320. doi: 10.1038/s41598-023-33279-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tithi TI, Tahsin MR, Anjum J, et al. An in vivo and in silico evaluation of the hepatoprotective potential of Gynura procumbens: a promising agent for combating hepatotoxicity. PLoS One. 2023;18:e0291125. doi: 10.1371/JOURNAL.PONE.0291125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose PW, Beran B, Bi C, et al. The RCSB Protein Data Bank: redesigned web site and web services. Nucleic Acids Res. 2011;39:D392-D401. doi: 10.1093/NAR/GKQ1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu C, Wu C, Ghoreishi D, et al. OPLS4: improving force field accuracy on challenging regimes of chemical space. J Chem Theor Comput. 2021;17:4291-4300. doi: 10.1021/ACS.JCTC.1C00302/SUPPL_FILE/CT1C00302_SI_002 [DOI] [PubMed] [Google Scholar]
- 32.Rehman NU, Ansari MN, Ahmad W, Amir M. GC–MS analysis and in vivo and ex vivo antidiarrheal and antispasmodic effects of the methanolic extract of Acacia nilotica. Mol. 2022;27:2107. doi: 10.3390/MOLECULES27072107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Thiessen PA, Bolton EE, et al. PubChem substance and compound databases. Nucleic Acids Res. 2016;44:D1202-D1213. doi: 10.1093/NAR/GKV951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jejurikar BLR, Jejurikar BL, Rohane SH. Drug designing in discovery studio. Asian J Research Chem. 2021;14:135-138. doi: 10.5958/0974-4150.2021.00025.0 [DOI] [Google Scholar]
- 35.Sharma A, Sinha S, Rathaur P, et al. Reckoning apigenin and kaempferol as a potential multi-targeted inhibitor of EGFR/HER2-MEK pathway of metastatic colorectal cancer identified using rigorous computational workflow. Mol Divers. 2022;26:3337-3356. doi: 10.1007/S11030-022-10396-7/METRICS [DOI] [PubMed] [Google Scholar]
- 36.Ahmad S, Usman Mirza M, Yean Kee L, et al. Fragment-based in silico design of SARS-CoV-2 main protease inhibitors. Chem Biol Drug Des. 2021;98:604-619. doi: 10.1111/CBDD.13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friesner RA, Murphy RB, Repasky MP, et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49:6177-6196. doi: 10.1021/JM051256O [DOI] [PubMed] [Google Scholar]
- 38.Repasky MP, Murphy RB, Banks JL, et al. Docking performance of the glide program as evaluated on the Astex and DUD datasets: a complete set of glide SP results and selected results for a new scoring function integrating WaterMap and glide. J Comput Aided Mol Des. 2012;26:787-799. doi: 10.1007/S10822-012-9575-9/METRICS [DOI] [PubMed] [Google Scholar]
- 39.Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/SREP42717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karami TK, Hailu S, Feng S, Graham R, Gukasyan HJ. Eyes on Lipinski’s rule of five: a new “rule of thumb” for physicochemical design space of ophthalmic drugs. J Ocul Pharmacol Therapeut. 2022;38:43-55. doi: 10.1089/JOP.2021.0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Wyk AS, Prinsloo G. Health, safety and quality concerns of plant-based traditional medicines and herbal remedies. South Afr J Bot. 2020;133:54-62. doi: 10.1016/J.SAJB.2020.06.031 [DOI] [Google Scholar]
- 42.Zivkovic Zaric R, Zaric M, Sekulic M, et al. Antimicrobial treatment of Serratia marcescens invasive infections: systematic review. Antibiotics. 2023;12:367. doi: 10.3390/ANTIBIOTICS12020367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giamarellou H. Multidrug-resistant Gram-negative bacteria: how to treat and for how long. Int J Antimicrob Agents. 2010;36 Suppl 2:S50-S54. doi: 10.1016/J.IJANTIMICAG.2010.11.014 [DOI] [PubMed] [Google Scholar]
- 44.Shamsudin NF, Ahmed QU, Mahmood S, et al. Antibacterial effects of flavonoids and their structure-activity relationship study: a comparative interpretation. Molecules. 2022;27:1149. doi: 10.3390/MOLECULES27041149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saha P, Talukdar AD, Nath R, et al. Role of natural phenolics in hepatoprotection: a mechanistic review and analysis of regulatory network of associated genes. Front Pharmacol. 2019;10:509. doi: 10.3389/FPHAR.2019.00509/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abd El-Ghffar EA, El-Nashar HAS, Eldahshan OA, Singab ANB. GC-MS analysis and hepatoprotective activity of the n-hexane extract of Acrocarpus fraxinifolius leaves against paracetamol-induced hepatotoxicity in male albino rats. Pharm Biol. 2017;55:444-449. doi: 10.1080/13880209.2016.1246575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elsayed HE, Ebrahim HY, Mady MS, Khattab MA, El-Sayed EK, Moharram FA. Ethnopharmacological impact of Melaleuca rugulosa (Link) Craven leaves extract on liver inflammation. J Ethnopharmacol. 2022;292:115215. doi: 10.1016/J.JEP.2022.115215 [DOI] [PubMed] [Google Scholar]
- 48.Babu BH, Shylesh BS, Padikkala J. Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia. 2001;72:272-277. doi: 10.1016/S0367-326X(00)00300-2 [DOI] [PubMed] [Google Scholar]
- 49.Li S, Tan HY, Wang N, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16:26087-26124. doi: 10.3390/IJMS161125942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blazka ME, Wilmer JL, Holladay SD, Wilson RE, Luster MI. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 1995;133:43-52. doi: 10.1006/TAAP.1995.1125 [DOI] [PubMed] [Google Scholar]
- 51.Li G, Chen MJ, Wang C, et al. Protective effects of hesperidin on concanavalin A-induced hepatic injury in mice. Int Immunopharm. 2014;21:406-411. doi: 10.1016/J.INTIMP.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 52.Turati F, Trichopoulos D, Polesel J, et al. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60:606-611. doi: 10.1016/J.JHEP.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 53.Ozturk Sarikaya SB. Acethylcholinesterase inhibitory potential and antioxidant properties of pyrogallol. J Enzym Inhib Med Chem. 2015;30:761-766. doi: 10.3109/14756366.2014.965700 [DOI] [PubMed] [Google Scholar]
- 54.Ojo OA, Ojo AB, Barnabas M, et al. Phytochemical properties and pharmacological activities of the genus Pennisetum: a review. Sci Afr. 2022;16:e01132. doi: 10.1016/J.SCIAF.2022.E01132 [DOI] [Google Scholar]
- 55.Reza ASMA, Haque MA, Sarker J, et al. Antiproliferative and antioxidant potentials of bioactive edible vegetable fraction of Achyranthes ferruginea Roxb. in cancer cell line. Food Sci Nutr. 2021;9:3777-3805. doi: 10.1002/FSN3.2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langmead L, Rampton DS. Review article: herbal treatment in gastrointestinal and liver disease—benefits and dangers. Aliment Pharmacol Ther. 2001;15:1239-1252. doi: 10.1046/J.1365-2036.2001.01053 [DOI] [PubMed] [Google Scholar]
- 57.Dey P, Olmstead BD, Sasaki GY, Vodovotz Y, Yu Z, Bruno RS. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J Nutr Biochem. 2020;84:108455. doi: 10.1016/J.JNUTBIO.2020.108455 [DOI] [PubMed] [Google Scholar]
- 58.Dey P. Gut microbiota in phytopharmacology: a comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol Res. 2019;147:104367. doi: 10.1016/J.PHRS.2019.104367 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Antimicrobial and Hepatoprotective Properties of Pods of Acacia nilotica (L.) Willd. ex Delile: In Vivo and In Silico Approaches by Mehak Idrees, Sana Javaid, Sumaira Nadeem, Faria Khurshid, Abida Parveen, Abdul Malik, Azmat Ali Khan, Suhail Akhtar, and Sabiha Fatima in Dose-Response.