ABSTRACT
Background
Current US federal action levels for domoic acid (DA) in seafood are based on acute toxicity observed in exposed adult humans. Life course considerations have not been incorporated. The potential for developmental neurotoxicity (DNT) at permissible DA levels has previously been noted, but not methodically assessed.
Methods
Studies of DNT following DA exposure in experimental and wild animals were identified through a comprehensive search strategy. Evidence from papers meeting inclusion criteria was evaluated for specific outcomes reported for doses at which adverse effects were observed. Exposure levels associated with DNT were compared with those known to cause adult toxicity. The findings are discussed in the context of the well‐characterized mechanism of DA neurotoxicity, as well as the toxicokinetics of DA across species and life stages.
Conclusions
DNT outcomes were reported with a no observed adverse effect level (NOAEL) 10 times lower than the NOAEL of 0.75 mg DA/kg for acute effects in adults. Apart from reviewing current regulatory action levels, public health outreach messaging to health care professionals and sensitive populations, such as pregnant or breastfeeding women, should be considered as a means of increasing awareness about risk for DNT from consumption of potentially DA‐contaminated seafood.
Keywords: developmental neurotoxicity, domoic acid, environmental health, life course considerations
1. Introduction
Many researchers have expressed concerns as to whether current safety considerations for the presence of domoic acid (DA) in seafood are sufficient to protect individual consumers throughout the course of their lives (Costa, Giordano, and Faustman 2010; Doucette and Tasker 2016; Grattan 2022; Panlilio et al. 2023; Petroff et al. 2021; Shum et al. 2020). The sensitivity of age‐based sub‐populations can vary due to the developmental stage of target tissues (e.g., brain), as well as with life‐stage changes in toxicokinetic (TK) factors such as rates of absorption and elimination (Lanphear 2015).
Compared to the adult brain, developing brains are generally more sensitive to the effects of neurotoxicants (Costa, Guizzetti, and Vitalone 2004; Lanphear 2015; Rodier 1995). Prenatal and early postnatal brain development are characterized by rapid neural cell proliferation and migration, maturation of receptor and transmitter systems, increasing numbers of synaptic connections, and production of myelin (Rodier 1995). Interference with any of these essential developmental processes can adversely impact eventual mature brain structure and/or function (Costa, Guizzetti, and Vitalone 2004). Simultaneously, an immature blood–brain barrier may not provide the developing brain with the same degree of protection from circulating toxins as afforded to an adult brain by a mature barrier (Costa, Guizzetti, and Vitalone 2004; Rodier 1995).
The neurotoxic effects of DA are consistent among adults of the species evaluated including humans, laboratory animals, and affected wildlife (Goldstein et al. 2008; Grant et al. 2010; Iverson and Truelove 1994). Experimental evidence indicates that developing brains are more sensitive than adult brains to DA‐induced neurotoxicity (Costa, Giordano, and Faustman 2010; Petroff et al. 2021).
For this commentary, we have compiled the empirical evidence from studies of DA‐induced developmental neurotoxicity (DNT) in experimental and wild animals. Specific impacts observed at the lowest DA doses with an adverse effect were compared across species and developmental stages. While the exact timing of key neurodevelopmental events with respect to the pre‐ versus post‐natal periods may vary among species (Clancy et al. 2008), the sequence of events appears to be highly conserved (Clancy, Darlington, and Finlay 2001). The results are discussed in the context of available background information on mechanisms of DA neurotoxicity and the TK of DA at various life stages. The detailed information presented in this commentary will be valuable for communicating risks of DA exposure throughout life.
1.1. Sources of DA Exposure
DA is a potent neurotoxin produced by algal diatoms. On the west coast of the US (OEHHA 2022a; Petroff et al. 2021) and in many other jurisdictions worldwide (Bates et al. 2018), diatoms of the genus Pseudo‐nitzschia are the most common source. Shellfish and fish can accumulate DA by feeding in waters contaminated with DA‐containing cells of Pseudo‐nitzschia (Bernstein et al. 2021; Lefebvre, Frame, and Kendrick 2012; Lefebvre et al. 2007). Accumulated DA can then be passed on to higher trophic levels (OEHHA 2022a) including seabirds (Gibble et al. 2021), marine mammals (Bowen et al. 2022; Goldstein et al. 2008; Kreuder 2005; Moriarty et al. 2021), and humans (Fritz et al. 1992).
1.2. Acute Effects of DA (1987 Amnesic Shellfish Poisoning Outbreak)
The acute effects of DA poisoning in adults came to wide attention following a 1987 outbreak involving over 100 people who consumed cultured mussels sourced from eastern Prince Edward Island, Canada (Perl, Bedard, Kosatsky, Hockin, Todd, Remis, et al. 1990). Symptoms ranged from gastrointestinal (GI) distress, to disorientation, seizure, coma, and death (Perl, Bedard, Kosatsky, Hockin, Todd, Remis, et al. 1990). DA poisoning is also referred to as “Amnesic Shellfish Poisoning” (ASP) because temporary or permanent loss of short‐term memory is a characteristic symptom at higher exposure levels (Grant et al. 2010; Perl, Teitelbaum, et al. 1990; Teitelbaum et al. 1990).
Ingested doses of DA were estimated for 10 individuals from the 1987 outbreak based on DA concentrations in meal remnants and estimated portions consumed (Perl, Bedard, Kosatsky, Hockin, Todd, Remis, et al. 1990). A single individual estimated to have consumed 20 mg of DA had no adverse GI or neurological effects (EFSA 2009). With doses normalized to 60 kg body weight (bw), the lowest observed adverse effect level (LOAEL) was determined to be 1.0 mg DA/kg bw, which was associated with mild gastrointestinal effects (Iverson and Truelove 1994). Severe illness requiring hospitalization was associated with an estimated dose of 4 mg DA/kg bw (EFSA 2009; Perl, Bedard, Kosatsky, Hockin, Todd, Remis, et al. 1990).
1.3. Basic Mechanism of Action (Kainic Acid Analog)
DA is a high‐affinity structural analog of kainic acid (Figure 1), and hence a selective agonist of kainate receptors (a subtype of ionotropic glutamate receptors) (Larm, Beart, and Cheung 1997). At sufficient concentrations, agonists of glutamate receptors can over‐stimulate neurons to death in a process known as excitotoxicity. Excitotoxicity contributes to impairment of learning and memory as well as to frank neurodegeneration in the hippocampus and neocortex (AOP‐48 2023; Larm, Beart, and Cheung 1997). DA has a higher affinity for kainate receptors than kainic acid itself, resulting in 3–20 fold greater potency for downstream effects (Larm, Beart, and Cheung 1997; Costa, Giordano, and Faustman 2010).
FIGURE 1.
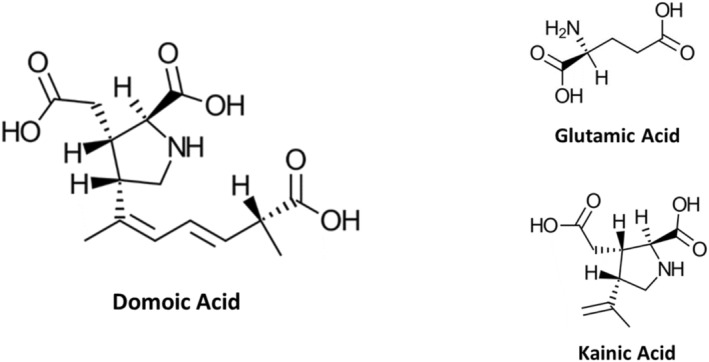
Chemical structures of domoic acid, and analogues glutamic acid and kainic acid.
The Adverse Outcome Pathway (AOP) for ionotropic glutamate receptors in adult brain (Figure 2) illustrates the sequence of key events from receptor binding through apical expression as deficits in learning and memory (AOP‐48 2023). The AOP is intended to apply to all types of glutamate ionotropic receptors, including kainate receptors, involved in basal excitatory synaptic transmission and synaptic plasticity, which under physiological conditions are critical for normal learning and memory. DA's action as an agonist for glutamate receptors is sufficiently well‐understood and specific to support its use as a positive control for certain in vitro assays used in large‐scale screening of compounds for potential DNT (USEPA 2020). While not specifically validated for developing brains, current evidence tends to support the pathway's applicability (AOP‐48 2023).
FIGURE 2.
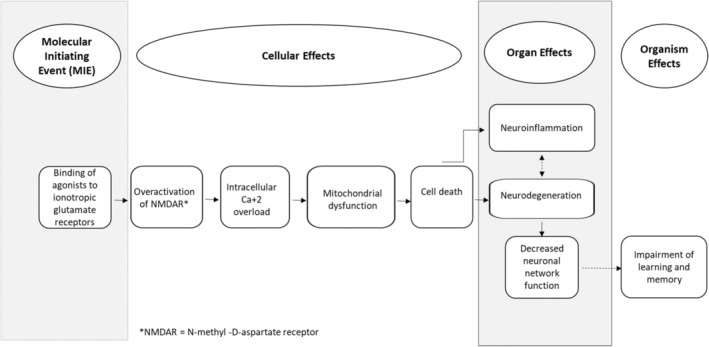
Schematic diagram of the adverse outcome pathway (AOP) for domoic acid and similar compounds. Modified from the AOP “Binding of agonists to ionotropic glutamate receptors in adult brain leading to excitotoxicity that mediates neuronal cell death, contributing to learning and memory impairment” (AOP 48, 2023) available online at: https://aopwiki.org/aops/48.
Data from humans, mice, and rats used to construct the AOP were characterized as strong evidence (AOP‐48 2023). Binding of DA to ionotropic glutamate receptors and the resulting adverse neurological effects have been documented broadly in additional vertebrate species including non‐human primates, marine mammals, birds, and finfish (Lefebvre, Frame, and Kendrick 2012; Lefebvre et al. 2007; Lefebvre 2001). While fish treated with DA by injection are susceptible to DA‐caused excitotoxicity, they may be less sensitive to oral doses of DA when compared to other species (Anderson et al. 2021; Lefebvre, Frame, and Kendrick 2012; Lefebvre et al. 2007; Lefebvre 2001). Fish die‐offs have not been observed to result from Pseudo‐nitzschia blooms, even when DA levels in fish digestive tract tissues were high (e.g., > 50 mg DA/kg viscera) (Bernstein et al. 2021; Lefebvre, Frame, and Kendrick 2012; Lefebvre et al. 2007).
Ionotropic glutamate receptors, including kainate receptors, have also been identified in tissues of the GI tract (Baj et al. 2019). These receptors are part of the “microbiota–gut–brain axis,” and modulation of their activity may influence brain as well as gut function. While vomiting and other GI effects have been reported from DA exposure in humans and non‐human primates, the specific involvement of ionotropic glutamate receptors in these symptoms has not been definitively established.
1.4. Basic Toxicokinetics (TK) of DA
DA is poorly absorbed from the GI tract, and is rapidly eliminated (e.g., half‐life < 110 min reported for non‐human primates) with approximately 75% metabolically unchanged in urine and feces (Suzuki and Hierlihy 1993; Truelove and Iverson 1994; Truelove et al. 1997; Jing et al. 2018; Costa, Giordano, and Faustman 2010). Distribution and elimination of DA has been studied in pregnant and non‐pregnant animals (Jing et al. 2018; Maucher Fuquay et al. 2012a; Shum et al. 2020), as well as in fetuses and neonates (Maucher Fuquay et al. 2012b; Maucher and Ramsdell 2005).
The results from TK studies of acutely dosed DA differ between the injection and oral routes of exposure. In adult female cynomolgus monkeys ( Macaca fascicularis ), a single intravenous (IV) dose of 5 μg DA/kg bw (0.005 mg/kg) had a mean elimination half‐life of 1.2 h (Jing et al. 2018). Acute oral doses of 0.075 and 0.15 mg DA/kg bw produced a mean terminal half‐life of 11.3 h with the difference between routes indicating “flip‐flop kinetics,” or a rate of oral absorption that is slower than the rate of elimination and hence rate‐limiting (Jing et al. 2018).
The TK of chronic oral dosing with DA at 0.075 and 0.15 mg/kg bw was studied in adult female cynomolgus monkeys before, during, and after pregnancy (Shum et al. 2020). At the time of delivery, DA was detected in infant plasma and amniotic fluid. TK modeling of maternal‐fetal DA disposition suggested that placental transport introduces DA to the fetus via cord blood. The fetus subsequently eliminates DA into the amniotic fluid, which is then recirculated by fetal swallowing (Figure 3). DA levels were 4.5–7.5X higher in amniotic fluid than in fetal plasma, suggesting that DA accumulates in amniotic fluid.
FIGURE 3.
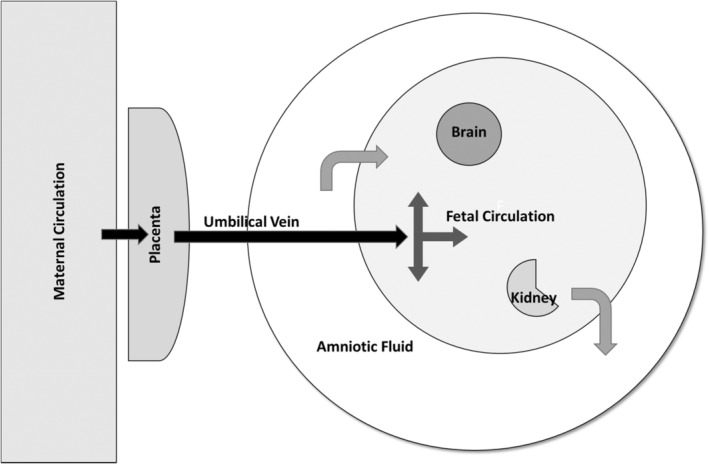
Schematic diagram of maternal‐fetal distribution of domoic acid (DA). DA in the maternal circulation is transported across the placenta and into the fetus via the umbilical vein. The fetal circulatory system transports DA to the immature fetal organs, including brain. The fetus eliminates DA into the amniotic fluid, which is swallowed by the fetus, thus recirculating available DA.
Distribution of DA in maternal and fetal tissues following gestational dosing is broadly consistent in experimental rats and monkeys (Maucher Fuquay et al. 2012a, 2012b; Shum et al. 2020), as well as in samples collected from fetal tissues and fluids of stranded sea lions (Brodie et al. 2006; Goldstein et al. 2009; Lefebvre et al. 2018). Following treatment of pregnant rats on gestation day (GD) 20 with 1.0 mg DA/kg bw IV, DA levels in maternal brain and cerebrospinal fluid (CSF) peaked at 15 min following treatment and were undetectable by 12–24 h (Maucher Fuquay et al. 2012a). Fetal plasma DA levels peaked at 60 min post‐dosing (Maucher Fuquay et al. 2012b). Post‐treatment DA levels in amniotic fluid and fetal brain did not show evidence of elimination over 24 h. Retention of DA in the amniotic fluid of these experimental animals (Maucher Fuquay et al. 2012b) reinforces the suggestion of continuous fetal re‐exposure, in turn increasing the relative risk for fetal harm from a given maternal dose of DA.
Experiments in lactating rats demonstrated the presence of DA in the milk of treated dams and in the plasma of nursing pups given supplemental milk with added DA (Maucher and Ramsdell 2005). While at one‐hour post‐dosing with 1.0 mg/kg bw DA by intraperitoneal injection (IP) DA levels were 16 times lower in milk than in maternal plasma; by 8 h post‐dosing, remaining DA levels were four times higher in milk than in maternal plasma. Although neonates given milk from these DA‐exposed females that did not attain measurable plasma levels of DA, neonates did absorb sufficient DA from consuming milk “spiked” with 1.0 mg DA/kg bw to result in measurable plasma DA levels.
1.5. Current Basis for US Regulation of DA in Seafood
Health Canada was the first entity to establish and implement an official regulatory limit of 20 μg DA/g (20 ppm) for DA in edible tissue of bivalve shellfish in 1989. Prior to that, Canadian health and regulatory entities had instituted an interim safety threshold in 1988 that restricted shellfish harvest when DA levels exceeded 20 μg DA/g (Todd 1990; Wekell, Hurst, and Lefebvre 2004). Health Canada organized a symposium on DA toxicity in Ottawa (April 1989), where scientists, health officials, and regulators met to discuss various facets of the 1987 DA poisoning event, define the acute DA toxicity clinical syndrome now known as ASP, and establish the scientific basis for a safe consumption threshold for DA in shellfish of 20 ppm. Health Canada applied a LOAEL approach to calculate the threshold where 50 mg DA was divided by an approximate 0.200 kg (wet weight) shellfish consumption rate, or 250 ppm, to which a safety factor of 12 was applied (Perl, Bedard, Kosatsky, Hockin, Todd, Remis, et al. 1990; Perl, Bedard, Kosatsky, Hockin, Todd, McNutt, et al. 1990; Perl, Teitelbaum, et al. 1990; Todd 1990; Wekell, Hurst, and Lefebvre 2004).
US Food and Drug Administration (FDA) critically reviewed the Health Canada regulatory limit at the FDA Pacific Region 1992 Domoic Acid Workshop in San Pedro, California, which was modeled after the 1989 Canadian symposium (DHHS 1993; USFDA 2021; Wekell, Hurst, and Lefebvre 2004). US FDA adopted the LOAEL approach using 60 mg DA as a LOAEL, divided by 0.250 kg (wet weight) shellfish consumption rate, or 240 ppm, and applied an uncertainty factor (UF) of 10. Twenty ppm was subsequently adopted and implemented as the FDA action level for DA in seafood (DHHS 1993). US FDA later evaluated additional DA data in Dungeness Crab and adopted a second action level in 1993 permitting 30 ppm DA in Dungeness Crab viscera (DHHS 1993; USFDA 2021).
Subsequent to the adoption of federal action limits, the Washington State Department of Health (DOH) conducted a literature review to determine a tolerable daily intake (TDI) for DA (Marien 1996). Overall, Washington DOH determined that the human and nonhuman primate toxicity data supported a no observed adverse effect level (NOAEL) of 0.75 mg DA/ kg bw for acute exposure of adult individuals (Marien 1996). Due to the similarities between humans and test monkeys in response to doses of DA (Iverson and Truelove 1994), no between‐species uncertainty factor (UF) was applied. Washington State health officials established a TDI of 0.075 mg DA/kg bw that incorporated a 10‐fold UF to account for variation in sensitivity within species (Marien 1996). Washington DOH concluded that the TDI was consistent with the US FDA action levels of 30 and 20 ppm for crab viscera and clams, respectively, and that the federal limits were sufficiently protective of public health from acute DA toxicity.
1.6. California State Agency Activities Regarding DA
The Office of Environmental and Health Hazard Assessment (OEHHA) is the lead California agency for evaluating health hazards posed by environmental contaminants and partners with other State agencies to ensure the safety of California fisheries from toxic substances, including DA (OEHHA 2019a). As Pseudo‐nitzschia blooms in California coastal waters are an ongoing concern, OEHHA assesses monitoring data for DA levels in California seafood samples analyzed by the California Department of Public Health (CDPH) laboratories. In consultation with CDPH, OEHHA makes formal health‐based recommendations to the California Department of Fish and Wildlife (CDFW) regarding delay of opening, closure, or re‐opening actions for California recreational and commercial fisheries under the authority of California Fish and Game Code section 5523 (CDFW) (2017).
OEHHA, CDPH, and CDFW coordinate DA‐related regulatory actions and provide the public with information in the form of responses to frequently asked questions (CDPH 2017; OEHHA 2018). These three entities, in cooperation with the California State Water Resources Control Board, comprise the Interagency harmful algal bloom (HAB)‐related Illness Workgroup which investigates and tracks potential HAB‐related illnesses in humans and animals throughout California, including DA‐induced ASP, which is a reportable illness in California (OEHHA 2022b, 2024). OEHHA has hosted two informational workshops on DA toxicity (2017 and 2019), which featured invited speakers from the research community (OEHHA 2017, 2019b).
In 2020, DA was one of 22 chemicals presented to the OEHHA Developmental and Reproductive Toxicant Identification Committee (DARTIC) for prioritization for consideration under California's Proposition 65 (OEHHA 2020). Chemicals included in that document had passed a data screen followed by a preliminary toxicological evaluation of available evidence for reproductive harm.
1.7. Empirical Data: Implications for DA‐Related DNT Risk
The extensive background information on DA summarized above affirms that life stage considerations are relevant to risk for DA's adverse neurological effects. Developing vertebrates are at particular risk of DA‐induced DNT due to the immaturity of developing brain tissues. Developing mammals face the additional complexities of placental transfer, recirculation between the fetus and amniotic fluid with slow elimination from the amniotic fluid, and potential postnatal exposure via milk from exposed maternal animals.
An earlier review reported on experimental evidence for DNT occurring at DA doses one to two orders of magnitude below levels causing neurotoxicity in adults (Costa, Giordano, and Faustman 2010). This present effort compiles and summarizes the currently available experimental data on the developmental toxicity of DA in developing nonhuman animals. Taking a comprehensive look at dose–response data for DNT associated with DA exposure early in life for several species will facilitate considerations of whether the current action levels provide protection during the most sensitive life stages.
2. Methods
2.1. Literature Identification and Selection
2.1.1. Search Strategies
The literature search strategy for prioritization candidates is detailed in a 2020 report by OEHHA (OEHHA 2020). The studies retrieved and reviewed for that document provided an initial nucleus for the DNT data presented in Tables.
The literature search for DA has been updated, and relevant new publications retrieved for inclusion. Additional efforts to identify relevant literature have included cross‐checking reference lists from published papers, and author‐based searches for papers published by researchers known to have worked on the DNT of DA.
2.1.2. Inclusion Criteria for Laboratory and Wild Animal Studies
Laboratory studies included in the data tables met the following criteria by reporting:
Original data.
At least one dose level of DA plus appropriate controls.
Single or repeated DA dosing restricted to the pre‐ and/or postnatal periods of brain development, with specific timing reported to differentiate between DNT and adult neurotoxicity resulting from acute or chronic DA exposures of mature subjects.
Assessment and reporting of DNT outcomes such as behavioral, histopathological, and/or biochemical effects.
DNT assessment must have occurred subsequent to DA dosing; while timing of dosing is restrictive to the period of neurodevelopment, assessment of DNT outcomes could have been conducted at any time following dosing, from immediately post‐dosing until later in life.
For wild marine mammals, only rough estimates of dosing and timing of DA exposure could be made. Tabulated studies reported data on young animals believed to have been exposed during the prenatal and/or neonatal (nursing) periods. All of the selected literature reported on California sea lions found stranded, with DA exposure inferred from the timing and extent of known toxigenic Pseudo‐nitzschia blooms capable of, or actively producing, DA (Smith et al. 2023). Some studies measured DA concentrations in affected animals' tissues and body fluids.
3. Results
Tables 1, 2, 3, 4, 5, 6, 7 summarize methods and results from whole‐animal studies of DNT in DA‐exposed animals. Each table represents studies grouped by time of exposure and species tested. Emphasis is placed on identifying the lowest dose at which DNT was observed for each study, and the specific effects reported at that dose. Where necessary to facilitate comparison between studies, units have been converted to mg/kg bw. Brief synopses of the tabulated information are presented below.
TABLE 1.
Gestational exposure only; Non‐human primates ( Macaca fascicularis ).
| Reference | Methods | Outcomes at LOAEL a | LOAEL/NOAEL b |
|---|---|---|---|
| Burbacher et al. 2019 | Oral doses of 0, 0.075, or 0.15 mg DA c /kg‐day during breeding and gestation. Evaluations of live offspring at birth. | No changes in measurements or health assessments of newborn infants. | LOAEL = NI d . |
| Grant et al. 2019 | Behavioral evaluations of infant cohort reported in Burbacher et al. 2019; Tests at 1‐2 PNM e . | Impairment on tests of recognition and differential visual attention to novel stimuli in the 0.15 mg/kg group. |
LOAEL = 0.15 mg/kg‐day (oral). NOAEL = 0.075 mg/kg‐day (oral). |
Note: The two papers tabulated above report studies of the same experimental animals evaluated at different times for different outcomes.
LOAEL = lowest observed adverse effect level. Outcomes for exposed animals significant at p < 0.05 or less.
NOAEL = no observed adverse effect level.
DA = domoic acid.
NI = not identified.
PNM = postnatal month.
TABLE 2.
Gestational exposure only; Rats.
| Reference | Methods | Outcomes at LOAEL | LOAEL/NOAEL |
|---|---|---|---|
| Levin et al. 2005 |
Subcutaneous injection (SC) of 0, 0.3, 0.6, or 1.2 mg DA/kg on GD a 13. T‐ and Figure 2 maze tests during PNW b 4‐8. Radial maze test during PNW 9‐13; scopolamine challenge after training. |
Impaired response in session 2 of T‐maze test in the 1.2 mg/kg group. Greater habituation of locomotor activity over 1 h in Figure 2 maze test in the 1.2 mg/kg group. Impaired choice accuracy on Radial‐arm maze test with scopolamine challenge to 0.6 and 1.2 mg/kg groups. |
LOAEL = 0.6 mg/kg (SC). NOAEL = 0.3 mg/kg (SC). |
GD = gestation day.
PNW = postnatal week.
TABLE 3.
Gestational exposure only; Mice.
| Reference | Methods | Outcomes at LOAEL | LOAEL/NOAEL |
|---|---|---|---|
| Shiotani et al. 2017 |
Oral gavage of 0, 1, or 3 mg DA/kg‐day on GD 10‐17. Behavioral test battery PND 5‐92. |
Results were analyzed by mixed effects ANOVA a . Test data for rotarod, elevated maze, PhenoTyper‐cage, Catwalk gait parameters, and Morris water maze all showed significant effects of DA dose. | 1 mg/kg‐day (oral) likely represents a LOAEL value, but paper does not clarify. |
| Mills et al. 2016 |
SC injection of 0 or 1.5 mg DA/kg on GD 16. Behavioral testing on PND 21, 25, and 35; MRI b on PND 32‐40. |
Reduced social interactions in male offspring. Altered functional connectivity patterns and alterations in certain brain regions resembling those associated with ASD c . |
LOAEL = 1.5 mg/kg (SC). NOAEL = NI. |
| Zuloaga et al. 2016 |
SC injection of 0 or 1.5 mg DA/kg GD 15. Behavioral testing on PND 21, 25, and 35. Brain microscopy and immuno‐histochemistry on PND 35. |
Diminished social investigation and ultrasonic vocalization in male offspring. Altered sensorimotor gating. |
LOAEL = 1.5 mg/kg (SC). NOAEL = NI. |
| Tanemura et al. 2009 |
Intraperitoneal injection (IP) of 0 or 1 mg DA/kg on GD 11.5, 14.5, or 17.5. Male offspring tested and examined at PNW 11. |
DA‐exposed male offspring showed impaired learning and memory, as well as altered anxiety‐related behaviors. Necropsies revealed myelination failure and overgrowth of neuronal processes of the limbic cortex neurons. While the magnitude of specific effects varied with day of treatment, all treated groups showed significant changes in some outcomes. |
LOAEL = 1 mg/kg (IP). NOAEL = NI. |
| Dakshinamurti et al. 1993 |
Intravenous injection (IV) of 0 or 0.6 mg DA/kg GD 13. EEG d monitoring PND 10‐30. |
Electrocortical inhibition. Reduced seizure thresholds in response to DA challenge. Abnormal histopathology of hippocampus and dentate gyrus. Reduced regional GABA e and increased glutamate levels. Increased kainate receptor binding and enhanced calcium influx. |
LOAEL = 0.6 mg/kg (IV). NOAEL = NI. |
ANOVA = analysis of variance.
MRI = magnetic resonance imaging.
ASD = autism spectrum disorder.
EEG = electroencephalogram.
GABA = gamma‐aminobutyric acid.
TABLE 4.
Zebrafish fertilized eggs or embryos.
| Reference | Methods | Outcomes at LOAEL | LOAEL/NOAEL |
|---|---|---|---|
| Panlilio et al. 2023 |
IV injection of 0, 0.14, or 0.18 ng DA at 48–53 hpf a (~2 dpf b ). Evaluations at 2.5, 4, and 5 dpf. |
Dose‐dependent reduction in number of myelinating oligodendrocytes. Loss of Mauthner neurons. Loss of intact middle primary motor neuron (MiP) axons. |
LOAEL = 0.14 ng (IV) [0.14E‐6 mg]. c NOAEL = NI. |
| Panlilio et al. 2021 |
IV injection of 0 or 0.14 ng DA on ~2 dpf. Testing at 2.5, 5, and 7 dpf. |
Reduced startle response. Altered kinematics of both short and long latency C‐bend startle responses. Sensory inputs were intact, but missing reticulospinal neurons. Reduced primary motor neuron axon collaterals. |
LOAEL = 0.14 ng (IV) [0.14E‐6 mg]. c NOAEL = NI. |
| Panlilio, Aluru, and Hahn 2020 |
IV injection of 0, or 0.09–0.18 ng DA at ~1, 2, or 4 dpf. Observations daily from 1 day post‐ dose through 5 dpf; additional observations at 5–7 dpf. |
Reduced startle response. Altered kinematics of both short and long latency C‐bend startle responses. Altered gene expression and disrupted myelin structure. Most sensitive time for all effects was 2 dpf. |
LOAEL = 0.09 ng (IV) [0.09E‐6 mg]. c NOAEL = NI. |
| Tiedeken and Ramsdell 2009 | Bath exposure of larvae at 7 dpf to DA at concentrations of 0, 0.25–1.0 mM. | Bath concentrations as low as 0.25 mM caused a “brief hyperactive response” followed by convulsive behavior. Expression of convulsive behavior included body contractions, circular swimming patterns, isolated tremors, and complete paralysis. |
LOAEL = 0.25 mM (bath exposure). NOAEL = NI. |
| Tiedeken and Ramsdell 2007 | Microinjection with 0 or 0.13–1.26 ng DA/mg egg weight at 3–4 hpf. | DA exposure reduced threshold to chemically‐induced seizures (by PTZ) d in the larvae at seven dpf, as well as increasing severity of seizure behavior. |
LOAEL = 0.13 ng/mg (microinjection) [0.13 mg/kg]. NOAEL = NI. |
| Tiedeken, Ramsdell, and Ramsdell 2005 | 0 or 0.12–17.0 mg DA/kg egg weight by microinjection to fertilized eggs at 6 hpf. |
Reduced hatching success at 0.4 mg/kg, 1.2 mg/kg and higher. Marked tonic–clonic type convulsions at 2 days after 1.2 mg/kg. Absence of touch response reflexes at 4 dpf after 4.0 mg/kg or higher. Rapid and constant pectoral fin movements from 5 dpf. |
LOAEL = 0.40 mg/kg (microinjection). NOAEL = 0.12 mg/kg (microinjection). |
hpf = hours post‐fertilization.
dpf = days post‐fertilization.
Dose expressed as ng/embryo.
PTZ = pentylenetetrazole.
TABLE 5.
Neonatal exposure; Rats.
| Reference | Methods | Outcomes at LOAEL | LOAEL/NOAEL |
|---|---|---|---|
| Marriott et al. 2016 |
SC injection of 0 or 20 μg DA/kg‐day on PND 8‐14. Post‐weaning animals housed either in groups or singly in social isolation. Evaluations of adult animals at approximately 4.5 months of age. |
Social isolation (without DA) led to lowered prepulse magnitude in male rats. DA exposure with or without post‐weaning social isolation led to no effect on pre‐pulse magnitude, but an additive increase in prepulse startle latency in both sexes. Alterations in prepulse inhibition indicate altered perceptual processing, used to assess neural alterations of neuropsychiatric disorders. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Thomsen et al. 2016 |
SC injection with 0, 20, or 60 μg DA/kg on PND 8‐14. Behavioral testing on PND 50, 75, and 98. Testing for α2‐adrenoceptor binding on PND 120. |
Differences on behavioral tests (open field, social interaction, and forced swim) only at the higher dose of DA. Altered α2‐adrenoceptor binding at both doses. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Jandová et al. 2014 |
SC injection of 0 or 30 μg DA/kg‐day on PND 10‐14. Behavioral observations on PND 35, 42, and 112. |
DA changed spontaneous activity patterns only during testing at 112 days. All activities recorded were affected, including: locomotion, rearing, and grooming. |
LOAEL = 30 μg/kg‐day (SC) [0.03 mg/kg‐day]. NOAEL = NI. |
| Gill et al. 2012 |
SC injection of 0 or 20 μg DA/kg‐day on PND 8‐14. Behavioral testing on PND 106‐140. Brain tissues taken for Western blot analysis on PND 241. |
DA increased indicators of behavioral stress including low‐grade seizure activity during maze testing. While initial learning of maze tasks was similar for DA and control groups, DA impaired responses to challenge reversal tasks, particularly for males. Western blot analysis revealed increases in adrenergic receptor (α2a and α2c) as well as increased mineralocorticoid receptor expression in DA‐treated males. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Adams et al. 2009 |
SC injection of 0 or 20 μg DA/kg‐day on PND 8‐14. Daily evaluation for startle response and eye opening from PND 8. Radial‐arm maze test from PND 34‐36; Morris water maze test from PND 130‐132. |
Altered performance on both radial‐arm maze and Morris water maze for treated animals. Performance improved for several parameters in both mazes, but impaired for treated females on the reversal task of the Morris water maze. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Gill et al. 2009 |
SC injection of 0 or 20 μg DA/kg‐day on PND 8‐14. Radiotelemetry transmitters surgically implanted on PND 90, for EEG recording of sleep stage durations. |
Decreased time in paradoxical sleep during 12 daylight hours. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Perry, Ryan, and Tasker 2009 |
SC injection of 0 or 20 μg DA/kg‐day on PND 8‐14. Animals sacrificed for biochemical analyses on PND 14 or 75, or behaviorally tested on PND 75. |
DA‐treated animals showed increased frequency of a behavioral syndrome (NIS‐L) consisting of repetitive and simultaneous squinting, mastication, and head bobbing during a test session. The test sessions in a novel water maze (NWM) considered to provide a mild to moderate stressor, which stimulated the NIS‐L response. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Adams, Doucette, and Ryan 2008 |
SC injection of 0 or 20 μg DA/kg‐day on PND 8‐14. Testing for prepulse inhibition (PPI) of the acoustic startle response began on PND 90. |
DA‐treated males showed lower %PPI, while treated females showed a higher baseline startle response at the beginning of the session. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Burt, Ryan, and Doucette 2008a |
SC injection of 0 or 20 μg DA/kg‐day on PND 8‐14. Tests for nicotine‐induced conditioned place preference post‐weaning on PND 21. |
Reduced sensitivity to nicotine‐induced conditioned place preference in DA‐treated animals. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Burt, Ryan, and Doucette 2008b |
SC injection of 0 or 20 μg DA/kg‐day on PND 8‐14. Assessment of developmental measures starting on PND 8. Behavioral testing from PND 18‐150. Nicotine‐induced conditioned place preference in DA‐treated animals assessed on PND 200‐240. |
Earlier eye opening in DA‐exposed females only. No effects on body weight gain, or timing of other developmental landmarks. Open field test results for male and/or female DA‐treated animals affected in one or more parameters. Testing on PND 18, 36, and 150. Time spent interacting with novel objects in a playground maze was increased for DA‐treated males only on PND 56. Place preference (time spent in a nicotine‐paired chamber) was increased for DA‐treated females only on PND 200‐240. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Bernard et al. 2007 |
SC injection of 0 or 20 μg DA/kg‐day on PND 8‐14. Hippocampi dissected out and prepared for evaluations. |
Alterations in densities of mossy fiber sprouting and associated changes in specific hippocampal regions with neonatal DA exposure. Results suggested regional‐specific cytoarchitectural changes indicative of age‐progressive abnormal development/synaptic plasticity. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Levin et al. 2006 |
SC injection of 0, 0.025, 0.05, and 0.1 mg DA/kg 2X/day (so doses of 0, 0.05, 0.1 and 0.2 mg/kg‐day) on PND 1 and 2. Behavioral testing during PNW 4‐13. |
High dose of 0.2 mg/kg‐day was lethal to all offspring. Hypoactivity in Figure‐8 maze tests at 0.1 mg/kg‐day for both sexes. |
LOAEL = 0.1 mg/kg‐day (SC). NOAEL = 0.05 mg/kg‐day (SC). |
| Tasker et al. 2005 |
SC injection of 0 or 20 μg DA/kg on PND 8‐14. Pups conditioned to an odor on PND 8. Odor preference, between conditioned and novel odors tested on PND 9 and 13. |
DA‐exposed rats tested on PND 13 preferred the conditioned odor over a novel odor. Co‐treatment with (RS)‐3‐(2‐Carboxypiperazin‐4‐yl)‐propyl‐1‐phosphonic acid (CPP), an antagonist of N‐methyl‐d‐aspartate (NMDA), eliminated the difference in preference, while saline and CPP controls preferred the novel test odor. |
LOAEL = 20 μg/kg‐day (SC) [0.02 mg/kg‐day]. NOAEL = NI. |
| Doucette et al. 2004 |
SC injection of 0, 5, or 20 μg DA/kg‐day on PND 8‐14. Observations and testing throughout study until 15 months of age. |
Seizure‐like syndrome when neonatally‐treated adult animals given different tests of spatial cognition. Increased hippocampal mossy fiber staining, and reductions in cell counts. |
LOAEL = 5.0 μg/kg‐day (SC) [0.005 mg/kg‐day]. NOAEL = NI. |
| Doucette et al. 2000 |
IP injection of 0 or 0.05–1.0 mg DA/kg on one of PND 0, 5, 14, or 22. Additional experiment with SC injection of 0.05–0.30 mg DA/kg on PND 8 and 14. Behavioral observations shortly after experimental treatment. |
The potency of DA‐mediated neurotoxicity decreased with increasing age. Lowest reported ED50 for behavioral score was 0.12 mg DA/kg bw IP on PND 0. No effect level was not specified. Second experiment reported ED50 of 0.08 mg DA/kg bw IP on PND 8, with no effect level not reported. |
LOAEL = 0.12 mg/kg (IP on PND 0). LOAEL = 0.08 mg/kg (SC on PND 8). NOAEL = NI (IP or SC). |
| Wang et al. 2000 |
SC injection of 0, 0.10, 0.17, 0.25, 0.33, 0.42, and 0.50 mg DA/kg on PND 7. Behavioral evaluations on all dose groups shortly following treatment. Electroencephalogram (EEG) studies and histopathology on 0.33 mg/kg group only. |
Dose‐dependent behavioral changes at all doses, starting 15 to 20 min post dosing. Histology of spinal cords from 0.33 mg/kg dose revealed pathological changes following DA exposure. Altered EEG findings for 0.33 dose group beginning between 10 and 20 min post DA treatment. |
LOAEL = 0.10 mg/kg (SC on PND 7). NOAEL = NI. |
| Xi, Peng, and Ramsdell 1997 |
IP injection of 0.02–0.70 mg DA/kg on PND 2, PND 5, or PND 10 (in several experiments). At 1 h post‐treatment, whole brain RNA extraction for c‐fos northern analysis. At 72 h post‐treatment, brain histochemistry. Behavioral observations on PND 5, following injection. |
LD50 for single dose: 0.25 mg DA/kg on PND 2 0.7 mg DA/kg on PND 10 Increased total brain c‐fos mRNA seen with 0.1 mg DA/kg on PND 5. Stereotypic behaviors observed with 0.1 mg DA/kg PND 5. Effects at 0.2 mg DA/kg onset more rapid and more severe. No effect at 0.02 mg DA/kg. |
LOAEL = 0.1 mg/kg (IP on PND 5). NOAEL = 0.02 mg/kg (IP on PND 5). |
TABLE 6.
Neonatal exposure; Mice.
| Reference | Methods | Outcomes at LOAEL | LOAEL/NOAEL |
|---|---|---|---|
| Sasaki et al. 2021 |
Gavage (oral) doses of 0 or 3.0 mg DA/kg at 2 or 10 weeks of age (PND 14 or PNW 10 (adult)). Behavioral testing at 12–13 weeks of age. |
No significant changes in performance on behavioral tests for animals exposed to DA on PND 14. Males exposed to DA as adults showed altered performance on parameters of light/dark transition and fear conditioning. Females in this group showed a significant effect on one parameter of the open field test. |
PND 14: LOAEL = NI. NOAEL = 3.0 mg/kg (oral). Adult: LOAEL = 3.0 mg/kg (oral). NOAEL = NI |
TABLE 7.
Wildlife; Marine mammals.
| Reference | Species and population | Outcomes observed |
|---|---|---|
| Krucik et al. 2023 |
California sea lion: case study of a stranded, malnourished 7‐month old California sea lion. Not able to be permanently released, the animal was raised and cared for in captivity. Exposure suspected to have occurred prenatally and/or pre‐weaning. |
Observations noted during the life of this animal were considered consistent with a hypothesis of early‐life environmental DA exposure associated with adult‐onset medial temporal lobe epilepsy. |
| Simeone et al. 2019 |
California sea lions with presumed exposure to DA were cared for at marine mammal centers. Study population of 171 animals (all ages) determined to be unsuitable for release. |
Neurological symptoms were significantly more likely among neonates than other age groups. The most common symptom was seizure. Of eight neonates dying during the study, six exhibited neurological disease. Five of these six were necropsied, with 3/5 showing central nervous system and/or hippocampal lesions; one of these three showed hippocampal‐focused encephalitis attributed specifically to DA toxicosis. |
| Goldstein et al. 2009 |
California sea lions on San Miguel Island, California. Biological samples were analyzed for DA content, as well as for other contaminants and infectious diseases, in a study population of 67 aborted and live‐born premature pups. Histopathology studies were conducted on various tissue samples (e.g., brain, placenta). |
DA‐producing algae were present in the environment and in sea lion feces. DA was detected in biological fluid samples from 15/79 of tested pups. Brain edema was observed in 6/9 prematurely born pups with detectable DA levels in the absence of other significant brain lesions. 2/5 placentas available for examination contained detectable levels of DA. Both had evidence of placental abruption. |
| Goldstein et al. 2008 |
Rescued California sea lions of all ages were studied while under care at The Marine Mammal Center in Sausalito, CA. In‐life symptoms of neurotoxicity were recorded and deceased animals were necropsied. DA exposure was not always documented by tissue analysis, but inferred from environmental conditions (i.e., Pseudo‐nitzschia bloom) at the time of rescue. |
19 immature animals (1 pup, 3 yearlings, and 15 juveniles or subadults) exhibiting chronic neurological symptoms died in captivity. Symptoms included seizures and severe behavioral abnormalities. Brain lesions noted among these animals included hemorrhage, edema, and mild meningoencephalitis in parahippocampal regions. |
3.1. Prenatal Exposure
3.1.1. Monkey
Two publications reported on a single study population of M. fascicularis that was exposed to DA during gestation, and followed from birth to 1–2 months of age (Burbacher et al. 2019; Grant et al. 2019). Maternal animals were given DA orally, with doses of 0, 0.075, or 0.15 mg/kg‐day throughout breeding and gestation. No adverse effects were noted for newborns, but testing at 1–2 months postnatal age revealed impairment of recognition memory in the 0.15 mg DA/kg bw‐day group (Burbacher et al. 2019; Grant et al. 2019). See Table 1 for more details of study methods and results.
3.1.2. Rodents
One study in rats (Levin et al. 2005) and five in mice (Dakshinamurti et al. 1993; Mills et al. 2016; Shiotani et al. 2017; Tanemura et al. 2009; Zuloaga et al. 2016) provided data on the DNT of DA following gestational exposure. See Tables 2 and 3 for brief summaries of study methods and outcomes.
For three rodent studies conducted by the subcutaneous (SC) route of exposure, the lowest LOAELs during gestation ranged between 0.6 to 1.5 mg/kg bw (Levin et al. 2005; Mills et al. 2016; Zuloaga et al. 2016). Reported outcomes included altered social behaviors and impaired maze performances.
A mouse gavage study administered DA daily on GD 10–17, at doses of 0, 1, or 3 mg DA/kg maternal bw‐day (Shiotani et al. 2017). Postnatal behavioral testing of prenatally‐exposed pups revealed both dose and sex influences on offspring performance. Significant changes were found in postweaning tests of motor coordination and indicators of anxiety behavior, with no identified NOAEL dose, while maternal animals showed no seizures or other effects at any tested dose.
A single IP injection of 1 mg DA/kg bw to pregnant mice was associated with behaviors indicating impaired learning and memory and increased anxiety in male offspring of 11 weeks postnatal age (Tanemura et al. 2009). The study also noted myelination failure and overgrowth of neuronal processes in limbic cortex neurons at necropsy of treated animals. In another study, a single IV dose of 0.6 mg DA/kg bw to pregnant dams altered postnatal electroencephalogram (EEG) results as well as histopathology and brain biochemistry (Dakshinamurti et al. 1993).
3.1.3. Zebrafish
Six studies used zebrafish as the test species for DA‐induced DNT (Panlilio, Aluru, and Hahn 2020; Panlilio et al. 2023, 2021; Tiedeken and Ramsdell 2007, 2009; Tiedeken, Ramsdell, and Ramsdell 2005). Routes of exposure included “bath” exposure, microinjection, and IV injection. LOAELs by route were: 0.09E‐6 mg DA/embryo by IV, 0.13E‐6 mg/kg DA by microinjection, and 0.25 mM DA in the bath solution for larvae. Effects reported included seizures, reduced startle response, altered gene expression, reduced presence of reticulospinal neurons and primarily motor neuron axon collaterals, and disrupted myelination. See Table 4 for more details of study methods and findings.
3.2. Neonatal/Postnatal Exposure
3.2.1. Rodents
Young pups were dosed with DA in 18 rodent studies, and later assessed using behavioral and/or physiological studies at hours to weeks post‐dosing. Only one study was conducted in mice, which was also the only study to use the gavage route (Sasaki et al. 2021). The other 17 studies used the rat model, with dosing by IP (Doucette et al. 2000; Xi, Peng, and Ramsdell 1997) and/or SC injection (Adams, Doucette, and Ryan 2008; Adams et al. 2009; Bernard et al. 2007; Burt, Ryan, and Doucette 2008a, 2008b; Doucette et al. 2004, 2000; Gill et al. 2009, 2012; Jandová et al. 2014; Levin et al. 2006; Marriott et al. 2016; Perry, Ryan, and Tasker 2009; Tasker et al. 2005; Thomsen et al. 2016; Wang et al. 2000). Twelve of the 18 studies used a 0.02 mg DA/kg bw‐day dose on each of postnatal days (PND) 8–14. Once established as reliably resulting in DNT, in the absence of general offspring or maternal toxicity, this dosing protocol was used for all but one study published from 2005 through 2016. Dose and timing procedures were selected to ensure detectable DNT.
Studies varied in the evaluations performed and in age(s) at testing. Significant adverse effects included alterations in perceptual processing, altered social behavior and spontaneous activity patterns, increased stress‐related behavior patterns and seizure activity, and altered maze performance with impaired responses to reversal challenges to previously learned mazes. Measurable effects were reported on open‐field behaviors as late as PND 150 following dosing with 0.02 mg DA/kg‐day on PND 8–14 (Burt, Ryan, and Doucette 2008b). Tables 5 and 6 provide more details of methods and outcomes for these studies, including histopathology and biochemical results.
3.2.2. Wild California Sea Lions ( Zalophus californianus )
DA toxicosis has been identified and studied in stranded marine mammals, most extensively in the California sea lion. Four studies of young sea lions ranged in type from a case study of a single individual to observations of larger populations of, or including, immature animals (Goldstein et al. 2008, 2009; Krucik et al. 2023; Simeone et al. 2019).
These wild animals were exposed to DA in utero and/or via their mother's milk due to maternal consumption of a natural diet including DA‐contaminated marine organisms. Older immature animals may also have been exposed by direct consumption of a DA‐contaminated diet. Exact dosing is unknown, but exposure was inferred from the presence of Pseudo‐nitzschia blooms of identified toxigenic size class and environmental monitoring in ocean waters near the sea lions' breeding and pupping locations (Smith et al. 2023). One study measured DA in bodily fluid samples from fetuses or pups (Goldstein et al. 2009). The others presumed DA‐exposure based on documented environmental sampling and field observations, along with pathological symptoms specifically characteristic of DA toxicosis (Krucik et al. 2023; Simeone et al. 2019) and/or from identification of DA in tissues collected from other animals rescued at the same time and location (Goldstein et al. 2008).
Neurological effects were so profound as to cause death or preclude release of surviving animals back to the wild after post‐stranding veterinary care. Effects included seizures (Goldstein et al. 2008; Simeone et al. 2019), adult‐onset medial temporal lobe epilepsy (Krucik et al. 2023), brain edema (Goldstein et al. 2008, 2009), and hippocampal lesions including one animal with hippocampal‐focused encephalitis (Simeone et al. 2019). See Table 7 for more information on study populations and observations.
4. Discussion
All the animal studies following gestational or larval exposure to DA (Tables 1, 2, 3, 4) reported DNT. While adverse effects were not detected at birth for prenatally‐exposed monkeys (Burbacher et al. 2019), the same cohort of exposed infants demonstrated DNT at 1–2 months postnatal age (Grant et al. 2019). All but one study (Sasaki et al. 2021) of DA exposure in early postnatal life (Tables 5, 6, 7) reported DNT effects of DA. While different studies evaluated various specific effects, all of the observed outcomes are consistent with consequences of the known action of DA as an agonist of glutamate kainate receptors. Precise comparisons among studies for NOAEL and LOAEL doses are complicated by species‐differences in DA TK parameters, which in turn influenced choices for experimental route of exposure. Most of the experimental animal studies were designed to investigate specific DNT outcomes, rather than to establish a dose–response relationship.
4.1. Gestational or Larval Experimental Exposures
The similarities between macaque monkeys and humans in TK models for oral DA exposure support experimental non‐human primates as a particularly relevant model for gestational exposure linked to DNT (Jing et al. 2018; Shum et al. 2020). Data from other laboratory and wild species provide considerable supporting evidence for DNT following developmental exposure to similar dose levels of DA.
Impairment of recognition memory was found in 1–2 month‐old M. fascicularis following repeated daily prenatal exposure to DA (Grant et al. 2019). The NOAEL from this study was 0.075 mg/kg bw‐day, which is 10 times lower than the adult NOAEL of 0.75 mg/kg bw used to establish the TDI by the State of Washington (Marien 1996).
The prevalence of parenteral routes of exposure in the studies of gestational exposure to DA in laboratory rats and mice may reflect the findings of reduced DA absorption from the rodent gut as compared to humans and other primates (Iverson and Truelove 1994). Only one of five rodent injection studies administered a range of DA doses to pregnant rats (Levin et al. 2005). The study reported a NOAEL dose for DA of 0.3 mg/kg on GD 13, with a LOAEL of 0.6 mg/kg. Other investigators chose a single dose, selected to induce DNT outcomes in the absence of other observed toxic effects in offspring or maternal animals, and given to mice on a single gestation day (Dakshinamurti et al. 1993; Mills et al. 2016; Tanemura et al. 2009; Zuloaga et al. 2016). Results were generally consistent across these studies, even including the one oral mouse study (Shiotani et al. 2017), with LOAEL doses ranging from 0.6–1.5 mg/kg bw. Observed effects of DA included altered maze performance, locomotor impairments, reduced social behaviors, altered functional connectivity patterns and sensorimotor gating, effects on learning and anxiety behaviors, reduced seizure thresholds, and altered histopathology including myelination failure.
All but one zebrafish study was conducted by injection, either into a fertilized egg or embryo (Tiedeken and Ramsdell 2007; Tiedeken, Ramsdell, and Ramsdell 2005) or into a larval vein (Panlilio, Aluru, and Hahn 2020; Panlilio et al. 2023, 2021). While TK data for DA in zebrafish are not available, observations of excitotoxicity in adult anchovies given DA by intracoelomic injection suggested that fish may differ from mammals in distribution of absorbed DA, rather than in resistance to DA neurotoxicity (Lefebvre 2001). Zebrafish demonstrated similar symptoms and sensitivity to DA as other species studied (Panlilio, Aluru, and Hahn 2020; Tiedeken, Ramsdell, and Ramsdell 2005). (Panlilio, Aluru, and Hahn 2020) expressly chose test doses “similar to those causing behavioral effects in developing [neonatal] rodents,” while also below levels “associated with acute toxicity in adult humans.”
4.2. Postnatal Experimental Exposures
Sasaki and colleagues administered a single oral dose of DA (3.0 mg/kg) to mice either on PND 14 or at 10 weeks postnatal age (adult), and conducted behavioral testing at 12–13 weeks postnatal age (Sasaki et al. 2021). In seeming contradiction to the generally greater sensitivity of immature individuals, effects on test performance were noted only in animals treated as adults. Treatment on PND 14, however, may have missed the critical window for irreversible developmental DA effects, while at the same time leaving 10 weeks for possible repair of transitory effects before testing at 12 weeks. The adult animals treated at 10 weeks had only two weeks recovery time before testing.
All of the early life experimental studies performed in rats used an injection method of DA exposure, with most giving repeated doses of 0 or 0.02 mg/kg‐day on each of PNDs 8–14 (Adams, Doucette, and Ryan 2008; Adams et al. 2009; Bernard et al. 2007; Burt, Ryan, and Doucette 2008a, 2008b; Doucette et al. 2004; Gill et al. 2009, 2012; Marriott et al. 2016; Perry, Ryan, and Tasker 2009; Tasker et al. 2005; Thomsen et al. 2016). The lowest identified adverse effect dose for postnatal rat studies found seizure‐like activity and altered hippocampal histopathology in adult animals following neonatal exposure to 0.005 mg/kg‐day on PND 8–14 (Doucette et al. 2004).
4.3. Wild Sea Lions, Pre‐ and Post‐Gestational Environmental Exposures
Wild California sea lions are large placental mammals, whose natural diet contains many of the same seafood species humans also enjoy eating. The Channel Islands off the coast of Santa Barbara, California, and surrounding waters are a primary breeding and pupping ground for these animals. Recurring Pseudo‐nitzschia blooms have affected the area in recent decades, causing serious harm to sea lions as well as other wild species (Brodie et al. 2006; Goldstein et al. 2008).
A survey of pregnant female sea lions stranded during California's Pseudo‐nitzschia blooms of 1998 and 2002 found 209 cases of reproductive failure attributed to DA exposure (Brodie et al. 2006). Types of reproductive failure included spontaneous abortion, premature live birth, fetal death, and maternal death prior to parturition. Another study specifically assessed DA contents in fetuses and fetal membranes from stranded pregnant females (Lefebvre et al. 2018). Although these studies did not assess DNT, they do provide context for evidence from surviving pups. In particular, the analysis of fetal body fluids demonstrated detectable levels of DA up to 8 days following maternal rescue (i.e., post cessation of environmental exposure).
Sea lion pups believed to have been exposed to DA, prenatally and/or neonatally, exhibited clinical symptoms and post‐mortem brain pathology consistent with DA toxicosis (Goldstein et al. 2008, 2009; Krucik et al. 2023; Simeone et al. 2019). Beyond evidence that DA exposure occurred or was likely, quantitative exposure assessment for environmentally‐exposed sea lions is not possible. Thus, direct comparisons cannot be made to adverse effect doses of DA identified for humans or experimental animal species. Additional complicating factors for assessment of sea lion data are the frequency of concurrent infections (e.g., bacterial, protozoal, viral), and contamination of the same habitat with chemicals such as DDTs and PCBs (Goldstein et al. 2009).
4.4. Life Course Considerations for Sensitivity to DA
The importance of a specific developmental stage to outcomes resulting from toxic exposures is a foundational principle of developmental toxicology (NRC 2000). The evidence summarized in this paper (Tables 1, 2, 3, 4, 5, 6, 7) supports a peak of susceptibility to DA during prenatal and/or early postnatal development, when developing vertebrate nervous tissues are most vulnerable.
Gestation and early infancy are not the only life stages with enhanced sensitivity to the neurological effects of DA. Exposures to DA late in life are also of elevated concern (Hendrix et al. 2023). An association between increased severity of effects and advanced age was noted in the initial sample of acutely poisoned adult humans (Perl, Bedard, Kosatsky, Hockin, Todd, Remis, et al. 1990; Wekell, Hurst, and Lefebvre 2004). All three patients who died in the hospital were over the age of 70, while only four of 19 hospitalized patients were under the age of 65. The younger hospitalized patients all had preexisting medical conditions, notably compromised kidney function. Experiments in mice compared sex and age for influence on sensitivity to effects of DA (Hendrix et al. 2023). Aged mice were found to be more susceptible to acute DA neurotoxicity than young adult mice, and females more sensitive than males. The older animals also accumulated higher concentrations of DA in serum and tissues compared to younger animals.
Concern has also been expressed for consequences of repeated, chronic exposures of healthy adult human populations to DA at levels below regulatory limits. A minimal, but detectable, memory decline was associated with repetitive sub‐acute, dietary exposures to DA at levels below established allowable concentrations in the seafood consumed (Grattan et al. 2021; Stuchal et al. 2020). These effects were observed in a cohort of 500 adult Native American tribal members residing in coastal areas of the Pacific Northwest, who were studied over a period of 8 years, and who otherwise exhibited stable cognition.
5. Conclusions
For any toxin, adverse effect dose levels can vary with exposure patterns, the sensitivity of target tissues, as well as with TK factors such as rates of absorption and elimination. Apart from genetic variation within and between populations, life course considerations are critical for determining the sensitive windows for exposure to toxins (Halfon et al. 2018; Schaffer, Smith, and Faustman 2017).
Internationally, government agencies as well as individual researchers have opined that current regulatory standards for DA in seafood, which have not been revisited since initial implementation, may be insufficient to protect exposed individuals at all life stages and/or patterns of consumption (COT 2001; EFSA 2009; Grattan et al. 2021; Wekell, Hurst, and Lefebvre 2004).
Current US federal action levels for DA are based on a LOAEL of 1.0 mg DA/kg bw, adjusted by a 10‐fold UF for within‐species variation to a tolerable dose of 0.1 mg DA/ kg bw (DHHS 1993). The European Union adopted the Health Canada and US FDA regulatory limit of 20 ppm for DA in bivalve shellfish in 1997 (COT 2001; EU. 1997). The United Kingdom Food Standard Agency Committee on Toxicity (COT) (COT 2001), and the European Food Safety Authority Panel on Contaminants in the Food Chain (EFSA) (EFSA 2009), reviewed the regulatory limit for DA. Both groups considered an UF of 10 to be inadequate. The COT concluded that, while the action level “may protect against major outbreaks,” it provides a “pragmatic guideline rather than a toxicologically based limit.” EFSA noted that sensitive indicators of adverse neurological effects were not assessed in affected people during the 1987 ASP outbreak. EFSA recommended lowering the action limit to 4.5 ppm in shellfish meat, based on a 60 kg body weight, a 400 g meal size, and an additional UF of three to extrapolate from a LOAEL.
In addition, or alternatively, to changing regulatory action levels, public health outreach to communities and groups at risk of DA‐toxicity has been recommended (Grattan et al. 2021). Clinicians caring for patients sickened after consumption of seafood, particularly shellfish, could benefit from raised awareness of DA toxicity. Initial symptoms of general acute gastrointestinal illness combined with the rapid clearance of DA from the body may complicate accurate diagnosis (CPCS 2016). An example of relevant outreach is provided by the Washington State Department of Health, which issued an interim health advisory for regular consumers of razor clams containing allowable levels of DA recommending that consumers “…eat no more than 15 razor clams each month for 12 consecutive months….” (WADOH 2023). While the interim advisory is intended for everyone, sensitive subpopulations are specified: “…especially women who are or might become pregnant, nursing mothers, children, the elderly, and people with compromised renal function.” Similar public health messaging on all potentially impacted types of seafood could be made more widely available through relevant agencies in affected states, and through educational materials for health professionals serving sensitive populations.
Conflicts of Interest
The authors declare no conflicts of interest.
Permission to Reproduce Material From Other Sources
AOP 48 (2023), from which Figure 2 is modified, is licensed under the Creative Commons BY‐SA license. “This license allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. If you remix, adapt, or build upon the material, you must license the modified material under identical terms.”
Acknowledgments
The authors would like to thank our OEHHA colleagues who provided critical commentary on drafts of this manuscript: Wesley Smith, Francisco Moran, and Martha Sandy. OEHHA recognizes the presenters, facilitators, and participants of the 2017 OEHHA‐University of California State of the Science Workshop, and the 2019 Domoic Acid Webinar. Their contributions advanced the scientific discourse on domoic acid toxicity and informed the framework for this manuscript. The authors are especially grateful for the technical guidance and cooperation of John S. Ramsdell, Chief of the Harmful Algal Bloom Monitoring and Reference Branch of the National Centers for Coastal Ocean Science of the National Oceanic and Atmospheric Administration (NCCOS‐NOAA). His valuable contributions with interpreting the zebrafish studies were greatly appreciated. Finally, the authors would like to express our gratitude to Vincent Cogliano, former OEHHA Deputy Director of Scientific Programs, whose support and enthusiasm were instrumental in helping this project get underway.
Contributor Information
Marlissa A. Campbell, Email: marlissa.campbell@gmail.com.
Shannon R. Murphy, Email: shannon.murphy@oehha.ca.gov.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- Adams, A. , Doucette T. A., and Ryan C. L.. 2008. “Altered Pre‐Pulse Inhibition in Adult Rats Treated Neonatally With Domoic Acid.” Amino Acids 35: 157–160. [DOI] [PubMed] [Google Scholar]
- Adams, A. L. , Doucette T. A., James R., and Ryan C. L.. 2009. “Persistent Changes in Learning and Memory in Rats Following Neonatal Treatment With Domoic Acid.” Physiology & Behavior 96, no. 4–5: 505–512. 10.1016/j.physbeh.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Anderson, D. M. , Fensin E., Gobler C. J., et al. 2021. “Marine Harmful Algal Blooms (HABs) in the United States: History, Current Status and Future Trends.” Harmful Algae 102: 101975. 10.1016/j.hal.2021.101975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOP‐48 . 2023. “Binding of Agonists to Ionotropic Glutamate Receptors in Adult Brain Causes Excitotoxicity that Mediates Neuronal Cell Death, Contributing to Learning and Memory Impairment.” (AOP: 48). https://aopwiki.org/aops/48.
- Baj, A. , Moro E., Bistoletti M., Orlandi V., Crema F., and Giaroni C.. 2019. “Glutamatergic Signaling Along the Microbiota‐Gut‐Brain Axis.” International Journal of Molecular Sciences 20, no. 6: 1482. https://www.mdpi.com/1422‐0067/20/6/1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, S. , Hubbard K. A., Lundholm N., Montresor M., and Pin Leaw C.. 2018. “Pseudo‐Nitzschia, Nitzschia, and Domoic Acid: New Research Since 2011.” Harmful Algae 79: 3–43. 10.1016/j.hal.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Bernard, P. B. , Macdonald D. S., Gill D. A., Ryan C. L., and Tasker R. A.. 2007. “Hippocampal Mossy Fiber Sprouting and Elevated trkB Receptor Expression Following Systemic Administration of Low Dose Domoic Acid During Neonatal Development.” Hippocampus 17, no. 11: 1121–1133. 10.1002/hipo.20342. [DOI] [PubMed] [Google Scholar]
- Bernstein, S. , Ruiz‐Cooley R. I., Kudela R., Anderson C. R., Dunkin R., and Field J. C.. 2021. “Stable Isotope Analysis Reveals Differences in Domoic Acid Accumulation and Feeding Strategies of Key Vectors in a California Hotspot for Outbreaks.” Harmful Algae 110: 102117. 10.1016/j.hal.2021.102117. [DOI] [PubMed] [Google Scholar]
- Bowen, L. , Knowles S., Lefebvre K., et al. 2022. “Divergent Gene Expression Profiles in Alaskan Sea Otters: An Indicator of Chronic Domoic Acid Exposure?” Oceans 3, no. 3: 401–418. https://www.mdpi.com/2673‐1924/3/3/27. [Google Scholar]
- Brodie, E. C. , Gulland F. M., Greig D. J., et al. 2006. “Domoic Acid Causes Reproductive Failure in California Sea Lions ( Zalophus californianus ).” Marine Mammal Science 22, no. 3: 700–707. [Google Scholar]
- Burbacher, T. M. , Grant K. S., Petroff R., et al. 2019. “Effects of Oral Domoic Acid Exposure on Maternal Reproduction and Infant Birth Characteristics in a Preclinical Nonhuman Primate Model.” Neurotoxicology and Teratology 72: 10–21. 10.1016/j.ntt.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt, M. A. , Ryan C. L., and Doucette T. A.. 2008a. “Altered Responses to Novelty and Drug Reinforcement in Adult Rats Treated Neonatally With Domoic Acid.” Physiology & Behavior 93, no. 1–2: 327–336. 10.1016/j.physbeh.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Burt, M. A. , Ryan C. L., and Doucette T. A.. 2008b. “Low Dose Domoic Acid in Neonatal Rats Abolishes Nicotine Induced Conditioned Place Preference During Late Adolescence.” Amino Acids 35, no. 1: 247–249. 10.1007/s00726-007-0584-2. [DOI] [PubMed] [Google Scholar]
- California Fish and Game Code . 2017. “California Fish and Game Code—FGC section 5523 et seq.” https://leginfo.legislature.ca.gov/.
- CDPH . 2017. “Domoic Acid Frequently Asked Questions.” https://www.cdph.ca.gov/Programs/CEH/DRSEM/Pages/EMB/Shellfish/Domoic‐Acid.aspx.
- Clancy, B. , Darlington R. B., and Finlay B. L.. 2001. “Translating Developmental Time Across Mammalian Species.” Neuroscience 105, no. 1: 7–17. https://www.sciencedirect.com/science/article/abs/pii/S0306452201001713. [DOI] [PubMed] [Google Scholar]
- Clancy, B. , Finlay B. L., Darlington R. B., and Anand K. J. S.. 2008. “Extrapolating Brain Development From Experimental Species to Humans.” Neurotoxicology 28, no. 5: 1–15. https://pmc.ncbi.nlm.nih.gov/articles/PMC2077812/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, L. G. , Giordano G., and Faustman E. M.. 2010. “Domoic Acid as a Developmental Neurotoxin.” Neurotoxicology 31, no. 5: 409–423. 10.1016/j.neuro.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, L. G. , Guizzetti M., and Vitalone A.. 2004. “Diet‐Brain Connections: Role of Neurotoxicants.” Environmental Toxicology and Pharmacology 19, no. 3: 395–400. 10.1016/j.etap.2004.12.001. [DOI] [PubMed] [Google Scholar]
- COT . 2001. “(Committee on Toxicity of Chemicals in Food, Consumer Products, and the Environment) Statement on Amnesic Shellfish Poisoning.” https://cot.food.gov.uk/sites/default/files/cot/cot‐asp.pdf.
- CPCS . 2016. “Amnesic Shellfish Poisoning.” California Poison Control System. https://calpoison.org/content/amnesic‐shellfish‐poisoning.
- Dakshinamurti, K. , Sharma S. K., Sundaram M., and Watanabe T.. 1993. “Hippocampal Changes in Developing Postnatal Mice Following Intrauterine Exposure to Domoic Acid.” Journal of Neuroscience 13, no. 10: 4486–4495. 10.1523/jneurosci.13-10-04486.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS . 1993. “Memorandum—Marine Biotoxins in Dungness Crab. Memorandum—Marine Biotoxins in Dungness Crab.” http://www.oceansciencetrust.org/wp‐content/uploads/2016/07/DA_Crabs_93.pdf.
- Doucette, T. A. , Bernard P. B., Husum H., Perry M. A., Ryan C. L., and Tasker R. A.. 2004. “Low Doses of Domoic Acid During Postnatal Development Produce Permanent Changes in Rat Behaviour and Hippocampal Morphology.” Neurotoxicity Research 6, no. 7–8: 555–563. 10.1007/bf03033451. [DOI] [PubMed] [Google Scholar]
- Doucette, T. A. , Strain S. M., Allen G. V., Ryan C. L., and Tasker R. A.. 2000. “Comparative Behavioural Toxicity of Domoic Acid and Kainic Acid in Neonatal Rats.” Neurotoxicology and Teratology 22, no. 6: 863–869. 10.1016/s0892-0362(00)00110-0. [DOI] [PubMed] [Google Scholar]
- Doucette, T. A. , and Tasker R. A.. 2016. “Perinatal Domoic Acid as a Neuroteratogen.” Current Topics in Behavioral Neurosciences 29: 87–110. 10.1007/7854_2015_417. [DOI] [PubMed] [Google Scholar]
- EFSA . 2009. “Scientific Opinion of the Panel on Contaminants in the Food Chain on a Request From the European Commission on Marine Biotoxins in Shellfish—Domoic Acid.” EFSA Journal 1181: 1–61. [Google Scholar]
- EU . 1997. “Council Directive 97/61/EC of 20 October 1997 Amending the Annex to Directive 91/492/EEC Laying Down the Health Conditions for the Production and Placing on the Market of Live Bivalve Molluscs.” Official Journal of the European Communities. L 295/35.
- Fritz, L. , Quilliam M. A., Wright J. L. C., Beale A. M., and Work T. M.. 1992. “An Outbreak of Domoic Acid Poisoning Attributed to the Pennate Diatom Pseudonitzschia australis .” Journal of Phycology 28: 439–442. [Google Scholar]
- Gibble, C. M. , Kudela R. M., Knowles S., Bodenstein B., and Lefebvre K. A.. 2021. “Domoic Acid and Saxitoxin in Seabirds in the United States Between 2007 and 2018.” Harmful Algae 103: 101981. 10.1016/j.hal.2021.101981. [DOI] [PubMed] [Google Scholar]
- Gill, D. A. , Bastlund J. F., Anderson N. J., and Tasker R. A.. 2009. “Reductions in Paradoxical Sleep Time in Adult Rats Treated Neonatally With Low Dose Domoic Acid.” Behavioural Brain Research 205, no. 2: 564–567. 10.1016/j.bbr.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Gill, D. A. , Perry M. A., McGuire E. P., Pérez‐Gómez A., and Tasker R. A.. 2012. “Low‐Dose Neonatal Domoic Acid Causes Persistent Changes in Behavioural and Molecular Indicators of Stress Response in Rats.” Behavioural Brain Research 230, no. 2: 409–417. 10.1016/j.bbr.2012.02.036. [DOI] [PubMed] [Google Scholar]
- Goldstein, T. , Mazet J. A. K., Zabka T. S., et al. 2008. “Novel Symptomatology and Changing Epidemiology of Domoic Acid Toxicosis in California Sea Lions ( Zalophus californianus ): An Increasing Risk to Marine Mammal Health.” Proceedings of the Royal Society B 275: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, T. , Zabka T. S., Delong R. L., et al. 2009. “The Role of Domoic Acid in Abortion and Premature Parturition of California Sea Lions ( Zalophus californianus ) on San Miguel Island.” California. Journal of Wildlife Diseases 45, no. 1: 91–108. 10.7589/0090-3558-45.1.91. [DOI] [PubMed] [Google Scholar]
- Grant, K. S. , Burbacher T. M., Faustman E. M., and Grattan L.. 2010. “Domoic Acid: Neurobehavioral Consequences of Exposure to a Prevalent Marine Biotoxin.” Neurotoxicology and Teratology 32, no. 2: 132–141. 10.1016/j.ntt.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, K. S. , Crouthamel B., Kenney C., et al. 2019. “Preclinical Modeling of Exposure to a Global Marine Bio‐Contaminant: Effects of In Utero Domoic Acid Exposure on Neonatal Behavior and Infant Memory.” Neurotoxicology and Teratology 73: 1–8. 10.1016/j.ntt.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan, L. M. 2022. “Invited Perspective: The Relevance of Animal Models of Domoic Acid Neurotoxicity to Human Health.” Environmental Health Perspectives 130, no. 9: 091302. 10.1289/EHP11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan, L. M. , Kaddis L., Tracy J. K., and Morris J. G.. 2021. “Long Term Memory Outcome of Repetitive, Low‐Level Dietary Exposure to Domoic Acid in Native Americans.” International Journal of Environmental Research and Public Health 18, no. 8: 3955. https://www.mdpi.com/1660‐4601/18/8/3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon, N. , Forrest C. B., Lerner R. M., and Faustman E. M.. 2018. Handbook of Life Course Health Development, edited by Halfon C. B. F. N., Lerner R. M., and Faustman E. M., 1st ed. Cham, Switzerland: Springer Cham. 10.1007/978-3-319-47143-3. [DOI] [Google Scholar]
- Hendrix, A. M. , Lefebvre K. A., Bowers E. K., Stuppard R., Burbacher T., and Marcinek D. J.. 2023. “Age and Sex as Determinants of Acute Domoic Acid Toxicity in a Mouse Model.” Toxins (Basel) 15, no. 4: 259. https://www.mdpi.com/2072‐6651/15/4/259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson, F. , and Truelove J.. 1994. “Toxicology and Seafood Toxins: Domoic Acid.” Natural Toxins 2: 334–339. 10.1002/nt.2620020514. [DOI] [PubMed] [Google Scholar]
- Jandová, K. , Kozler P., Langmeier M., Marešová D., Pokorný J., and Riljak V.. 2014. “Influence of Low‐Dose Neonatal Domoic Acid on the Spontaneous Behavior of Rats in Early Adulthood.” Physiological Research 63, no. Suppl 4: S521–S528. [DOI] [PubMed] [Google Scholar]
- Jing, J. , Petroff R., Shum S., et al. 2018. “Toxicokinetics and Physiologically Based Pharmacokinetic Modeling of the Shellfish Toxin Domoic Acid in Nonhuman Primates.” Drug Metabolism and Disposition 46, no. 2: 155–165. 10.1124/dmd.117.078485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuder, C. M. , Miller M. A., Lowenstine L. J., et al. 2005. “Evaluations of Cardiac Lesions and Risk Factors Associated With Myocarditis and Dilated Cardiomyopathy in Southern Sea Otters ( Enhydra lutris nereis ).” American Journal of Veterinary Research 66, no. 2: 289–299. [DOI] [PubMed] [Google Scholar]
- Krucik, D. D. R. , Cook P., Cathey M., et al. 2023. “Adult‐Onset Epilepsy and Hippocampal Pathology in a California Sea Lion ( Zalophus californianus ): A Case Study of Suspected In Utero Exposure to Domoic Acid.” Neurotoxicology 96: 13–18. 10.1016/j.neuro.2023.02.010. [DOI] [PubMed] [Google Scholar]
- Lanphear, B. P. 2015. “The Impact of Toxins on the Developing Brain.” Annual Review of Public Health 36: 211–230. [DOI] [PubMed] [Google Scholar]
- Larm, J. A. , Beart P. M., and Cheung N. S.. 1997. “Neurotoxin Domoic Acid Produces Cytotoxicity via Kainate‐ and AMPA‐Sensitive Receptors in Cultured Cortical Neurones.” Neurochemistry International 31, no. 5: 677–682. 10.1016/s0197-0186(97)00030-2. [DOI] [PubMed] [Google Scholar]
- Lefebvre, K. A. , Frame E. R., and Kendrick P. S.. 2012. “Domoic Acid and Fish Behavior: A Review.” Harmful Algae 13: 126–130. 10.1016/j.hal.2011.09.011. [DOI] [Google Scholar]
- Lefebvre, K. A. , Hendrix A., Halaska B., et al. 2018. “Domoic Acid in California Sea Lion Fetal Fluids Indicates Continuous Exposure to a Neuroteratogen Poses Risks to Mammals.” Harmful Algae 79: 53–57. 10.1016/j.hal.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre, K. A. , Noren D. P., Schultz I. R., Bogard S. M., Wilson J., and Eberhart B.‐T. L.. 2007. “Uptake, Tissue Distribution and Excretion of Domoic Acid After Oral Exposure in Coho Salmon ( Oncorhynchus kisutch ).” Aquatic Toxicology 81, no. 3: 266–274. 10.1016/j.aquatox.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Lefebvre, K. A. D. , Dovel S. L., and Silver M. W.. 2001. “Tissue Distribution and Neurotoxic Effects of Domoic Acid in a Prominent Vector Species, the Northern Anchove Engraulis mordax .” Marine Biology 138: 693–700. 10.1007/s002270000509. [DOI] [Google Scholar]
- Levin, E. D. , Pang W. G., Harrison J., Williams P., Petro A., and Ramsdell J. S.. 2006. “Persistent Neurobehavioral Effects of Early Postnatal Domoic Acid Exposure in Rats.” Neurotoxicology and Teratology 28, no. 6: 673–680. 10.1016/j.ntt.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Levin, E. D. , Pizarro K., Pang W. G., Harrison J., and Ramsdell J. S.. 2005. “Persisting Behavioral Consequences of Prenatal Domoic Acid Exposure in Rats.” Neurotoxicology and Teratology 27, no. 5: 719–725. 10.1016/j.ntt.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Marien, K. 1996. “Establishing Tolerable Dungeness Crab ( Cancer magister ) and Razor Clam ( Siliqua patula ) Domoic Acid Contaminant Levels.” Environmental Health Perspectives 104, no. 11: 1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott, A. L. , Tasker R. A., Ryan C. L., and Doucette T. A.. 2016. “Alterations to Prepulse Inhibition Magnitude and Latency in Adult Rats Following Neonatal Treatment With Domoic Acid and Social Isolation Rearing.” Behavioural Brain Research 298: 310–317. 10.1016/j.bbr.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Maucher Fuquay, J. , Muha N., Wang Z., and Ramsdell J. S.. 2012a. “Elimination Kinetics of Domoic Acid From the Brain and Cerebrospinal Fluid of the Pregnant Rat.” Chemical Research in Toxicology 25, no. 12: 2805–2809. 10.1021/tx300434s. [DOI] [PubMed] [Google Scholar]
- Maucher Fuquay, J. , Muha N., Wang Z., and Ramsdell J. S.. 2012b. “Toxicokinetics of Domoic Acid in the Fetal Rat.” Toxicology 294, no. 1: 36–41. 10.1016/j.tox.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Maucher, J. M. , and Ramsdell J. S.. 2005. “Domoic Acid Transfer to Milk: Evaluation of a Potential Route of Neonatal Exposure.” Environmental Health Perspectives 113, no. 4: 461–464. 10.1289/ehp.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, B. D. , Pearce H. L., Khan O., Jarrett B. R., Fair D. A., and Lahvis G. P.. 2016. “Prenatal Domoic Acid Exposure Disrupts Mouse Pro‐Social Behavior and Functional Connectivity MRI.” Behavioural Brain Research 308: 14–23. 10.1016/j.bbr.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty, M. E. , Tinker M. T., Miller M. A., et al. 2021. “Exposure to Domoic Acid Is an Ecological Driver of Cardiac Disease in Southern Sea Otters✰.” Harmful Algae 101: 101973. 10.1016/j.hal.2020.101973. [DOI] [PubMed] [Google Scholar]
- NRC . 2000. Scientific Frontiers in Developmental Toxicology and Risk Assessment, edited by N. R. C. C. o. D. Toxicology . Washington, DC: National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK225674/. [PubMed] [Google Scholar]
- OEHHA . 2017. “Domoic Acid Workshop: Evaluating the State of the Science and Implications for Human Toxicity.” https://oehha.ca.gov/fish/events/domoic‐acid‐workshop.
- OEHHA . 2018. “Frequently Asked Questions about Domoic Acid in Seafood.” https://oehha.ca.gov/fish/fact‐sheet/frequently‐asked‐questions‐about‐domoic‐acid‐seafood.
- OEHHA . 2019a. “Domoic Acid (a Marine Biotoxin) in Fish and Shellfish.” https://oehha.ca.gov/fish/general‐info/domoic‐acid‐marine‐biotoxin‐fish‐and‐shellfish.
- OEHHA . 2019b. “Domoic Acid Webinar: Research on Effects of Repeat Low‐Level Exposures and its Implications for Human Toxicity.” https://oehha.ca.gov/fish/events/domoic‐acid‐webinar‐research‐effects‐repeat‐low‐level‐exposures‐and‐its‐implications.
- OEHHA . 2020. “Prioritization: Chemicals Identified for Consultation with the Developmental and Reproductive Toxicant Identification Committee.” https://www.google.com/url?client=internal‐element‐cse&cx=001779225245372747843:v3sx‐oyt7xc&q=https://oehha.ca.gov/media/downloads/crnr/dartprioritization100120.pdf&sa=U&ved=2ahUKEwiL5tv7j‐z_AhWOhu4BHWPpB2YQFnoECAQQAg&usg=AOvVaw1Thlijw0lbAK4uFt48HsAq.
- OEHHA . 2022a. “Marine Harmful Algal Blooms.” https://oehha.ca.gov/media/epic/downloads/04marinehabs.pdf.
- OEHHA . 2022b. “Title 17, California Code of Regulations (CCR) §2500, §2593, §2641.5‐2643.20, and §2800‐2812 Reportable Diseases and Conditions.” https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/ReportableDiseases.pdf.
- OEHHA . 2024. “Marine Harmful Algal Bloom (HAB)‐Related Illness Tracking.” https://oehha.ca.gov/fish/general‐info/marine‐harmful‐algal‐bloom‐hab‐related‐illness‐tracking.
- Panlilio, J. M. , Aluru N., and Hahn M. E.. 2020. “Developmental Neurotoxicity of the Harmful Algal Bloom Toxin Domoic Acid: Cellular and Molecular Mechanisms Underlying Altered Behavior in the Zebrafish Model.” Environmental Health Perspectives 128, no. 11: 117002. 10.1289/ehp6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio, J. M. , Hammar K. M., Aluru N., and Hahn M. E.. 2023. “Developmental Exposure to Domoic Acid Targets Reticulospinal Neurons and Leads to Aberrant Myelination in the Spinal Cord.” Scientific Reports 13, no. 1: 2587. 10.1038/s41598-023-28166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio, J. M. , Jones I. T., Salanga M. C., Aluru N., and Hahn M. E.. 2021. “Developmental Exposure to Domoic Acid Disrupts Startle Response Behavior and Circuitry in Zebrafish.” Toxicological Sciences 182, no. 2: 310–326. 10.1093/toxsci/kfab066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl, T. M. , Bédard L., Kosatsky T., et al. 1990. “Amnesic Shellfish Poisoning: A New Clinical Syndrome Due to Domoic Acid.” Canada Diseases Weekly Report 16, no. Suppl 1E: 7–8. [PubMed] [Google Scholar]
- Perl, T. M. , Bédard L., Kosatsky T., Hockin J. C., Todd E. C., and Remis R. S.. 1990. “An Outbreak of Toxic Encephalopathy Caused by Eating Mussels Contaminated With Domoic Acid.” New England Journal of Medicine 322, no. 25: 1775–1780. [DOI] [PubMed] [Google Scholar]
- Perl, T. M. , Teitelbaum J., Hockin J., and Todd E. C.. 1990. “Domoic Acid Toxicity. Panel Discussion: Definition of the Syndrome.” Canada Diseases Weekly Report 16 Suppl 1E: 41–45. [PubMed] [Google Scholar]
- Perry, M. A. , Ryan C. L., and Tasker R. A.. 2009. “Effects of Low Dose Neonatal Domoic Acid Administration on Behavioural and Physiological Response to Mild Stress in Adult Rats.” Physiology & Behavior 98, no. 1–2: 53–59. 10.1016/j.physbeh.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Petroff, R. , Hendrix A., Shum S., Grant K. S., Lefebvre K. A., and Burbacher T. M.. 2021. “Public Health Risks Associated With Chronic, Low‐Level Domoic Acid Exposure: A Review of the Evidence.” Pharmacology & Therapeutics 227: 107865. 10.1016/j.pharmthera.2021.107865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier, P. M. 1995. “Developing Brain as a Target of Toxicity.” Environmental Health Perspectives 103: 73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, T. , Saito H., Hiradate Y., Hara K., and Tanemura K.. 2021. “Behavioural Effects in Mice Orally Exposed to Domoic Acid or Ibotenic Acid Are Influenced by Developmental Stages and Sex Differences.” Biochemical and Biophysical Research Communications 558: 175–182. 10.1016/j.bbrc.2021.04.080. [DOI] [PubMed] [Google Scholar]
- Schaffer, R. M. , Smith M. N., and Faustman E. M.. 2017. “Developing the Regulatory Utility of the Exposome: Mapping Exposures for Risk Assessment Through Lifestage Exposome Snapshots (LEnS).” Environmental Health Perspectives 125, no. 8: 085003. 10.1289/EHP1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotani, M. , Cole T. B., Hong S., et al. 2017. “Neurobehavioral Assessment of Mice Following Repeated Oral Exposures to Domoic Acid During Prenatal Development.” Neurotoxicology and Teratology 64: 8–19. 10.1016/j.ntt.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Shum, S. , Jing J., Petroff R., et al. 2020. “Maternal‐Fetal Disposition of Domoic Acid Following Repeated Oral Dosing During Pregnancy in Nonhuman Primate.” Toxicology and Applied Pharmacology 398: 115027. 10.1016/j.taap.2020.115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone, C. , Fauquier D., Skidmore J., et al. 2019. “Clinical Signs and Mortality of Non‐Released Stranded California Sea Lions Housed in Display Facilities: The Suspected Role of Prior Exposure to Algal Toxins.” Veterinary Record 185, no. 10: 304. 10.1136/vr.105371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. , Cram J. A., Berndt M. P., Hoard V., Shultz D., and Deming A. C.. 2023. “Quantifying the Linkages Between California Sea Lion ( Zalophus californianus ) Strandings and Particulate Domoic Acid Concentrations at Piers Across Southern California.” Frontiers in Marine Science 10: 1278293. 10.3389/fmars.2023.1278293. [DOI] [Google Scholar]
- Stuchal, L. D. , Grattan L. M., Portier K. M., et al. 2020. “Dose‐Response Assessment for Impaired Memory From Chronic Exposure to Domoic Acid Among Native American Consumers of Razor Clams.” Regulatory Toxicology and Pharmacology 117: 104759. 10.1016/j.yrtph.2020.104759. [DOI] [PubMed] [Google Scholar]
- Suzuki, C. A. , and Hierlihy S. L.. 1993. “Renal Clearance of Domoic Acid in the Rat.” Food and Chemical Toxicology 31, no. 10: 701–706. [DOI] [PubMed] [Google Scholar]
- Tanemura, K. , Igarashi K., Matsugami T. R., Aisaki K., Kitajima S., and Kanno J.. 2009. “Intrauterine Environment‐Genome Interaction and children's Development (2): Brain Structure Impairment and Behavioral Disturbance Induced in Male Mice Offspring by a Single Intraperitoneal Administration of Domoic Acid (DA) to Their Dams.” Journal of Toxicological Sciences 34: Sp279–Sp286. 10.2131/jts.34.sp279. [DOI] [PubMed] [Google Scholar]
- Tasker, R. A. , Perry M. A., Doucette T. A., and Ryan C. L.. 2005. “NMDA Receptor Involvement in the Effects of Low Dose Domoic Acid in Neonatal Rats.” Amino Acids 28, no. 2: 193–196. 10.1007/s00726-005-0167-z. [DOI] [PubMed] [Google Scholar]
- Teitelbaum, J. S. , Zatorre R. J., Carpenter S., et al. 1990. “Neurologic Sequelae of Domoic Acid Intoxication Due to the Ingestion of Contaminated Mussels.” New England Journal of Medicine 322, no. 25: 1781–1787. 10.1056/NEJM199006213222505. [DOI] [PubMed] [Google Scholar]
- Thomsen, M. B. , Lillethorup T. P., Jakobsen S., et al. 2016. “Neonatal Domoic Acid Alters In Vivo Binding of [(11)C]Yohimbine to α(2)‐adrenoceptors in Adult Rat Brain.” Psychopharmacology 233, no. 21–22: 3779–3785. 10.1007/s00213-016-4416-5. [DOI] [PubMed] [Google Scholar]
- Tiedeken, J. A. , and Ramsdell J. S.. 2007. “Embryonic Exposure to Domoic Acid Increases the Susceptibility of Zebrafish Larvae to the Chemical Convulsant Pentylenetetrazole.” Environmental Health Perspectives 115, no. 11: 1547–1552. 10.1289/ehp.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeken, J. A. , and Ramsdell J. S.. 2009. “DDT Exposure of Zebrafish Embryos Enhances Seizure Susceptibility: Relationship to Fetal p,p'‐DDE Burden and Domoic Acid Exposure of California Sea Lions.” Environmental Health Perspectives 117, no. 1: 68–73. 10.1289/ehp.11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeken, J. A. , Ramsdell J. S., and Ramsdell A. F.. 2005. “Developmental Toxicity of Domoic Acid in Zebrafish ( Danio rerio ).” Neurotoxicology and Teratology 27, no. 5: 711–717. 10.1016/j.ntt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Todd, E. C. 1990. “Chronology of the Toxic Mussels Outbreak.” Canada Diseases Weekly Report 16, no. Suppl 1E: 3–4. [PubMed] [Google Scholar]
- Truelove, J. , and Iverson F.. 1994. “Serum Domoic Acid Clearance and Clinical Observations in the Cynomolgus Monkey and Sprague‐Dawley Rat Following a Single i.v. Dose.” Bulletin of Environmental Contamination and Toxicology 52, no. 4: 479–486. [DOI] [PubMed] [Google Scholar]
- Truelove, J. , Mueller R., Pulido O., Martin L., Fernie S., and Iverson F.. 1997. “30‐Day Oral Toxicity Study of Domoic Acid in Cynomolgus Monkeys: Lack of Overt Toxicity at Doses Approaching the Acute Toxic Dose.” Natural Toxins 5, no. 3: 111–114. . [DOI] [PubMed] [Google Scholar]
- USEPA . 2020. “Use of New Approach Methodologies to Derive Extrapolation Factors and Evaluate Developmental Neurotoxicity for Human Health Risk Assessment.” Agency Issue Paper.
- USFDA . 2021. “Appendix 5: FDA and EPA Safety Levels and Regulations and Guidance.” https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwipi7jf_6zrAhWFtp4KHdQOCTMQFjAAegQIBRAB&url=https%3A%2F%2Fwww.fda.gov%2Fmedia%2F80400%2Fdownload&usg=AOvVaw3gZChY_lOemec‐gay1fSuD.
- WADOH . 2023. “Domoic Acid in Razor Clams.” Interim Health Advisory on Eating Razor Clams, Washington State Department of Health. https://doh.wa.gov/community‐and‐environment/shellfish/recreational‐shellfish/illnesses/biotoxins/domoic‐acid‐razor‐clams.
- Wang, G. J. , Schmued L. C., Andrews A. M., Scallet A. C., Slikker W. Jr., and Binienda Z.. 2000. “Systemic Administration of Domoic Acid‐Induced Spinal Cord Lesions in Neonatal Rats.” Journal of Spinal Cord Medicine 23, no. 1: 31–39. 10.1080/10790268.2000.11753506. [DOI] [PubMed] [Google Scholar]
- Wekell, J. C. , Hurst J., and Lefebvre K. A.. 2004. “The Origin of the Regulatory Limits for PSP and ASP Toxins in Shellfish.” Journal of Shellfish Research 23, no. 3: 927–930. [Google Scholar]
- Xi, D. , Peng Y. G., and Ramsdell J. S.. 1997. “Domoic Acid Is a Potent Neurotoxin to Neonatal Rats.” Natural Toxins 5, no. 2: 74–79. . [DOI] [PubMed] [Google Scholar]
- Zuloaga, D. G. , Lahvis G. P., Mills B., Pearce H. L., Turner J., and Raber J.. 2016. “Fetal Domoic Acid Exposure Affects Lateral Amygdala Neurons, Diminishes Social Investigation and Alters Sensory‐Motor Gating.” Neurotoxicology 53: 132–140. 10.1016/j.neuro.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


