Abstract
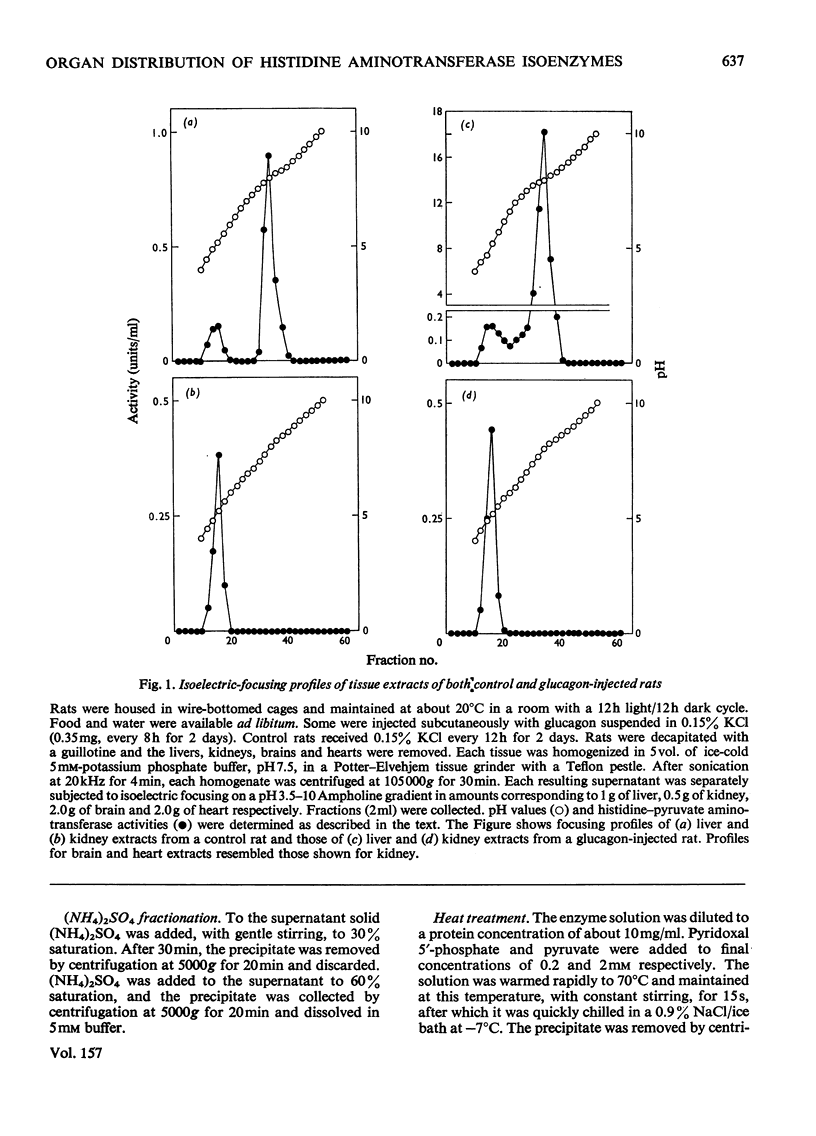
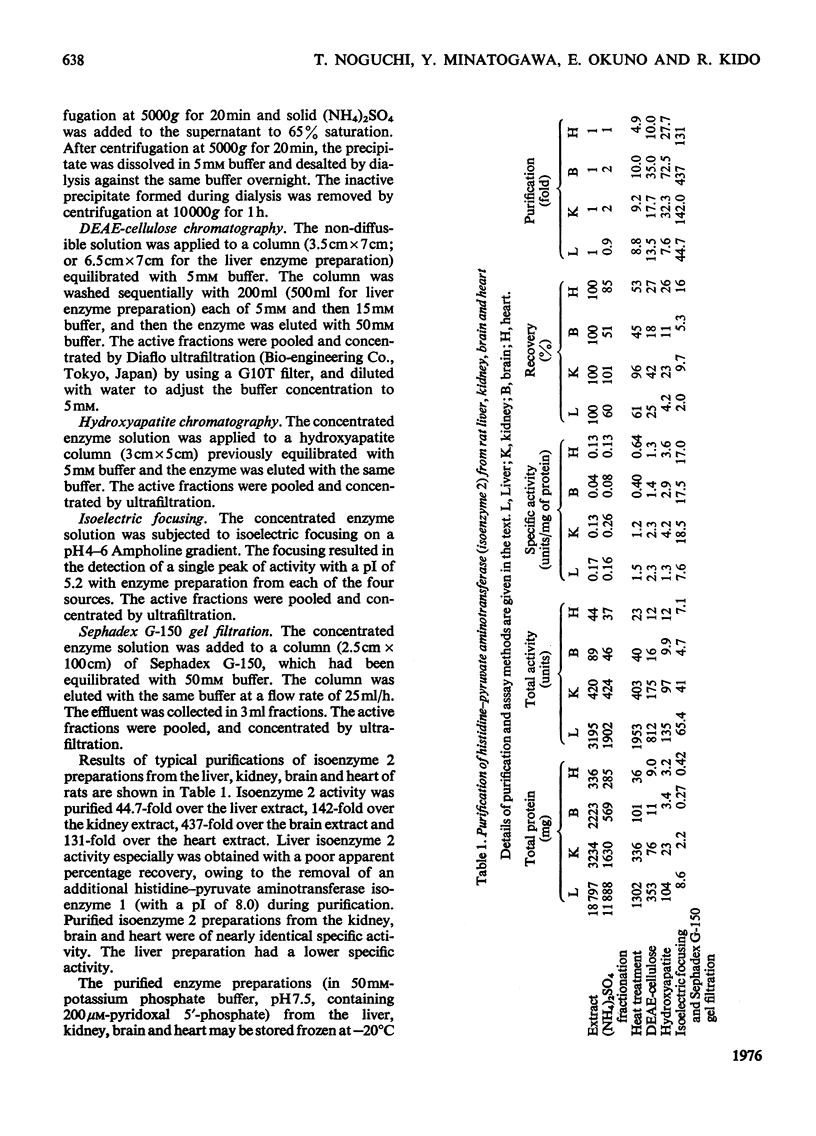
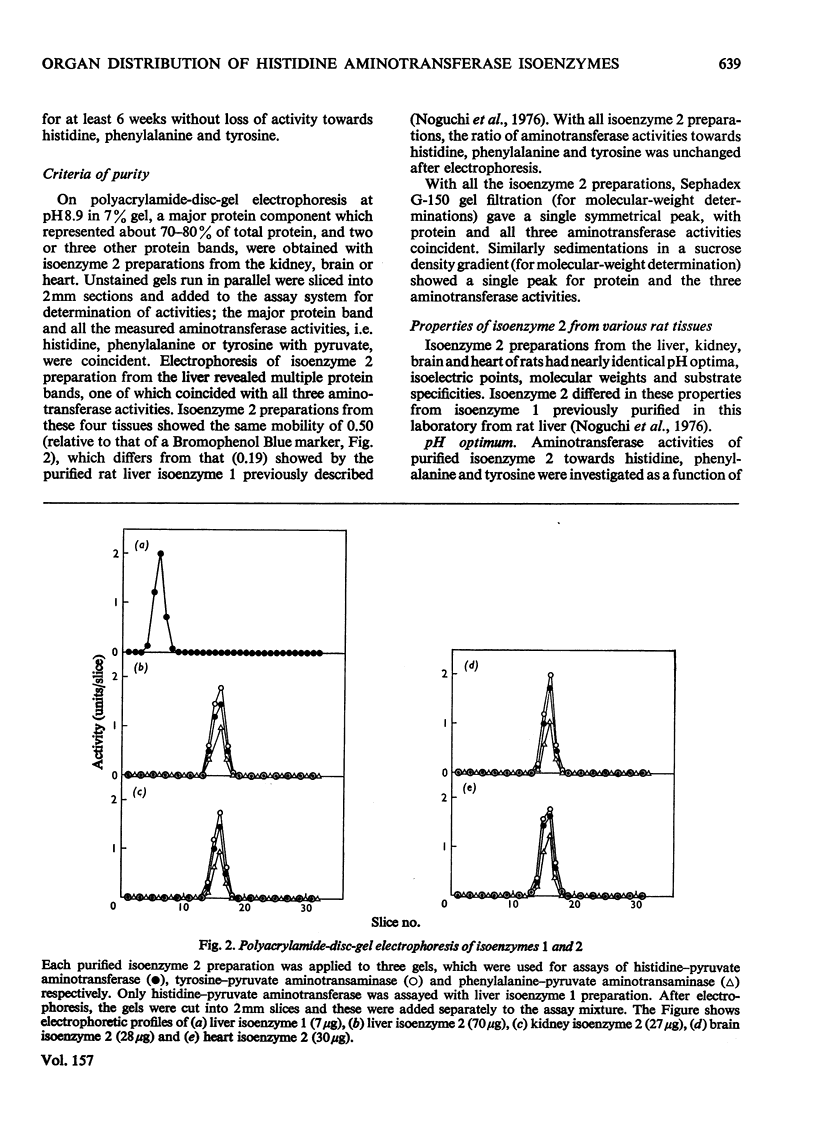
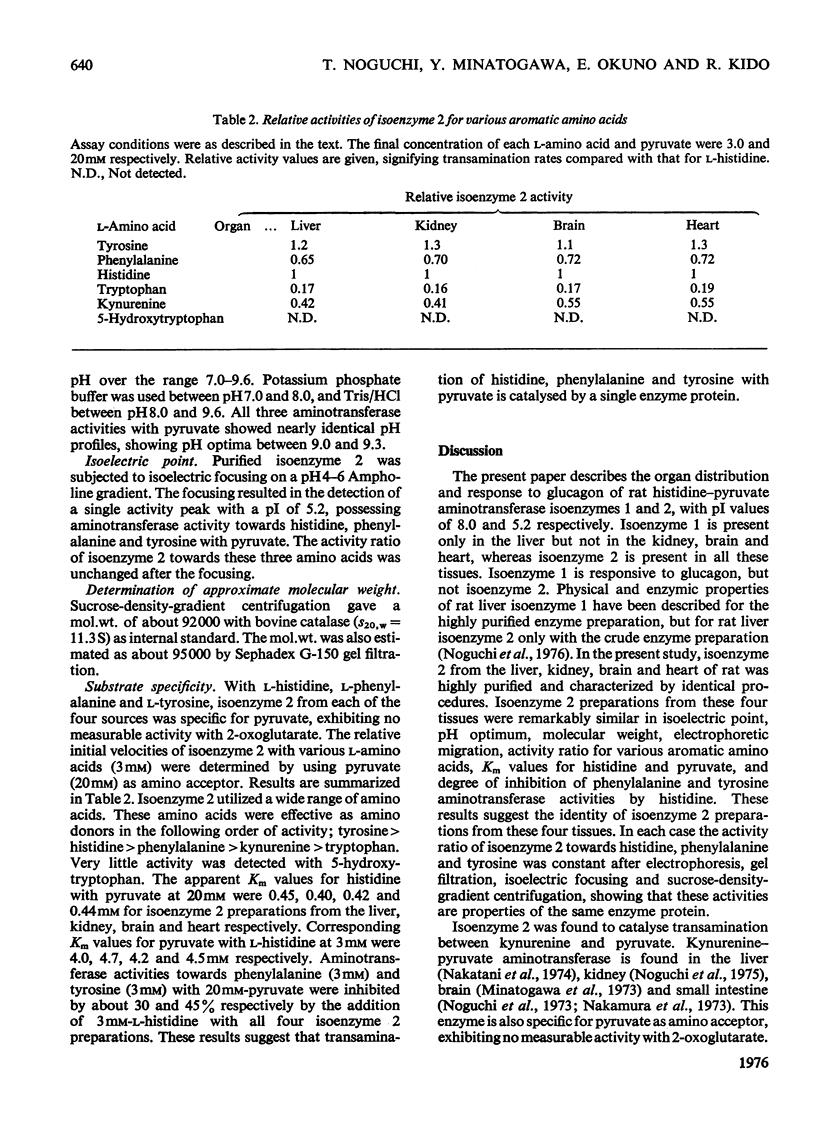
The organ distribution of rat histidine-pyruvate aminotransferase isoenzymes 1 and 2 was examined by using an isoelectric-focusing technique. Isoenzyme 1 (pI8.0) is present only in the liver and its activity is increased by the injection of glucagon, whereas isoenzyme 2 (pI5.2) is distributed in all tissues (liver, kidney, brain and heart) tested, and is not affected by glucagon injection. Isoenzyme 2 of the liver, kidney, brain and heart was purified by the same procedure and characterized. Isoenzyme 2 preparations from these four tissues were nearly identical in physical and enzymic properties. These properties differed from those previously found for the highly purified isoenzyme 1 preparation of rat liver. Isoenzyme 2 was active with pyruvate but not with 2-oxoglutarate as amino acceptor. Amino donors were effective in the following order of activity: tyrosine greater than histidine greater than phenylalanine greater than kynurenine greater than tryptophan. Very little activity was found with 5-hydroxytryptophan. The apparent Km for histidine was about 0.45 mM. The Km for pyruvate was about 4.5 mM with histidine as amino donor. The amino-transferase activities of isoenzyme 2 towards phenylalanine and tyrosine were inhibited by histidine. The ratio of aminotransferase activities towards these three amino acids was constant through gel filtration, electrophoresis, isoelectric focusing and sucrose-density-gradient centrifugation of the purified isoenzyme 2 preparations. These results suggest that these three activities are properties of the same enzyme protein. Sephadex G-150 gel filtration and sucrose-density-gradient centrifugation yielded mol.wts. of approx. 95000 and 92000 respectively. The pH optimum was between 9.0 and 9.3.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- KNOX W. E. The relation of liver kynureninase to tryptophan metabolism in pyridoxine deficiency. Biochem J. 1953 Feb;53(3):379–385. doi: 10.1042/bj0530379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., PITT B. M., CIVEN M., KNOX W. E. The assay of aromatic amino acid transaminations and keto acid oxidation by the enol borate-tautomerase method. J Biol Chem. 1958 Sep;233(3):668–673. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASON M. The kynurenine transaminase of rat kidney. J Biol Chem. 1954 Dec;211(2):839–844. [PubMed] [Google Scholar]
- Minatogawa Y., Noguchi T., Kido R. Aromatic amino acid transaminases in rat brain. J Neurochem. 1973 May;20(5):1479–1481. doi: 10.1111/j.1471-4159.1973.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Morris M. L., Lee S. C., Harper A. E. A comparison of the responses of mitochondrial and cytosol histidine-pyruvate aminotransferases to nutritional and hormonal treatments. J Biol Chem. 1973 Feb 25;248(4):1459–1465. [PubMed] [Google Scholar]
- Nakamura J., Noguchi T., Kido R. Aromatic amino acid transaminase in rat intestine. Biochem J. 1973 Dec;135(4):815–818. doi: 10.1042/bj1350815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani M., Morimoto M., Noguchi T., Kido R. Subcellular distribution and properties of kynurenine transaminase in rat liver. Biochem J. 1974 Nov;143(2):303–310. doi: 10.1042/bj1430303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Nakamura J., Kido R. Kynurenine pyruvate transaminase and its inhibitor in rat intestine. Life Sci. 1973 Oct 1;13(7):1001–1010. doi: 10.1016/0024-3205(73)90091-x. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Nakatani M., Minatogawa Y., Morimoto M., Kido R. Subcellular distribution and properties of kynurenine pyruvate transaminase in rat kidney. Hoppe Seylers Z Physiol Chem. 1975 Aug;356(8):1245–1250. doi: 10.1515/bchm2.1975.356.2.1245. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Okuno E., Minatogawa Y., Kido R. Purification, characterization and identification of rat liver histidine-pyruvate aminotransferase isoenzymes. Biochem J. 1976 Apr 1;155(1):107–115. doi: 10.1042/bj1550107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOLTER H., BALDRIDGE R. C. MULTIPLE FORMS OF HISTIDINE-PYRUVATE TRANSAMINASE IN RAT LIVER. Biochim Biophys Acta. 1964 Aug 19;90:287–290. doi: 10.1016/0304-4165(64)90191-6. [DOI] [PubMed] [Google Scholar]
- Tottmar S. O., Pettersson H., Kiessling K. H. The subcellular distribution and properties of aldehyde dehydrogenases in rat liver. Biochem J. 1973 Dec;135(4):577–586. doi: 10.1042/bj1350577a. [DOI] [PMC free article] [PubMed] [Google Scholar]


