Abstract
We have recently demonstrated that the binding subunit (B-oligomer) of pertussis toxin (PTX-B) deactivates CCR5 and inhibits entry of R5 human immunodeficiency virus type 1 (HIV-1) strains in activated primary T lymphocytes (M. Alfano et al., J. Exp. Med. 190:597–605, 1999). We now present evidence that PTX-B also affects a postentry step of HIV-1 replication. While PTX-B inhibited fusion induced by R5 but not that induced by X4 envelopes, it blocked infection of T cells with recombinant HIV-1 particles pseudotyped with R5, X4, and even murine leukemia virus or vesicular stomatitis virus envelopes. It also suppressed HIV-1 RNA synthesis in cultures of infected peripheral blood mononuclear cells when new infections had been inhibited by zidovudine, and it reduced Tat-dependent expression of the luciferase reporter gene controlled by the HIV-1 long terminal repeat (LTR). Surprisingly, PTX-B did not affect expression from the cytomegalovirus promoter, nor did it reduce the basal (Tat-independent) expression from the LTR promoter. These results indicate that PTX-B inhibits HIV-1 infection at both the entry and the postentry stages of viral replication, with the postentry activity specifically affecting transcription or stability of Tat-stimulated HIV-1 mRNAs.
Pertussis toxin (PTX) is a pentameric protein which can be functionally divided into two (A and B) subunits. The A (active) monomer exhibits ADP ribosyltransferase activity responsible for ribosylation and inactivation of Gi-like proteins, whereas the role of the B (binding)-oligomer (PTX-B) was initially perceived to be limited to binding of PTX to target cells (21). It is now clear, however, that PTX-B alone initiates signal transduction (1, 17–19) in target cells. Although the signal-transducing receptor for PTX has not been unequivocally identified yet, it appears to belong to the class of sialylated glycoproteins, with likely candidates being a 43-kDa protein in T lymphocytes (16) and the CD11b-CD18 integrin in myelomonocytic cells (20, 22). Recently, we demonstrated that PTX-B inhibits an early stage of replication of R5 human immunodeficiency type 1 (HIV-1) strains, likely the entry of the viral core into target cells (1). Importantly, PTX-B did not diminish CCR5 or CD4 expression on peripheral blood mononuclear cells (PBMC), nor did it affect binding of CCR5 ligands (macrophage inflammatory protein 1β [MIP-1β] or R5 gp120) to the cells. However, functional activities of CCR5 were inhibited: PTX-B-treated PBMC did not exhibit Ca2+ flux when treated with MIP-1β or RANTES (while preserving Ca2+ response to stroma-derived factor 1α (SDF-1α), a CXCR4 ligand) and did not display the R5 Env-induced capping of CCR5 characteristic of HIV-1 infection. The effect of PTX-B on CCR5 could be reversed by protein kinase C inhibitor, implicating a signaling mechanism likely originating from the PTX-B receptor. These findings suggested that PTX-B induces selective heterologous desensitization of one of the major HIV-1 coreceptors, CCR5, thus affecting infection by the viruses that use this receptor (1). That study also underscored differences between primary cells, where HIV-1 infection depends on chemokine coreceptor signaling and capping (1, 11), and cell lines, where HIV-1 coreceptor activity of chemokine receptors does not appear to involve signaling (3, 4, 8, 10).
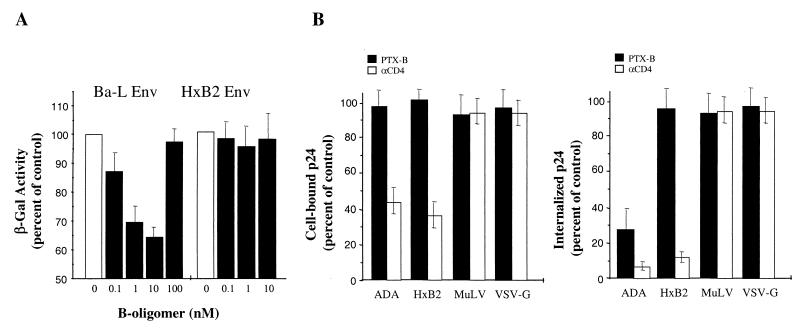
A previous study (1) relied on PCR analysis of early reverse transcription products, thus not allowing discrimination between various possible mechanisms of PTX-B activity, such as inhibition of virus-cell fusion, uncoating, or initiation of reverse transcription. To directly measure the effect of PTX-B on HIV-1 Env-mediated fusion, we used the classical cell fusion assay (2). Primary activated PBMC were infected with vaccinia virus expressing T7 RNA polymerase (vTF7-3 [9]) and coincubated at a ratio of 5:1 in the presence or absence of PTX-B with HeLa cells coinfected with vaccinia viruses expressing HIV-1 Env (vCB-41 for X4 EnvLAV and vCB-43 for R5 EnvBa-L [5]) and the Escherichia coli lacZ gene linked to the T7 promoter (vCB-21R-lacZ [2]) for 2.5 h to allow cell fusion. Cell fusion was assessed by measuring β-galactosidase (β-Gal) activity in detergent cell lysates by colorimetric assay. Results of a representative experiment are presented in Fig. 1A. Treatment of PBMC with PTX-B reduced in a dose-dependent fashion cell fusion induced by R5 EnvBa-L (to 65% of control at 10 nM PTX-B), while X4 EnvLAV-mediated fusion was not affected. A classical bell-shaped dose response was observed, and at high PTX-B concentrations the inhibitory effect disappeared. Some variation in the magnitude of the inhibitory effect observed with cells from different donors might be explained by different levels of expression of the PTX-B receptor (M. Alfano, unpublished observation). This result indicates that the inhibitory activity of PTX-B at the early stage of HIV-1 infection is targeted at the step of virus-cell fusion. A lower activity of PTX-B in this assay than in the virus-based entry assay with primary cells, where members of our group routinely observed inhibition by 70 to 80% (1), can be explained by lower dependence of cell-cell fusion on the ability of fusion receptors to cap, possibly due to tight contacts between the cells in cocultures. Alternatively, overexpression of HIV-1 Env using vaccinia vector may partially overcome CCR5 deficiency. To evaluate the effect of PTX-B on fusion in the context of virus infection, we measured cell-associated p24 after inoculation of PBMC with HIV-1. We used recombinant viruses containing HIV-1 core pseudotyped with envelopes derived from the R5 HIV-1 strain ADA, the X4 HIV-1 strain HxB2, amphotropic murine leukemia virus (MuLV), or vesicular stomatitis virus protein G (VSV-G) (6). When cells were inoculated with pseudotyped viruses (20 ng of p24 per 106 cells) in the presence or absence of PTX-B (1 nM) and incubated for 1 h at 0°C to allow virus attachment, PTX-B did not change the amount of cell-associated p24, while anti-CD4 monoclonal antibody (MAb) significantly reduced attachment of HIV-1 Env-pseudotyped viruses (Fig. 1B, left). This result is consistent with the lack of effect of PTX-B on CD4 or chemokine receptor expression (not shown) and with our earlier finding that PTX-B does not reduce R5 gp120 binding to CCR5 or entry of X4 HIV-1 into PBMC (1). However, when cells were transferred to 37°C for 2 h to allow virus internalization and then trypsinized to remove noninternalized virus, PTX-B reduced by approximately 70% the amount of internalized p24 for EnvADA-pseudotyped virus, without affecting p24 internalization for other viruses (Fig. 1B, right). As expected, anti-CD4 MAb reduced the amount of internalized p24 for both HIV-1 Env-pseudotyped viruses but not for EnvMuLV- or VSV-G-pseudotyped viruses. Results of this experiment support our conclusion that the PTX-B-mediated effect on CCR5 manifests itself in selective inhibition of fusion between R5 HIV-1 and target cells.
FIG. 1.
Effects of PTX-B on cell-cell and virus-cell fusion mediated by different envelopes. (A) Analysis of Env-mediated cell-cell fusion. Fusion between PBMC (3-day PHA blasts cultured for 7 days in interleukin 2) infected with vaccinia virus expressing T7 RNA polymerase and HeLa cells coinfected with vaccinia viruses expressing HIV-1 Env (X4 EnvLAV or R5 EnvBa-L) and the E. coli lacZ gene linked to the T7 promoter was assessed by a colorimetric assay in detergent cell lysates. The background activity detected in cocultures of T7 RNA polymerase-expressing PBMC with HeLa cells expressing only the E. coli lacZ gene linked to the T7 promoter was subtracted from all values. Results are expressed as percentages of β-Gal activity in experimental cells (treated with indicated concentrations of PTX-B) relative to that in control (untreated) cells and are the means ± the standard deviations (SD) of three independent measurements with cells from the same donor. (B) Analysis of cell-associated p24. PBMC were inoculated in the presence of PTX-B (1 nM) or anti-CD4 MAb (Leu3a, 5 μg/ml) with HIV-1 pseudotypes carrying indicated envelopes. Virus attachment was assessed by measuring cell-associated p24 following a 1-h incubation at 4°C (left panel). Virus-cell fusion was assessed by measuring the amount of internalized p24 following a 2-h incubation at 37°C and subsequent trypsinization of the cells to remove noninternalized virus. The data (p24 values relative to control, untreated samples) are the means ± SD of three independent experiments.
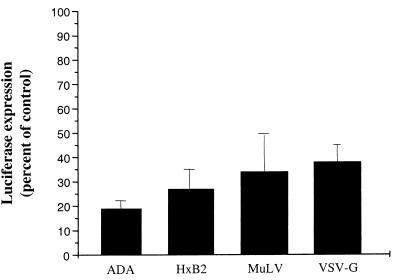
In addition to exerting inhibitory activity on infection by R5 HIV-1 strains at the step of virus-cell fusion, PTX-B was also found to block replication of X4 viruses at a postfusion step of infection (1). To substantiate this activity of PTX-B, we analyzed its effects in a single-cycle assay with Env-pseudotyped, luciferase-expressing HIV-1 recombinants (6, 7). Cells were infected with pseudotyped virus (5 ng of p24 per 106 cells) in the presence (1 nM) or absence of PTX-B. After 4 days, cells were washed and lysed in reporter lysis buffer (Promega), and the luciferase activity was measured in relative light units using a Dynex MLX microplate luminometer. As expected, PTX-B inhibited infection with HIV-1 pseudotyped with R5 EnvADA, as measured by luciferase activity in cell lysates (Fig. 2). It also inhibited infection with HIV-1 pseudotyped with X4 EnvHxB2, despite the fact that X4 Env-mediated fusion was not affected (Fig. 1). Furthermore, infection with EnvMuLV- and VSV-G-pseudotyped viruses, which enter cells via CD4- and chemokine receptor-independent fusion and receptor-mediated endocytosis, respectively, was also inhibited. Given that PTX-B did not diminish entry of any virus other than EnvADA-pseudotyped virus (Fig. 1B), this above-mentioned result indicates an additional, postentry inhibitory activity of PTX-B. This activity explains PTX-B-mediated inhibition of replication of X4 viruses in long-term cultures (1).
FIG. 2.
Effects of PTX-B on PBMC infection with different HIV-1 pseudotypes. PHA-activated PBMC cultures were inoculated with HIV-1 pseudotypes carrying envelopes of HIV-1 strains ADA (R5) or HxB2 (X4) or of amphotropic MuLV or VSV-G. Luciferase expression was measured on day 4 postinfection, and the results are presented as percentages of expression in experimental cells (treated with 1 nM PTX-B) relative to that in control (no PTX-B) cells.
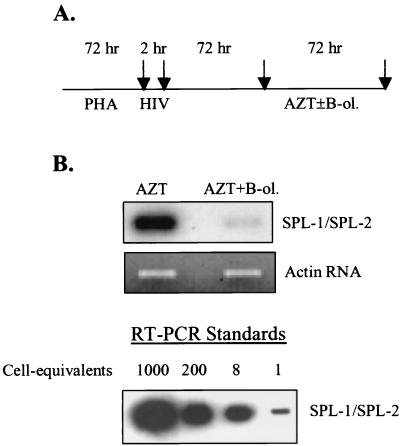
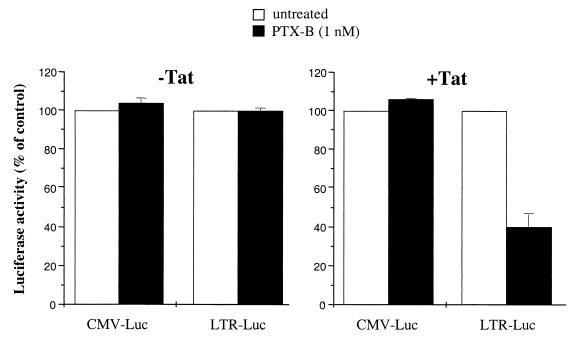
To narrow down possible mechanisms of postentry inhibition by PTX-B, we analyzed synthesis of HIV-1-specific mRNA in infected PBMC cultures under conditions in which new rounds of infection had been blocked by zidovudine (AZT). The scheme of this experiment is shown in Fig. 3A. Phytohemagglutinin (PHA)-activated cells were infected with the X4 strain HIV-1LAI and incubated for 3 days to allow establishment of infection. AZT at a concentration of 1 μM (a dose that completely inhibited de novo infection) was then added with or without PTX-B (1 nM). Production of spliced HIV-1 mRNA was estimated by reverse transcription (RT)-PCR using random hexamers for reverse transcription and an SPL-1–SPL-2 primer pair specific for double-spliced HIV-1 RNA and revealed by Southern blot hybridization (sense, 5′-CTTAGGCATCTCCTATGGCA-3′; antisense, 5′-CGGGCCTGTCGGGTTCCCTC-3′; probe, 5′-CAGGAAGAAGCGGAGACAGC-3′). The β-actin RNA was amplified from the same samples to control RNA loading (sense, 5′-GACTTAGTTGCGTTACACCC-3′; antisense, 5′-CCTCCCCTGTGTGGACTTGG-3′). RT-PCR standards were prepared by amplification of RNA extracted from various numbers of tumor necrosis factor alpha (TNF-α)-induced U1 cells (each cell contains 2 copies of HIV-1 provirus) using the same primers and PCR conditions as for experimental samples. While no quantitative conclusions can be derived from this standard curve, it indicates that signals falling within the range from 1 to 8 cell-equivalents would be easily discriminated. Primers used for HIV-1 RNA amplification were derived from Tat-encoding cDNA and do not amplify unspliced (genomic) viral RNA (Fig. 3B). Therefore, the amount of amplified product reflects the steady-state level of spliced HIV-1 mRNA. As shown in Fig. 3B, PTX-B reduced the amount of spliced HIV-1 mRNA in this system, suggesting that the inhibitory effect is confined either to splicing or to the transcription and stability of viral mRNAs. Unfortunately, we could not discriminate between these two possibilities within the model shown in Fig. 3A, as analysis of unspliced HIV-1 mRNAs was confounded by contribution of input viral RNA to the resultant signal. We therefore resorted to analysis of reporter gene expression in the Jurkat T-cell line, which is responsive to PTX-B effects (M. Alfano, unpublished observation). Jurkat cells were cotransfected by electroporation with 500 ng of pRL-CMV DNA (Promega) encoding Renilla luciferase and 10 μg of HIV-1 LTR-Luc (kindly provided by Ben Berkhout [12]) encoding firefly luciferase. Some samples were also cotransfected with 1 μg of pcDNA1/Tat (kindly provided by Chiara Bovolenta); Tat stimulates HIV-1 long terminal repeat (LTR)-driven expression by interacting with the Tat-responsive (TAR) region in the RNA (15). Transfected cells were cultured in the presence or absence of B-oligomer (1 nM) for 48 h and then harvested and processed using the Dual-Luciferase assay system (Promega) as suggested by the manufacturer. Results presented in Fig. 4 (left) demonstrate that in the absence of Tat, PTX-B did not reduce the basal, Tat-independent expression from LTR, nor did it affect expression from the cytomegalovirus (CMV) promoter. In the presence of Tat, however, PTX-B inhibited expression of the luciferase reporter driven by HIV-1 LTR but not by the CMV promoter (Fig. 4, right). Because LTR-driven luciferase expression does not depend on splicing, this result indicates that PTX-B affects transcription and stability but not splicing of HIV-1 DNA. It also suggests that this inhibitory effect is specific for Tat-dependent transcription and might involve modulation of activity of one of the components of the Tat-containing transcription complex (14).
FIG. 3.
Effects of PTX-B on HIV-1 mRNA expression. PHA-activated PBMC cultures were infected with the X4 isolate HIV-1LAI. (A) Cells were cultured according to the scheme shown. AZT was used at a concentration of 1 μM, which completely blocked de novo infection, and PTX-B (B-ol.) was used at a concentration of 1 nM. Arrows indicate times at which the medium was changed and cells were thoroughly washed. (B) After the final wash, total RNA was analyzed by RT-PCR using an SPL-1–SPL-2 primer pair specific for spliced HIV-1 mRNA (product detected by Southern blot hybridization, upper panel) or β-actin mRNA (product detected by ethidium bromide staining of the gel, bottom panel). Results are for one representative experiment out of two performed with cells from different donors. The standards were prepared by extracting RNA from indicated numbers of TNF-α-stimulated U1 cells (each cell containing 2 copies of integrated HIV-1 provirus) and amplifying it in parallel with experimental samples.
FIG. 4.
Effects of PTX-B on luciferase reporter gene expression. Jurkat cells were cotransfected with pRL-CMV DNA (CMV-Luc) encoding Renilla luciferase and LTR-Luc (LTR of HIV-1LAI) encoding firefly luciferase (left panel) or with the same plasmids plus pcDNA1/Tat encoding HIV-1 Tat protein (right panel). After 48 h of incubation, luciferase activity was measured by the Dual-Luciferase assay system. Results are the averages of two independent experiments, both done in duplicate.
In a previous report (1), it was demonstrated that PTX-B inhibits HIV-1 infection by at least two mechanisms, one dependent on CCR5 and the other one not. This study demonstrates that CCR5-dependent inhibition by PTX-B involves repression of virus-cell fusion, while the second, CCR5-independent mechanism affects the transcription and stability of HIV-1 mRNA. The latter activity appears specific for viral TAR-containing RNAs, consistent with the very low cytotoxicity of PTX-B (1), manifested also in its well-known mitogenic activity (21). Importantly, both inhibitory activities appear to be mediated by signaling events originating from an as-yet-uncharacterized PTX receptor. Future studies will identify molecular mechanisms responsible for the intriguing specificity of the PTX-B effect on HIV-1 LTR-driven transcription and RNA stability, which might operate through signal-dependent modification of a factor involved in the function of Tat-dependent transcription elongation complex.
The dose-response curve of PTX-B activity had a characteristic bell shape, indicating a biphasic response (Fig. 1A). Indeed, at high PTX-B concentrations (0.1 to 1 μM) the inhibitory effect disappeared, and a recent study (13) even found a stimulating activity of PTX-B on HIV-1 infection at those concentrations. That activity appears to be manifested at an early stage of viral infection, likely at the step of virus-cell fusion, and does not seem to depend on signaling (13).
The described activities of PTX-B make it an attractive candidate for anti-HIV therapy, as it can inhibit virus production both after de novo infection and after reactivation of latent virus. The latter scenario becomes especially important in view of emerging evidence that persistence and expression of the virus in resting T cells might be the reason for the failure of highly active antiretroviral therapy to eliminate HIV from the body (23).
Acknowledgments
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: vTF7-3 from Tom Fuerst and Bernard Moss and vCB21R-lacZ, vCB-41, and vCB-43 from Christopher C. Broder, Paul E. Kennedy, and Edward A. Berger. pNL-Luc, pSV-Env(MuLV), and pSV-Env(VSV-G) plasmids were a gift from Nathaniel Landau. pEnvΔCT and pEnvHXB2 were kindly provided by Heinrich Gottlinger. HIV-LTR-CAT and pcDNA1/Tat were gifts from Ben Berkhout and Chiara Bovolenta, respectively.
This work was supported in part by NIH grant R01 AI 38245 (to M.B.) and by funds from The Picower Institute for Medical Research.
REFERENCES
- 1.Alfano M, Schmidtmayerova H, Amella C A, Pushkarsky T, Bukrinsky M. The B-oligomer of pertussis toxin deactivates CC chemokine receptor 5 and blocks entry of M-tropic HIV-1 strains. J Exp Med. 1999;190:597–606. doi: 10.1084/jem.190.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Broder C C, Berger E A. Cell type-specific fusion cofactors determine human immunodeficiency virus type 1 tropism for T-cell lines versus primary macrophages. J Virol. 1996;70:5487–5494. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkhatib G, Locati M, Kennedy P E, Murphy P M, Berger E A. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 4.Aramori I, Ferguson S S, Bieniasz P D, Zhang J, Cullen B, Cullen M G. Molecular mechanism of desensitization of the chemokine receptor CCR-5: receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO J. 1997;16:4606–4616. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broder C C, Berger E A. Fusogenic selectivity of the envelope glycoprotein is a major determinant of human immunodeficiency virus type 1 tropism for CD4+ T-cell lines vs. primary macrophages. Proc Natl Acad Sci USA. 1995;92:9004–9008. doi: 10.1073/pnas.92.19.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 8.Farzan M, Choe H, Martin K A, Sun Y, Sidelko M, Mackay C R, Gerard N P, Sodroski J, Gerard C. HIV-1 entry and macrophage inflammatory protein-1β-mediated signaling are independent functions of the chemokine receptor CCR5. J Biol Chem. 1997;272:6854–6857. doi: 10.1074/jbc.272.11.6854. [DOI] [PubMed] [Google Scholar]
- 9.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosling J, Monteclaro F S, Atchison R E, Arai H, Tsou C L, Goldsmith M A, Charo I F. Molecular uncoupling of C-C chemokine receptor 5-induced chemotaxis and signal transduction from HIV-1 coreceptor activity. Proc Natl Acad Sci USA. 1997;94:5061–5066. doi: 10.1073/pnas.94.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyengar S, Hildreth J E, Schwartz D H. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J Virol. 1998;72:5251–5255. doi: 10.1128/jvi.72.6.5251-5255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeeninga R E, Hoogenkamp M, Armand-Ugon M, de Baar M, Verhoef K, Berkhout B. Functional differences between the long terminal repeat transcriptional promoters of human immunodeficiency virus type 1 subtypes A through G. J Virol. 2000;74:3740–3751. doi: 10.1128/jvi.74.8.3740-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Momoi Y, Ichiyama K, Chowdhury I H, Koyanagi Y, Yamamoto N. Pertussis toxin enhances human immunodeficiency virus type 1 replication. AIDS Res Hum Retrovir. 2000;16:373–379. doi: 10.1089/088922200309250. [DOI] [PubMed] [Google Scholar]
- 14.Rana T M, Jeang K T. Biochemical and functional interactions between HIV-1 Tat protein and TAR RNA. Arch Biochem Biophys. 1999;365:175–185. doi: 10.1006/abbi.1999.1206. [DOI] [PubMed] [Google Scholar]
- 15.Roebuck K A, Saifuddin M. Regulation of HIV-1 transcription. Gene Expr. 1999;8:67–84. [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers T S, Corey S J, Rosoff P M. Identification of a 43-kilodalton human T lymphocyte membrane protein as a receptor for pertussis toxin. J Immunol. 1990;145:678–683. [PubMed] [Google Scholar]
- 17.Rosoff P M, Walker R, Winberry L. Pertussis toxin triggers rapid second messenger production in human T lymphocytes. J Immunol. 1987;139:2419–2423. [PubMed] [Google Scholar]
- 18.Stewart S J, Prpic V, Johns J A, Powers F S, Graber S E, Forbes J T, Exton J H. Bacterial toxins affect early events of T lymphocyte activation. J Clin Investig. 1989;83:234–242. doi: 10.1172/JCI113865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thom R E, Casnellie J E. Pertussis toxin activates protein kinase C and a tyrosine protein kinase in the human T cell line Jurkat. FEBS Lett. 1989;244:181–184. doi: 10.1016/0014-5793(89)81188-3. [DOI] [PubMed] [Google Scholar]
- 20.Wong W S, Luk J M. Signaling mechanisms of pertussis toxin-induced myelomonocytic cell adhesion: role of tyrosine phosphorylation. Biochem Biophys Res Commun. 1997;236:479–482. doi: 10.1006/bbrc.1997.6986. [DOI] [PubMed] [Google Scholar]
- 21.Wong W S, Rosoff P M. Pharmacology of pertussis toxin B-oligomer. Can J Physiol Pharmacol. 1996;74:559–564. doi: 10.1139/cjpp-74-5-559. [DOI] [PubMed] [Google Scholar]
- 22.Wong W S, Simon D I, Rosoff P M, Rao N K, Chapman H A. Mechanisms of pertussis toxin-induced myelomonocytic cell adhesion: role of Mac-1 (CD11b/CD18) and urokinase receptor (CD87) Immunology. 1996;88:90–97. doi: 10.1046/j.1365-2567.1996.d01-646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4(+) T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]