Abstract
Background
Acephalic spermatozoa syndrome is a rare but severe type of teratozoospermia. The familial trait of acephalic spermatozoa syndrome suggests that genetic factors play an important role. However, known mutations account for only some acephalic spermatozoa syndrome patients, and more studies are needed to elucidate its pathogenesis. The current study aimed to elucidate the pathogenesis of acephalic spermatozoa syndrome caused by PMFBP1 mutation.
Results
We identified a homozygous splice site mutation (NM_031293.2, c.2089-1G > T) in PMFBP1 through Sanger sequencing. Western blotting and immunofluorescence analyses revealed that this splice site mutation resulted in the absence of PMFBP1 protein expression in the patient's sperm cells. We generated an in vitro model carrying the splice site mutation in PMFBP1 and confirmed, through RT‒PCR and Sanger sequencing, that it led to a deletion of 4 base pairs from exon 15.
Conclusion
A homozygous splice site mutation results in a deletion of 4 bp from exon 15 of PMFBP1, thereby affecting the expression of the PMFBP1 protein. The absence of PMFBP1 protein expression can lead to acephalic spermatozoa syndrome. This finding elucidates the underlying cause of acephalic spermatozoa syndrome associated with this specific mutation (NM_031293.2, c.2089-1G > T) in PMFBP1.
Keywords: Acephalic spermatozoa syndrome, Polyamine-modulated factor 1 binding protein 1 (PMFBP1), Splice-site mutation
Résumé
Contexte
Le syndrome des spermatozoïdes acéphaliques est un type rare mais grave de tératozoospermie. Le caractère familial du syndrome des spermatozoïdes acéphaliques suggère que les facteurs génétiques jouent un rôle important. Cependant, les mutations connues ne représentent que quelques patients atteints du syndrome des spermatozoïdes acéphaliques, et d’autres études sont nécessaires pour en élucider la pathogenèse. La présente étude visait à élucider la pathogenèse du syndrome des spermatozoïdes acéphales causé par la mutation PMFBP1.
Résultats
Nous avons identifié une mutation homozygote du site d’épissage (NM_031293.2, c.2089-1G > T) dans PMFBP1 par séquençage Sanger. Les analyses par Western blot et par immunofluorescence ont révélé que cette mutation du site d’épissage entraînait l’absence d’expression de la protéine PMFBP1 dans les spermatozoïdes du patient. Nous avons généré un modèle in vitro portant la mutation du site d’épissage dans PMFBP1 et avons confirmé, par RT-PCR et séquençage Sanger, qu’elle conduisait à une délétion de 4 paires de bases de l’exon 15.
Conclusions
Une mutation homozygote du site d’épissage entraîne une délétion de 4 paires de bases de l’exon 15 de PMFBP1, affectant ainsi l’expression de la protéine PMFBP1. L’absence d’expression de la protéine PMFBP1 peut entraîner un syndrome des spermatozoïdes acéphales. Cette découverte élucide la cause sous-jacente du syndrome des spermatozoïdes acéphaliques associée à cette mutation spécifique (NM_031293.2, c.2089-1G > T) dans PMFBP1.
Mots-clés
Syndrome des Spermatozoïdes acéphaliques; Protéine de Liaison 1 du Facteur 1 modulé par les Polyamines (PMFBP1); Mutation du Site d’Epissage.
Introduction
Infertility is a global issue that affects approximately 15% of couples worldwide. Importantly, male factors contribute to nearly half of all infertility cases, highlighting the importance of addressing male infertility alongside female infertility [1, 2]. One common cause of male infertility is teratozoospermia, which refers to morphological defects in spermatozoa. Among the various types of teratozoospermia, acephalic spermatozoa syndrome (ASS) is rare yet severe [3–9]. This condition is characterized by the absence of the cephalic region in spermatozoa [5]. During ejaculation, patients with ASS typically exhibit a paucity of normal spermatozoa in their seminal fluid. Instead, they frequently present an abundance of headless sperm tails (pinhead sperm), a limited quantity of tailless sperm heads, and aberrant connections between the head and tail segments. These anomalous sperm exhibit intact structures, such as the nucleus, acrosome, and midpiece; however, the alignment of the midpiece with the rest of the sperm is disrupted [5].
ASS is often associated with genetic factors. In recent years, researchers have made progress in identifying specific genes that play a role in ASS. Mutations in several genes, such as PMFBP1, SUN5, TSGA10, BRDT, HOOK1, DNAH6, ACTRT1, SPATC1L and SPATA20, have been identified as causative factors for some patients [10–23]. However, it is important to note that these mutations account for only a portion of individuals diagnosed with ASS. To gain further insights into the pathogenesis of this condition and potentially identify additional contributing factors or genetic variations involved in ASS development, more extensive studies are needed.
In this study, we aim to elucidate the pathogenesis of ASS caused by PMFBP1 mutation. We identified a specific genetic mutation associated with ASS. A homozygous splice site mutation (NM_031293.2, c.2089-1G > T) was found in the PMFBP1 gene. Further analysis via western blotting and immunofluorescence techniques revealed that this splice site mutation led to the absence of the PMFBP1 protein in the patient's sperm cells. Additionally, a minigene construction of PMFBP1 was generated, and its mRNA expression level was subsequently confirmed through in vitro studies. These tests confirmed that the splice-site mutation caused a deletion of 4 base pairs from exon 15 of the PMFBP1 gene. Our findings explain the pathogenesis that PMFBP1 mutation induced ASS.
Materials and methods
Patient information
A 32-year-old man with a history of primary infertility for more than 5 years whose parents were consanguineous sought to identify genetic factors at the Reproductive Medicine Center of Zhuzhou Hospital Affiliated to Xiangya School of Medicine, CSU, in June 2023. He also has a sister who has a normal baby. The patient's karyotype and Y chromosome microdeletion were examined. The bilateral testicular size, spermatic vein, and prostate were assessed via color ultrasound imaging. This study was approved by the Ethics Committee of Zhuzhou Hospital Affiliated to Xiangya School of Medicine, CSU, and written informed consent was obtained from the participant.
Computer-assisted semen and sperm morphology analysis
The patient abstained from sexual activity for a period of 3 to 5 days prior to the extraction procedure. The patient's semen was analyzed three times at the same reproductive center, following the guidelines outlined in the “WHO laboratory manual for the examination and processing of human semen” (Sixth Edition) [24]. The following parameters were observed: semen volume, pH, sperm concentration, sperm motility, and proportion of abnormal spermatozoa. (Table 1). Sperm morphology was assessed via Papanicolaou staining according to the “WHO laboratory manual for the examination and processing of human semen” (Sixth Edition). The morphology of sperm is divided into head anomalies, PI anomalies and flagellar anomalies.
Table 1.
Semen parameters of the patient with ASS
| Proband | 1st | 2nd | 3rd | |||
|---|---|---|---|---|---|---|
| Volume (ml) | 2.8 | 3.4 | 3.1 | |||
| pH | 7.5 | 7.4 | 7.5 | |||
| Concentration (106/ml) | 5.2 | 6.5 | 10.4 | |||
| Motility B + C (%)a | 7.2 | 7.6 | 8.2 | |||
| Sperm morphology(%) | Abnormal head–tail junction | 2 | Abnormal head–tail junction | 3 | Abnormal head–tail junction | 2.3 |
| Decaudated | 1 | Decaudated | 1 | Decaudated | 1.7 | |
| Acephalic | 97 | Acephalic | 96 | Acephalic | 96 | |
aMotility B+C:B=spermatozoa with progressive motility and C= small circular motion or tail swing
Sanger sequencing of PMFBP1 and SUN5
Patient family (patient, patient parents and his sister) peripheral blood was collected and stored at -80 °C in a refrigerator for preservation purposes. Genomic DNA extraction from the collected blood sample was conducted via the QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. The specific coding regions within the PMFBP1 and SUN5 genes were subsequently amplified using the obtained genomic DNA as a template. The PCR products were then detected via an ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA), followed by Sanger sequencing to validate any mutations present in PMFBP1 and Sad1 and SUN5.
Western blotting analysis
Fresh semen was subjected to three rounds of centrifugation at 6000 rpm for 1 min in PBS. The resulting supernatant was discarded, and the precipitate was dissolved in loading buffer at a concentration of 1 mM at 100 °C for 10 min. The proteins were subsequently separated via SDS‒PAGE and transferred onto a nitrocellulose membrane by electroblotting at 100 V for an hour and a half at 4 °C. Prior to incubation with primary antibodies (anti-PMFBP1 diluted to 1:1000 or anti-α-tubulin diluted to 1:1000; Sigma), the membrane was blocked with a solution containing BSA at room temperature for one hour. To detect the antigen content, secondary antibodies (goat anti-rabbit IgG or goat anti-mouse IgG diluted to 1:5000; ProteinTech Group, Hubei, China) were used.
Immunofluorescence analysis
The sperm samples were subjected to immunofluorescence analysis following previously established protocols [9, 11]. The patient's sperm samples were first fixed onto glass slides. The fixed samples were then permeabilized to allow better antibody penetration into the cells. Next, the primary antibodies (4′, 6-diamidino-2-phenylindole(DAPI), anti-PMFBP1 and anti-CD46 Thermo) were applied to the samples at a dilution of 1:200. After incubation with these primary antibodies, any unbound antibodies were washed away thoroughly. To detect the bound primary antibodies and visualize their respective target proteins within the sperm cells, secondary fluorescently labeled antibodies were employed.
Minigene construction
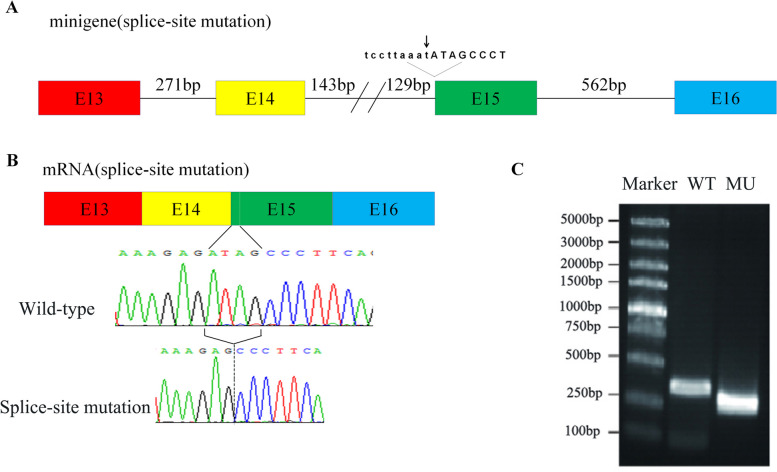
Eligible DNA was used to amplify exons 13–16 and their short intronic flanking regions (Fig. 4A). The amplified fragments were subsequently inserted into the pcDNA3.1 vector by subcloning. Site-directed mutagenesis was performed via a mutagenesis kit to introduce the desired mutation according to the manufacturer's instructions. The primers used in this study are listed in Table 2, and all the constructs were verified via Sanger sequencing analysis.
Fig. 4.
Determination of the removal after transcription. A The boxes with different colors represent the four exons. Minigenes were created using exons 13–16, complete introns 13 and 15, and two partial intron 14. The arrow indicates the mutation site. B Sanger sequencing confirmed that the splice-site mutation in PMFBP1 led to a deletion of 4 base pairs within exon 15. C RT‒PCR analysis revealed a reduced transcriptional output for the splice-site mutant minigenes compared with their wild-type counterparts(WT:wild-type; MU:splice-site mutation)
Table 2.
Primers used to construct minigenes
| F/Ra | Primer Sequence (5’ to 3’) | |
|---|---|---|
| 1b | F | TTACAGGCGTTGGAGGAAAC |
| R | AAGTAGAGCTGTAGGTGGCC | |
| 2c | F | GTTAACACGGACTCTGCAGG |
| R | CGATCTTTGCTGCCTCTGTC | |
| 3d | F | AGCTCTACTTGTTAACACGGACTCTGCAGG |
| R | CCGTGTTAACAAGTAGAGCTGTAGGTGGCC | |
| 4d | F | CTATAGGGAGACCCAAGCTTCACAGGGAGCAAGGCTCCA |
| R | ACGTCATATGGATAGGATCCCTCCAGCTCACTCTCCTGGT | |
| 5e | F | ATCACGTGACCTCAGAGACAAAGAGCCTGCAGCAAA |
| R | TCTGAGGTCACGTGATTGAGCTTCCGGAAAAGACAAAG | |
| 6f | F | AGACAAGACGTTGAAAGAG |
| R | CGTGATTGAGCTTCTCGAG |
aF represents the forward primer. R represents the reverse primer
bIt was used to amplify exons 13–14 with complete intron 13 and partial intron 14
cExons 15–16 with complete intron 15 and partial intron 14
dThese sequences were used to connect two amplifications and construct wild-type minigenes
eMutant minigenes were constructed
fIt was used to determine the removal resulting from splice-site mutation
Transfection
HEK293T cells, derived from human embryonic kidney cells, were cultured in Dulbecco's modified Eagle growth medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, HyClone), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). The cells were maintained at 37 °C with a CO2 concentration of 5%. Next, the expression plasmids with wild-type and mutant minigenes were separately introduced into the cells through transfection. The final concentration of each transfected plasmid was 0.01 μg/μl. Finally, the cells were harvested after an additional period of 48 h.
RT‒PCR and Sanger sequencing
After 48 h of transfection, the HEK-293 T cells were harvested, and total RNA was extracted via the TRIzol method. Subsequently, DNaseI treatment was performed to remove any contaminating DNA. Following that, 800–1000 ng of total RNA was reverse transcribed using the Revert Aid H Minus First Strand cDNA Synthesis Kit as per the manufacturer's instructions. PCRs amplification with specific primers targeting the partial PMFBP1 transcript was then carried out. Finally, Sanger sequencing analysis was conducted on the resulting PCR products.
Results
Clinical findings
On the basis of routine semen analysis (Table 1) and abnormal sperm morphology (Fig. 1), the patient was diagnosed with ASS in accordance with previous studies. Notably, sperm morphology analysis revealed a high prevalence of headless sperm tails (pinhead sperm), a limited quantity of tailless sperm heads, and aberrant connections between the head and tail segments (Fig. 1). The patient's karyotype was determined to be 46; XY, and Y chromosome microdeletion analysis revealed no evident abnormalities. Color ultrasound examination confirmed that the bilateral testes were normal in size without any apparent varicose veins or prostate abnormalities, ruling out other potential causes contributing to infertility.
Fig. 1.
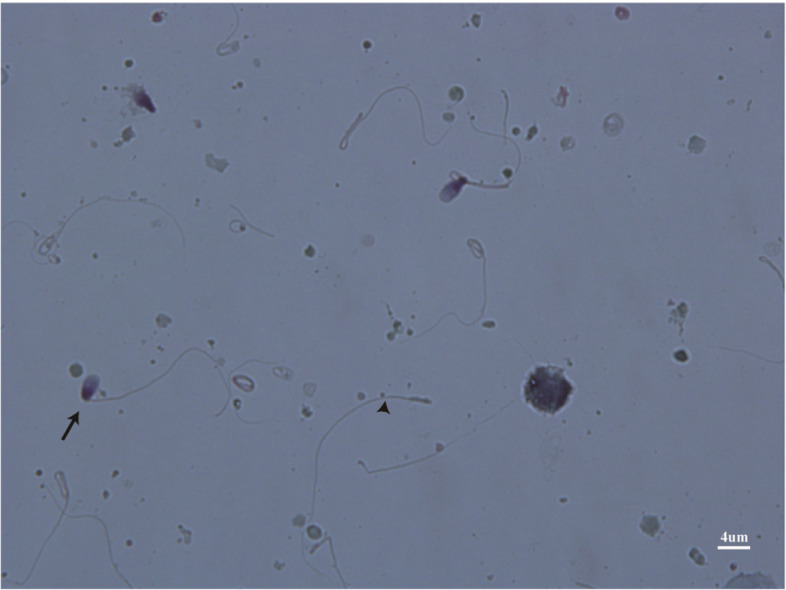
Papanicolaou staining of the spermatozoa from the patient. The sperm morphology analysis revealed a high prevalence of headless sperm tails (pinhead sperm) (black arrowhead), a limited quantity of tailless sperm heads, and aberrant connections between the head and tail segments (black arrow). 100 times oil microscope observation, scale bars = 4 µm
Genetic findings
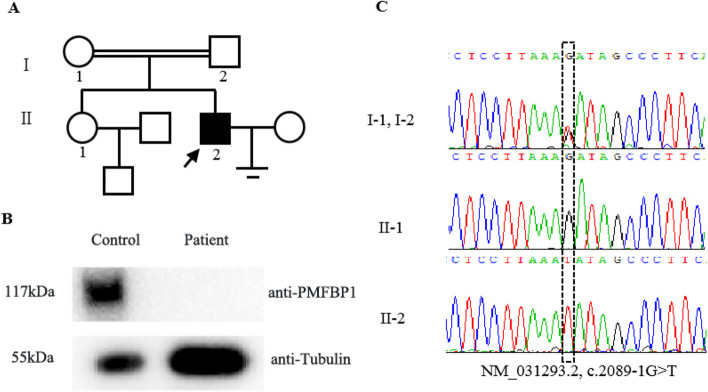
To determine the causative factor of ASS in the patient, DNA was extracted from peripheral blood samples. Previous studies have established that PMFBP1 and SUN5 are the primary genes associated with ASS. Therefore, Sanger sequencing was performed to analyze the coding regions of SUN5 and PMFBP1 in the patient family(patient, patient parents and his sister). No mutations were detected in SUN5. However, Sanger sequencing revealed a homozygous splice site mutation (NM_031293.2, c.2089-1G > T) in PMFBP1 in the patient (Fig. 2C). Furthermore, this splice site mutation was inherited from both parents (Fig. 2A).
Fig. 2.
Pedigrees of a family with inherited PMFBP1 mutations and protein expression of PMFBP1 in the spermatozoa from the patient. A Proband (Π-2) has a homozygous splice site mutation in PMFBP1. B Western blotting was performed to test the expression level of PMFBP1 in the control and patient sperm. Our findings revealed a complete absence of PMFBP1 protein expression in patient sperm, whereas control samples presented detectable levels of the PMFBP1 protein. C Sanger sequencing revealed a homozygous splice site mutation (NM_031293.2, c.2089-1G > T) in PMFBP1 in the patient
Western blotting detected the expression of PMFBP1
To evaluate the impact of splice site mutations on PMFBP1 protein expression, we conducted western blot analysis to assess the presence of the PMFBP1 protein in patient sperm and control samples. Our findings revealed a complete absence of PMFBP1 protein expression in patient sperm, whereas control samples presented detectable levels of the PMFBP1 protein (Fig. 2B).
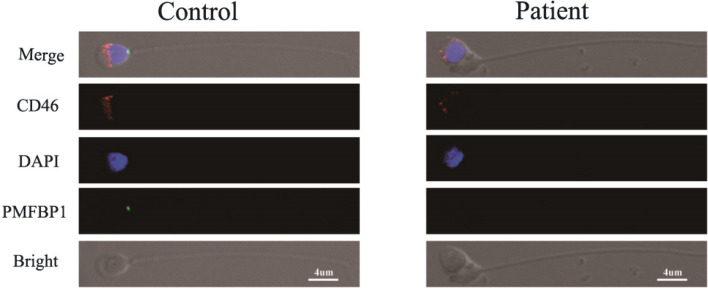
Immunofluorescence localization of the PMFBP1 protein
The localization of PMFBP1 was determined through immunofluorescence analysis. Our findings indicate that the PMFBP1 protein is localized at the head–tail junction of spermatozoa in control samples but is not detected in patient spermatozoa (Fig. 3). The fluorescence patterns observed for CD46 and DAPI, which are used to stain the acrosome and nucleus, respectively, were similar between the control and patient groups (Fig. 3). These results provide further evidence supporting the hypothesis that a splice-site mutation in PMFBP1 leads to an absence of expression of this protein in patients.
Fig. 3.
Immunofluorescence analysis of the spermatozoa from the control and from the patient. The acrosome, nucleus, and neck of the sperm were stained with CD46 (red), DAPI (blue) and PMFBP1 antibodies (green), respectively. The localization of PMFBP1 in sperm from control and patient samples was determined by immunofluorescence staining. The PMFBP1 protein is localized at the head–tail junction of spermatozoa in control samples but is not detected in patient spermatozoa. 40 times oil microscope observation, scale bar = 4 µm
RT‒PCR and Sanger sequencing in vitro
To elucidate the underlying mechanism of the splice site mutation in PMFBP1, we transfected both wild-type and mutant minigenes into HEK-293 T cells. Subsequent RT‒PCR analysis revealed a reduced transcriptional output for the mutant minigenes compared with their wild-type counterparts (Fig. 4C). Furthermore, Sanger sequencing confirmed that the splice-site mutation in PMFBP1 led to a deletion of 4 base pairs within exon 15 (Fig. 4B).
Discussion
ASS is a rare and severe form of male infertility that poses significant challenges for affected individuals and their families [3–9]. In this study, we encountered a primary infertile patient who was diagnosed with ASS through semen analysis and Papanicolaou staining techniques. By performing Sanger sequencing on the DNA sample, we identified a homozygous splice-site mutation (2089-1G > T; NM_031293.2) within the PMFBP1 gene as the likely pathogenic factor responsible for causing ASS in this particular patient.
According to previous studies, PMFBP1 and SUN5 mutations are primarily responsible for ASS [11–15, 18]. The PMFBP1 gene is located on human chromosome 16 and consists of 27 exons. The coding region of the gene spans 3,024 bases and encodes a protein consisting of 1,007 amino acids [25]. PMFBP1 is specifically expressed in both human and mouse testes [12, 26]. It localizes to the junction area between the head and flagella of sperm cells, and its expression is lost in patients carrying PMFBP1 mutations [11, 12]. Functionally, PMFBP1 interacts with SUN5 and SPATA6 to facilitate the connection between the head and tail of sperm cells. In male mice, mutations in PMFBP1 disrupt this cooperation, leading to headless sperm. Therefore, PMFBP1 mutation is believed to play a significant role in acephalospermia, which can cause male infertility [11, 12]. The separation in subtype II is positioned between the nucleus and the proximal centriole [27]. Reproductive success was observed in clinical pregnancies achieved through intracytoplasmic spermine injection (ICSI) methods in individuals with subtype II mutations within PMFBP1 [26, 27].
In our study, we analyzed the protein expression of PMFBP1 in patient samples compared with controls via western blotting. Our results revealed an absence of detectable levels of the PMFBP1 protein in patient sperm, whereas control samples presented positive expression. These findings indicate that the splice site mutation leads to an absence of functional PMFBP1 protein production. Immunofluorescence analysis further confirmed that the splice site mutation resulted in the absence of detectable levels of the PMFPB1 protein.
Furthermore, to further investigate the functional consequences of the splice site mutation in PMFBP1, we constructed minigenes. These minigenes were then transfected into HEK-293 T cells. The results of our experiments revealed that this particular splice-site mutation led to a deletion of 4 base pairs in exon 15 when tested in vitro. This deletion ultimately resulted in an ASS phenotype, which is characterized by certain developmental abnormalities. On the basis of our observations, we hypothesized that this specific mutation introduces a premature stop codon after the translation of six amino acids (NM_031293.2, c.2089-1G > T, p.I697Pfs7*). Importantly, such mutations can have significant implications for protein structure and function. To confirm these findings and gain further insights into the effects of this mutation on mRNA splicing and protein production, we attempted RT‒PCR analysis using sperm mRNA from the studied patients. However, due to the limited availability of sperm heads from this individual, we were unable to extract sufficient sperm mRNA for successful analysis. Despite these limitations, our initial experiments with minigenes provided valuable information regarding the impact of this splice site mutation on PMFBP1 expression. Further studies utilizing alternative approaches or larger sample sizes may be necessary to fully elucidate its role in disease development and progression.
Limitations
Because ASS is rare, our study was limited by the small size of our sample, which can be considered the primary constraint of this study. However, given the rarity of our targeted population, a limited sample size was unavoidable. Another limitation of this study is the inability to obtain sufficient sperm heads from this individual. Furthermore, the inability to detect the expression of sperm mRNA was identified as an additional limitation. Finally, the pathogenesis of ASS caused by different mutation sites is different. However, the mechanism by which specific mutation sites lead to acephalospermia is well established.
Conclusion
Our study identified a homozygous splice-site PMFBP1 mutation in an ASS patient, which led to the removal of 4 base pairs in exon 15 and subsequently resulted in the absence of expression of the PMFBP1 protein. Our findings add to the growing body of evidence supporting the involvement of PMFBP1 in male infertility disorders such as ASS. Understanding how this mutation affects sperm development at both the cellular and molecular levels may provide valuable insights into potential therapeutic targets or interventions aimed at improving fertility outcomes for affected individuals.
Acknowledgements
We would like to sincerely thank the patients and their families for their participation.
Abbreviations
- ACTRT1
Actin-related protein T1
- ASS
Acephalic spermatozoa syndrome
- BRDT
Bromodomain testis associated
- DAPI
4′, 6-Diamidino-2-phenylindole
- DNAH6
Dynein axonemal heavy chain 6
- HOOK1
Hook microtubule tethering protein 1
- ICSI
Intracytoplasmic sperm injection
- HEK293T
Human embryonic kidney cells
- PMFBP1
Polyamine modulated factor 1 binding protein 1
- SUN5
Sad1 and UNC84 domain containing 5
- SPATC1L
Spermatogenesis and centriole-associated 1 like
- SPATA20
Spermatogenesis-associated gene 20
- SPATA6
Spermatogenesis-associated gene 6
- TSGA10
Testis-specific gene antigen 10
Authors’ contributions
HQ.X. and LY.L. conceived and designed the experiments. WY.M., T.W. and J.Z. collected the samples. HQ.X., KL.Y. and T.W. performed the experiments. HQ.X. and LY.L. analyzed the data. HQ.X. and LY.L. wrote the article. All the authors read and approved the final article.
Funding
Hunan Province Natural Science Foundation (Grant/Award Number:2024JJ9553) supported our study.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Zhuzhou Hospital Affiliated to Xiangya School of Medicine, CSU, (Permit Number: KY2024094-01).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–26. 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perotti ME, Giarola A, Gioria M. Ultrastructural study of the decapitated sperm defect in an infertile man. J Reprod Fertil. 1981;63(2):543–9. 10.1530/jrf.0.0630543. [DOI] [PubMed] [Google Scholar]
- 4.Baccetti B, Selmi MG, Soldani P. Morphogenesis of ‘decapitated’ spermatozoa in a man. J Reprod Fertil. 1984;70(2):395–7. 10.1530/jrf.0.0700395. [DOI] [PubMed] [Google Scholar]
- 5.Chemes HE, Puigdomenech ET, Carizza C, Olmedo SB, Zanchetti F, Hermes R. Acephalic spermatozoa and abnormal development of the head-neck attachment: a human syndrome of genetic origin. Hum Reprod. 1999;14(7):1811–8. 10.1093/humrep/14.7.1811. [DOI] [PubMed] [Google Scholar]
- 6.Toyama Y, Iwamoto T, Yajima M, Baba K, Yuasa S. Decapitated and decaudated spermatozoa in man, and pathogenesis based on the ultrastructure. Int J Androl. 2000;23(2):109–15. 10.1046/j.1365-2605.2000.t01-1-00217.x. [DOI] [PubMed] [Google Scholar]
- 7.Chemes HE, Carizza C, Scarinci F, Brugo S, Neuspiller N, Schwarsztein L. Lack of a head in human spermatozoa from sterile patients: a syndrome associated with impaired fertilization. Fertil Steril. 1987;47(2):310–6. 10.1016/s0015-0282(16)50011-9. [DOI] [PubMed] [Google Scholar]
- 8.Baccetti B, Burrini AG, Collodel G, Magnano AR, Piomboni P, Renieri T, et al. Morphogenesis of the decapitated and decaudated sperm defect in two brothers. Gamete Res. 1989;23(2):181–8. 10.1002/mrd.1120230205. [DOI] [PubMed] [Google Scholar]
- 9.Porcu G, Mercier G, Boyer P, Achard V, Banet J, Vasserot M, et al. Pregnancies after ICSI using sperm with abnormal head-tail junction from two brothers: case report. Hum Reprod. 2003;18(3):562–7. 10.1093/humrep/deg121. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Sha YW, Xu X, Mei LB, Qiu PP, Ji ZY, et al. DNAH6 is a novel candidate gene associated with sperm head anomaly. Andrologia. 2018;e12953. 10.1111/and.12953 [DOI] [PubMed]
- 11.Sha YW, Wang X, Xu X, Ding L, Liu WS, Li P, et al. Biallelic mutations in PMFBP1 cause acephalic spermatozoa. Clin Genet. 2019;95(2):277–86. 10.1111/cge.13461. [DOI] [PubMed] [Google Scholar]
- 12.Zhu F, Liu C, Wang F, Yang X, Zhang J, Wu H. Mutations in PMFBP1 cause acephalic spermatozoa syndrome. Am J Hum Genet. 2018;103(2):188–99. 10.1016/j.ajhg.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu F, Wang F, Yang X, Zhang J, Wu H, Zhang Z. Biallelic SUN5 mutations cause autosomal-recessive acephalic spermatozoa syndrome. Am J Hum Genet. 2016;99(4):942–9. 10.1016/j.ajhg.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sha YW, Xu X, Ji ZY, Lin SB, Wang X, et al. Genetic contribution of SUN5 mutations to acephalic spermatozoa in Fujian China. Gene. 2018;647:221–5. 10.1016/j.gene.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Zhang J, Zhu F, Yang X, Cui Y, Liu J. Patients with acephalic spermatozoa syndrome linked to SUN5 mutations have a favorable pregnancy outcome from ICSI. Hum Reprod. 2018;33(3):372–7. 10.1093/humrep/dex382. [DOI] [PubMed] [Google Scholar]
- 16.Sha YW, Sha YK, Ji ZY, Mei LB, Ding L, et al. TSGA10 is a recurrent candidate gene associated with acephalic spermatozoa. Clin Genet. 2018;93(4):776–83. 10.1111/cge.13140. [DOI] [PubMed] [Google Scholar]
- 17.Ye Y, Wei X, Sha Y, Li N, Yan X, Cheng L, et al. Loss-of-function mutation in TSGA10 causes acephalic spermatozoa phenotype in human. Mol Genet Genomic Med. 2020;8(7):e1284. 10.1002/mgg3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G, Wang N, Zhang H, Yin S, Dai H, Lin G, Li WN. Recurrent mutations in PMFBP1, TSGA10 and SUN5: expanding the spectrum of mutations that may cause acephalic spermatozoa. Clin Genet. 2020;97(6):938–9. 10.1111/cge.13747. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Sha Y, Wang X, Li P, Wang J, Kee K, Wang BB. Whole-exome sequencing identified a homozygous BRDT mutation in a patient with acephalic spermatozoa. Oncotarget. 2017;8(12):19914–22. 10.18632/oncotarget.15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Zhu Y, Zhu Z, Zhi E, Lu K, Wang X, et al. Detection of heterozygous mutation in hook microtubule-tethering protein 1 in three patients with decapitated and decaudated spermatozoa syndrome. J Med Genet. 2018;55(3):150–7. 10.1136/jmedgenet-2016-104404. [DOI] [PubMed] [Google Scholar]
- 21.Sha Y, Liu W, Li L, et al. Pathogenic variants in ACTRT1 cause acephalic spermatozoa syndrome. Front Cell Dev Biol. 2021;9:676246. 10.3389/fcell.2021.676246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YZ, Li N, Liu WS, et al. Biallelic mutations in spermatogenesis and centriole-associated 1 like (SPATC1L) cause acephalic spermatozoa syndrome and male infertility. Asian J Androl. 2021;23:1–6. 10.4103/aja.aja5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Jiang C, Dai S, et al. Identification of nonfunctional SPATA20 causing acephalic spermatozoa syndrome in humans. Clin Genet. 2023;103(3):310–9. 10.1111/cge.14268. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. WHo laboratory manual for the examination and processing of human semen[M]. 16th ed. Geneva: WHO Press; 2121. [Google Scholar]
- 25.Wu C, Jin X, Tsueng G, et al. BioGPS: Building your own mash-up of gene annotations and expression profiles. Nucleic Acids Res. 2016;44(D1):D313–6. 10.1093/nar/gkv1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohuchi J, Arai T, Kon Y, Asano A, Yamauchi H, Watanabe T. Characterization of a recurrent gene sperm-tail-associated protein (Stap) in mouse post-meiotic testicular germ cells. Mol Reprod Dev. 2001;59(4):350–8. 10.1002/mrd.1041. [DOI] [PubMed] [Google Scholar]
- 27.Nie H, Tang YG, Qin WB. Beyond Acephalic Spermatozoa: The Complexity of Intracytoplasmic Sperm Injection Outcomes. Biomed Res Int. 2020;2020:6279795. 10.1155/2020/6279795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.