Abstract
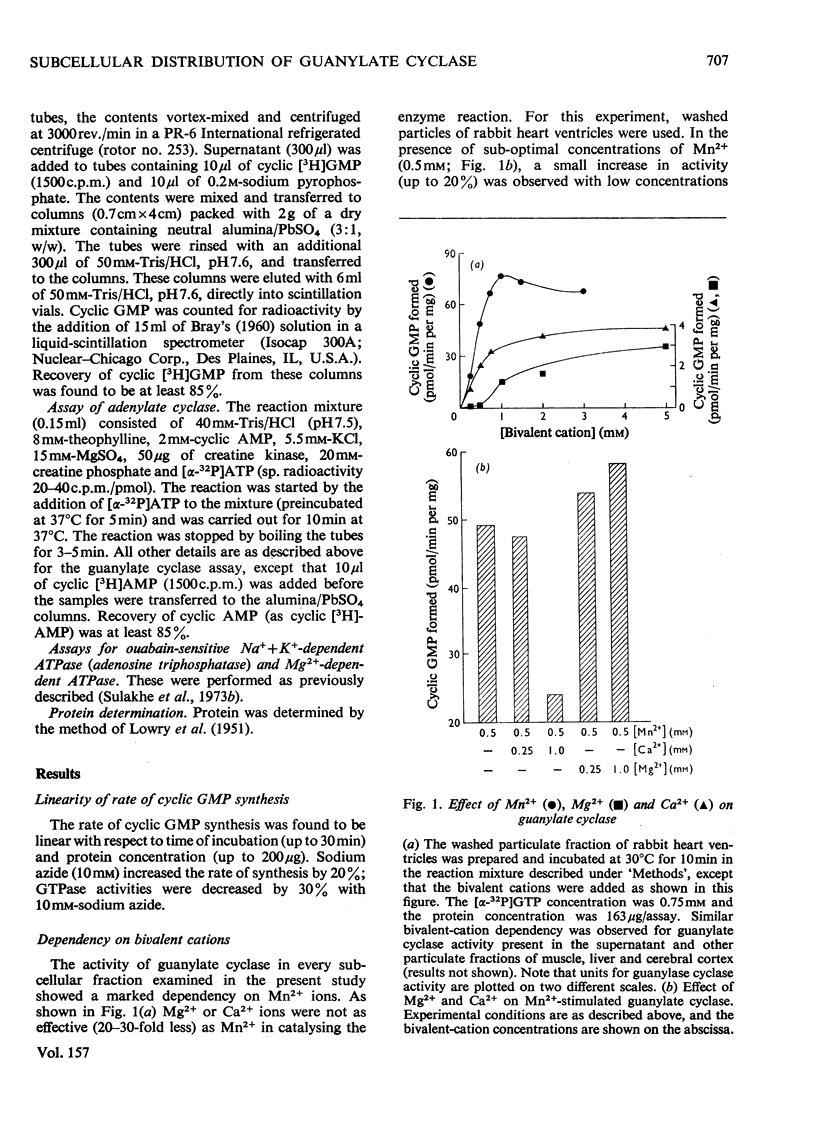
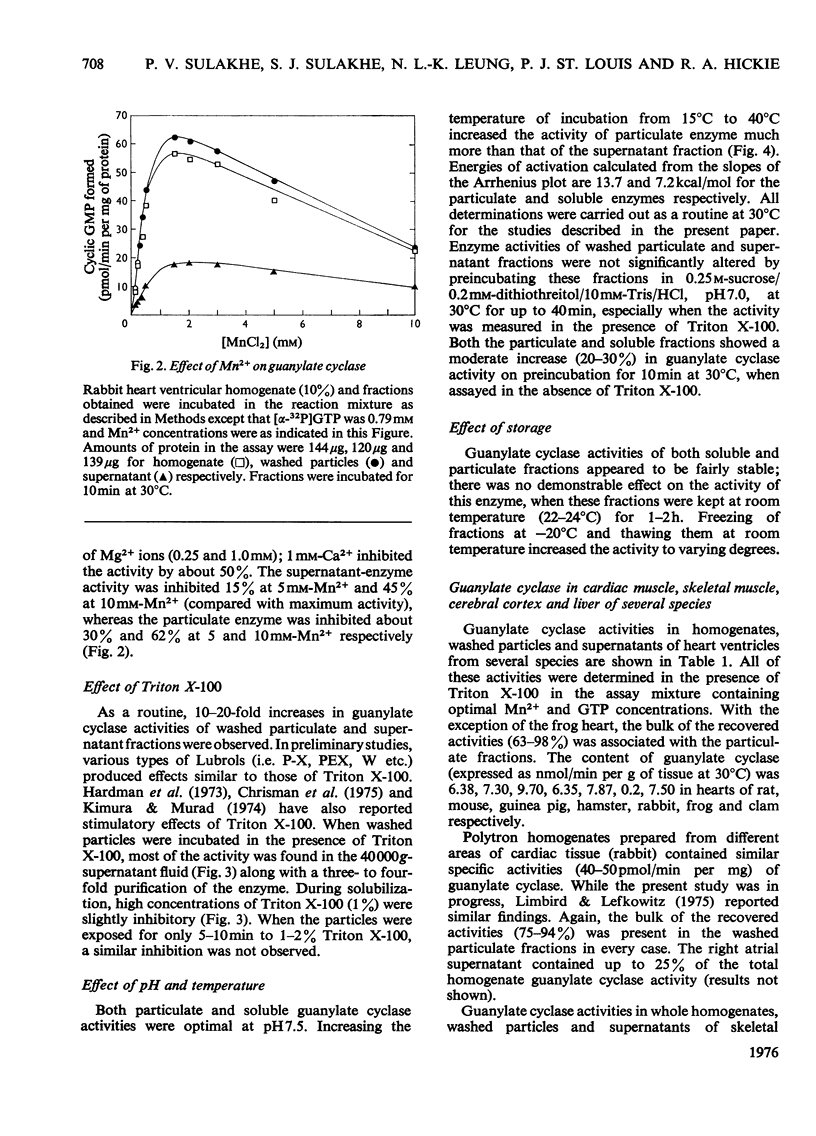
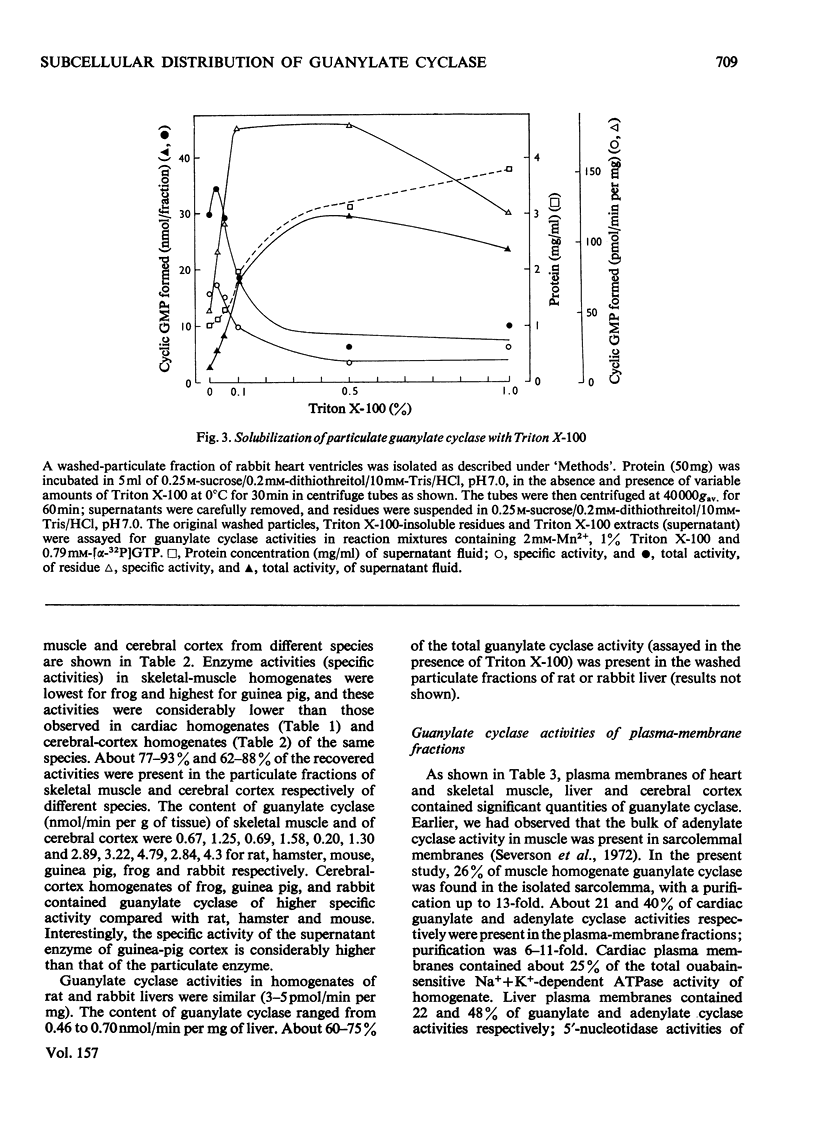
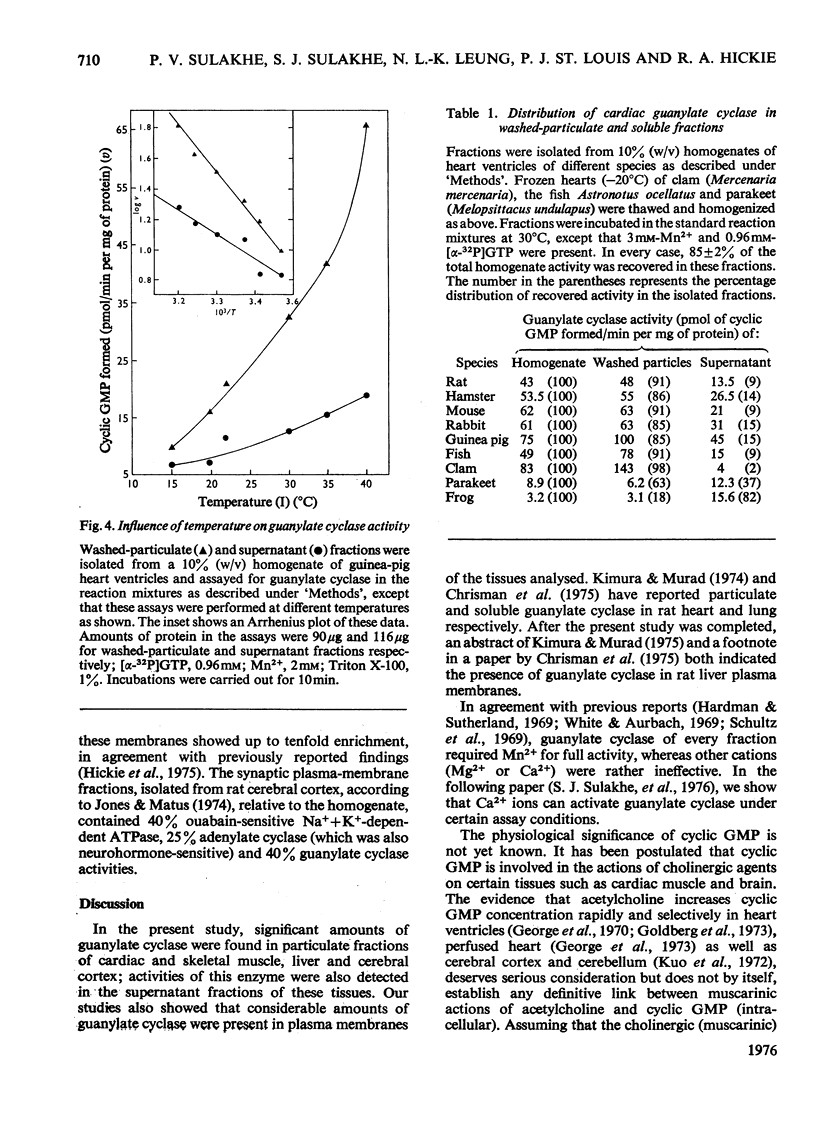
1. Guanylate cyclase of every fraction studied showed an absolute requirement for Mn2+ ions for optimal activity; with Mg2+ or Ca2+ reaction was barely detectable. Triton X-100 stimulated the particulate enzyme much more than the supernatant enzyme and solubilized the particulate-enzyme activity. 2. Substantial amounts of guanylate cyclase were recovered with the washed particulate fractions of cardiac muscle (63-98%), skeletal muscle (77-93%), cerebral cortex (62-88%) and liver (60-75%) of various species. The supernatants of these tissues contained 7-38% of total activities. In frog heart, the bulk of guanylate cyclase was present in the supernatant fluid. 3. Plasma-membrane fractions contained 26, 21, 22 and 40% respectively of the total homogenate guanylate cyclase activities present in skeletal muscle (rabbit), cardiac muscle (guinea pig), liver (rat) and cerebral cortex (rat). In each case, the specific activity of this enzyme in plasma membranes showed a five- to ten-fold enrichment when compared with homogenate specific activity. 4. These results suggest that guanylate cyclase, like adenylate cyclase, and ouabain-sensitive Na+ + K+-dependent ATPase (adenosine triphosphatase), is associated with the surface membranes of cardiac muscle, skeletal muscle, liver and cerebral cortex; however, considerable activities are also present in the supernatant fractions of these tissues which contain very little adenylate cyclase or ouabain-sensitive Na+ + K+-dependent ATPase activities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartfai T., Anner J., Schultzberg M., Montelius J. Partial purification and characterization of a muscarinic acetylcholine receptor from rat cerebral cortex. Biochem Biophys Res Commun. 1974 Jul 24;59(2):725–733. doi: 10.1016/s0006-291x(74)80040-9. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Chrisman T. D., Garbers D. L., Parks M. A., Hardman J. G. Characterization of particulate and soluble guanylate cyclases from rat lung. J Biol Chem. 1975 Jan 25;250(2):374–381. [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Wilkerson R. D., Kadowitz P. J. Influence of acetylcholine on contractile force and cyclic nucleotide levels in the isolated perfused rat heart. J Pharmacol Exp Ther. 1973 Jan;184(1):228–235. [PubMed] [Google Scholar]
- Goldberg N. D., O'Dea R. F., Haddox M. K. Cyclic GMP. Adv Cyclic Nucleotide Res. 1973;3:155–223. [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Hardman J. G., Sutherland E. W. Guanyl cyclase, an enzyme catalyzing the formation of guanosine 3',5'-monophosphate from guanosine trihosphate. J Biol Chem. 1969 Dec 10;244(23):6363–6370. [PubMed] [Google Scholar]
- Hickie R. A., Jan S. H., Datta A. Comparative adenylate cyclase activities in homogenate and plasma membrane fractions of Morris hepatoma 5123tc (h). Cancer Res. 1975 Mar;35(3):596–600. [PubMed] [Google Scholar]
- Ishikawa E., Ishikawa S., Davis J. W., Sutherland E. W. Determination of guanosine 3',5'-monophosphate in tissues and of guanyl cyclase in rat intestine. J Biol Chem. 1969 Dec 10;244(23):6371–6376. [PubMed] [Google Scholar]
- Jones D. H., Matus A. I. Isolation of synaptic plasma membrane from brain by combined flotation-sedimentation density gradient centrifugation. Biochim Biophys Acta. 1974 Aug 9;356(3):276–287. doi: 10.1016/0005-2736(74)90268-5. [DOI] [PubMed] [Google Scholar]
- Kimura H., Murad F. Evidence for two different forms of guanylate cyclase in rat heart. J Biol Chem. 1974 Nov 10;249(21):6910–6916. [PubMed] [Google Scholar]
- Kimura H., Murad F. Localization of particulate guanylate cyclase in plasma membranes and microsomes of rat liver. J Biol Chem. 1975 Jun 25;250(12):4810–4817. [PubMed] [Google Scholar]
- Kuo J. F., Lee T. P., Reyes P. L., Walton K. G., Donnelly T. E., Jr, Greengard P. Cyclic nucleotide-dependent protein kinases. X. An assay method for the measurement of quanosine 3',5'-monophosphate in various biological materials and a study of agents regulating its levels in heart and brain. J Biol Chem. 1972 Jan 10;247(1):16–22. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Myocardial guanylate cyclase: properties of the enzyme and effects of cholinergic agonists in vitro. Biochim Biophys Acta. 1975 Jan 23;377(1):186–196. doi: 10.1016/0005-2744(75)90299-5. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Schultz G., Böhme E., Munske K. Guanyl cyclase. Determination of enzyme activity. Life Sci. 1969 Dec 15;8(24):1323–1332. doi: 10.1016/0024-3205(69)90189-1. [DOI] [PubMed] [Google Scholar]
- Schultz G., Jakobs K. H., Böhme E., Schultz K. Einfluss verschiedener Hormone auf die Bildung von Adenosin-3':5'-monophosphat und Guanosin-3':5'-monophosphat durch partikuläre Präparationen aus der Rattenniere. Eur J Biochem. 1972 Jan 21;24(3):520–529. doi: 10.1111/j.1432-1033.1972.tb19714.x. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Drummond G. I., Sulakhe P. V. Adenylate cyclase in skeletal muscle. Kinetic properties and hormonal stimulation. J Biol Chem. 1972 May 10;247(9):2949–2958. [PubMed] [Google Scholar]
- Sulakhe P. V., Drummond G. I., Ng D. C. Adenosine triphosphatase activities of muscle sarcolemma. J Biol Chem. 1973 Jun 25;248(12):4158–4162. [PubMed] [Google Scholar]
- Sulakhe P. V., Drummond G. I., Ng D. C. Calcium binding by skeletal muscle sarcolemma. J Biol Chem. 1973 Jun 25;248(12):4150–4157. [PubMed] [Google Scholar]
- Sulakhe P. V., Leung N. L., St Louis P. J. Stimulation of calcium accumulation in cardiac sarcolemma by protein kinase. Can J Biochem. 1976 May;54(5):438–445. doi: 10.1139/o76-063. [DOI] [PubMed] [Google Scholar]
- Sulakhe S. J., Leung N. L., Sulakhe P. V. Properties of particulate, membrane-associated and soluble guanylate cyclase from cardiac muscle, skeletal muscle, cerebral cortex and liver. Biochem J. 1976 Sep 1;157(3):713–719. doi: 10.1042/bj1570713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. A., Aurbach G. D. Detection of guanyl cyclase in mammalian tissues. Biochim Biophys Acta. 1969;191(3):686–697. doi: 10.1016/0005-2744(69)90362-3. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Muscarinic cholinergic binding in rat brain. Proc Natl Acad Sci U S A. 1974 May;71(5):1725–1729. doi: 10.1073/pnas.71.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]


