Abstract
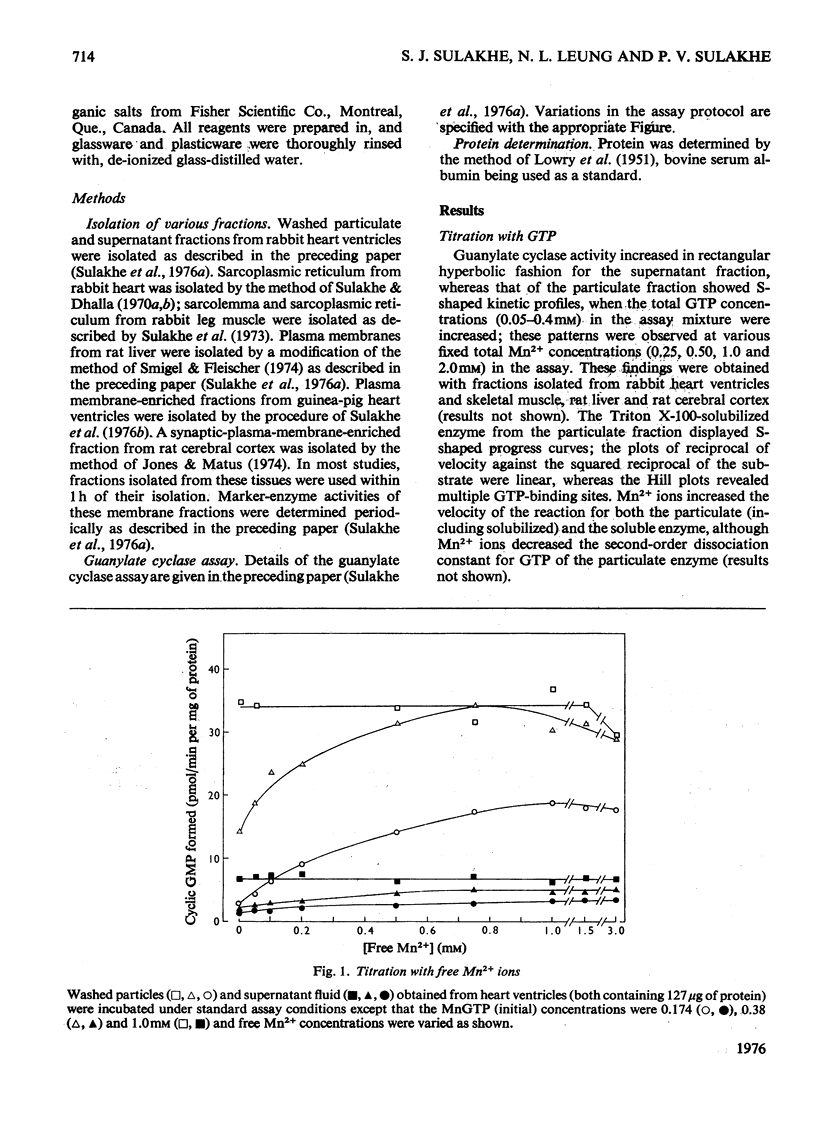
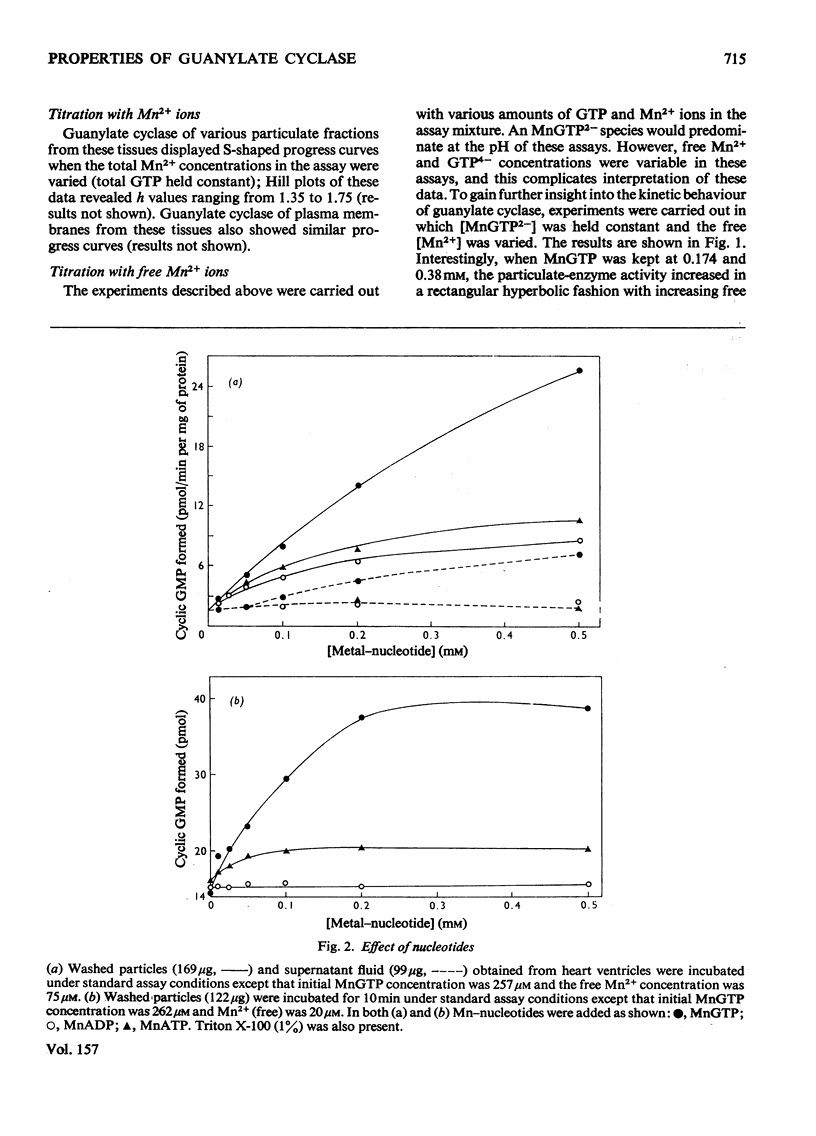
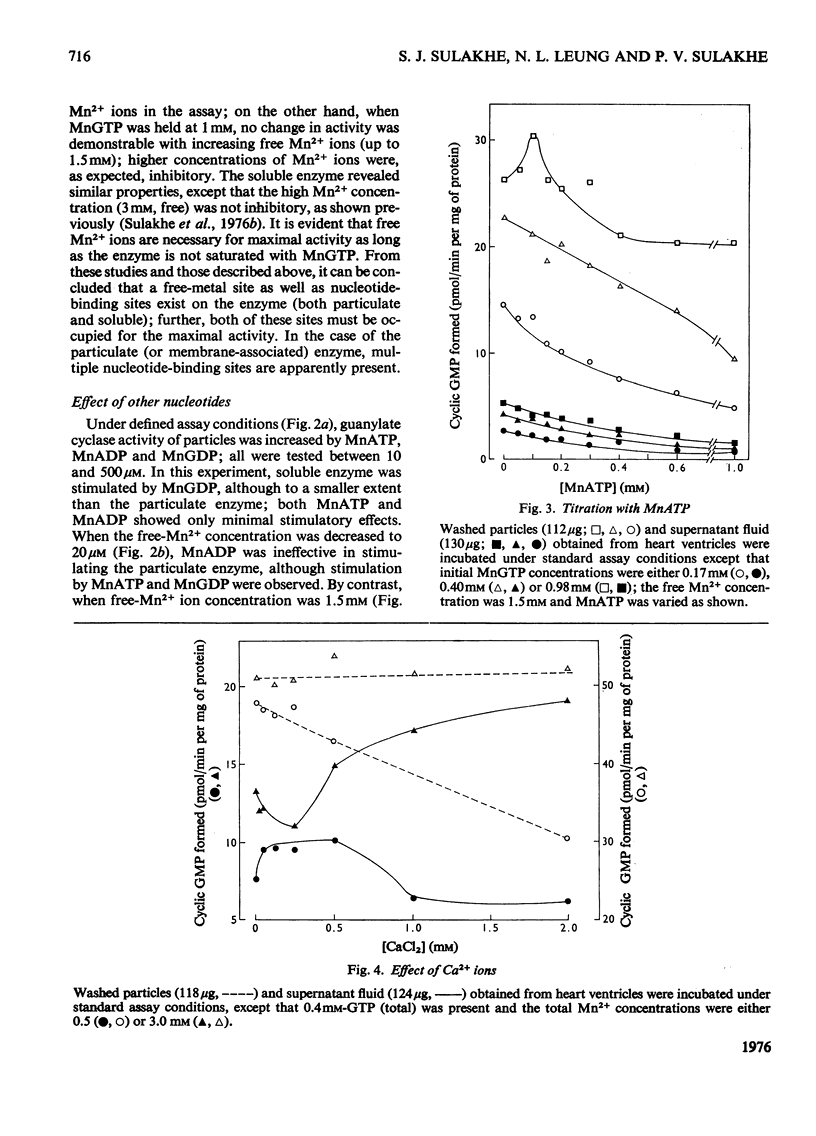
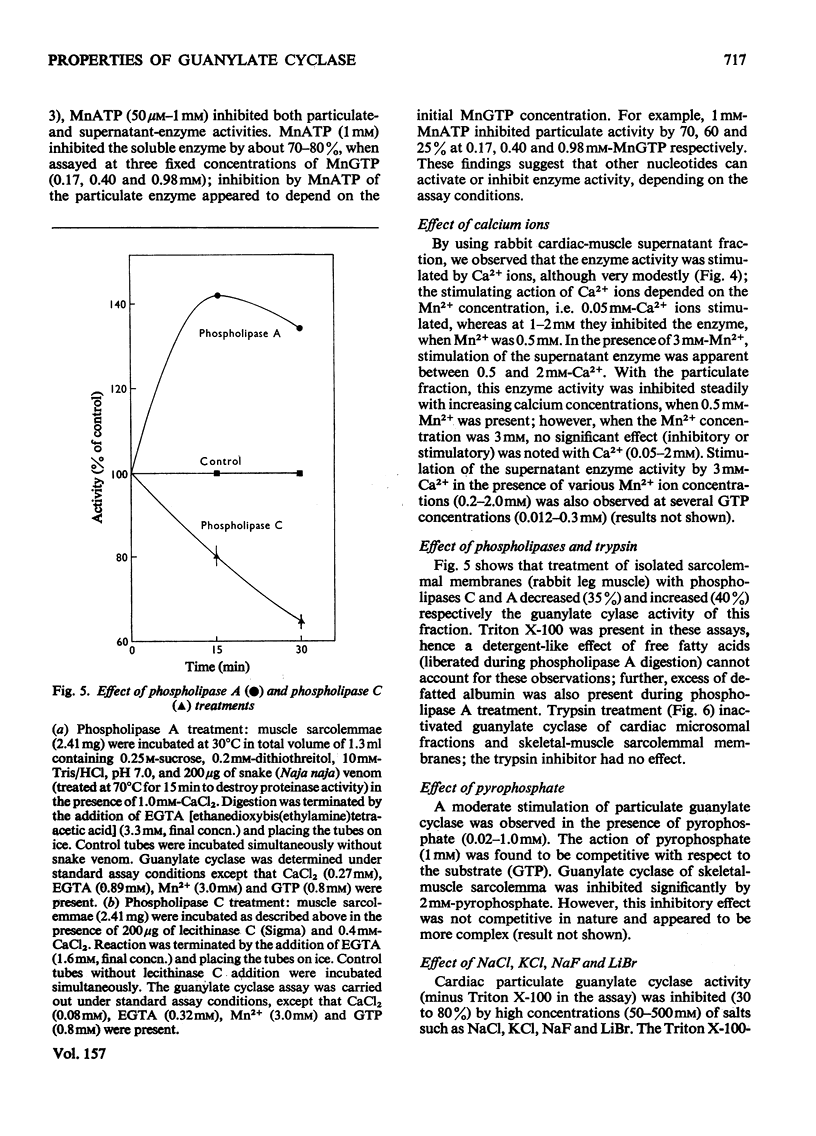
1. Guanylate cyclase of washed particles and plasma membranes showed S-shaped progress curves when titrated with either GTP or Mn2+ ions; similar results were obtained with Triton X-100-solubilized enzyme preparation from washed particles. Hill plots of these data revealed multiple metal-nucleotide and free-metal binding sites. 2. Guanylate cyclase of supernatant fractions displayed typical Michaelis-Menten properties when enzyme required excess of (free) Mn2+ (over GTP) for maximal activities; Ka (free Mn2+) was about 0.15-0.25 mM at subsaturating concentrations of GTP. 4 MnATP, MnADP, and MnGDP were found to increase the activities of both particulate and superantant enzyme, when MnGTP concentration was below saturation and free Mn2+ ion concentration was low (less than 100 muM); MnATP (50muM-1 mM) inhibited both these activities at high free Mn2+ concentration (1.5 mM) and inhibition of the particulate enzyme was greater than that of supernatant enzyme. 5. Ca2+ ions stimulated supernatant-enzyme activity; the stimulatory concentration of Ca2+ ions depended on the concentration of Mn2+ and GTP. 6. A modest stimulation of particulate guanylate cyclase by pyrophosphate (0.02-1 mM) was observed; the pyrophosphate effect appeared to be competitive with respect to GTP. At a higher concentration (2 mM), pyrophosphate produced a marked inhibition of particulate enzyme; the nature of inhibitory effect appeared complex. 7. Inorganic salts (e.g. NaCl, KCl, LiBr, NaF) produced inhibition of particulate enzyme; the degree of inhibition of Triton X-100-stimulated activity was less than that of unstimulated activity. 9. Treatment of sarcolemmal or microsomal membranes with either phospholipase C or trypsin decreased, whereas phospholipase A increased, the activity of guanylate cyclase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrisman T. D., Garbers D. L., Parks M. A., Hardman J. G. Characterization of particulate and soluble guanylate cyclases from rat lung. J Biol Chem. 1975 Jan 25;250(2):374–381. [PubMed] [Google Scholar]
- Dousa T. P. Effect of renal medullary solutes on vasopressin-sensitive adenyl cyclase. Am J Physiol. 1972 Mar;222(3):657–662. doi: 10.1152/ajplegacy.1972.222.3.657. [DOI] [PubMed] [Google Scholar]
- Dousa T., Hechter O. The effect of NaCl and LiCl on vasopressin-sensitive adenyl cyclase. Life Sci I. 1970 Jul 1;9(13):765–770. doi: 10.1016/0024-3205(70)90286-9. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Chrisman T. D., Suddath J. L., Hardman J. G. Formation of pyrophosphate by soluble guanylate cyclase from rat lung. Arch Biochem Biophys. 1975 Jan;166(1):135–138. doi: 10.1016/0003-9861(75)90372-0. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Dyer E. L., Hardman J. G. Effects of cations on guanylate cyclase of sea urchin sperm. J Biol Chem. 1975 Jan 25;250(2):382–387. [PubMed] [Google Scholar]
- Jones D. H., Matus A. I. Isolation of synaptic plasma membrane from brain by combined flotation-sedimentation density gradient centrifugation. Biochim Biophys Acta. 1974 Aug 9;356(3):276–287. doi: 10.1016/0005-2736(74)90268-5. [DOI] [PubMed] [Google Scholar]
- Kimura H., Murad F. Evidence for two different forms of guanylate cyclase in rat heart. J Biol Chem. 1974 Nov 10;249(21):6910–6916. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Myocardial guanylate cyclase: properties of the enzyme and effects of cholinergic agonists in vitro. Biochim Biophys Acta. 1975 Jan 23;377(1):186–196. doi: 10.1016/0005-2744(75)90299-5. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Krans H. M., Kozyreff V., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. VI. Evidence for a role of membrane lipids. J Biol Chem. 1971 Jul 25;246(14):4447–4454. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y., Londos C., Harwood J. P., Martin B. R., Rendell M., Berman M. Role of adenine and guanine nucleotides in the activity and response of adenylate cyclase systems to hormones: evidence for multisite transition states. Adv Cyclic Nucleotide Res. 1975;5:3–29. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Drummond G. I., Sulakhe P. V. Adenylate cyclase in skeletal muscle. Kinetic properties and hormonal stimulation. J Biol Chem. 1972 May 10;247(9):2949–2958. [PubMed] [Google Scholar]
- Sulakhe P. V., Dhalia N. S. Excitation-contraction coupling in heart. VII. Calcium accumulation in subcellular particles in congestive heart failure. J Clin Invest. 1971 May;50(5):1019–1027. doi: 10.1172/JCI106573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulakhe P. V., Dhalla N. S. Adenylate cyclase of heart sarcotubular membranes. Biochim Biophys Acta. 1973 Feb 15;293(2):379–396. doi: 10.1016/0005-2744(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Sulakhe P. V., Dhalla N. S. Excitation-contraction coupling in heart. 3. Evidence against the involvement of adenosine cyclic 3',5'-monophosphate in calcium transport by sarcotubular vesicles of canine myocardium. Mol Pharmacol. 1970 Nov;6(6):659–666. [PubMed] [Google Scholar]
- Sulakhe P. V., Drummond G. I., Ng D. C. Calcium binding by skeletal muscle sarcolemma. J Biol Chem. 1973 Jun 25;248(12):4150–4157. [PubMed] [Google Scholar]
- Sulakhe P. V., Leung N. L., St Louis P. J. Stimulation of calcium accumulation in cardiac sarcolemma by protein kinase. Can J Biochem. 1976 May;54(5):438–445. doi: 10.1139/o76-063. [DOI] [PubMed] [Google Scholar]
- Sulakhe P. V., Sulakhe S. J., Leung N. L., St Louis P. J., Hickie R. A. Guanylate cyclase. Subcellular distribution in cardiac muscle, skeletal muscle, cerebral cortex and liver. Biochem J. 1976 Sep 1;157(3):705–712. doi: 10.1042/bj1570705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Iorio J. M., Katz A. M. Control of cardiac sarcolemmal adenylate cyclase and sodium, potassium-activated adenosinetriphosphatase activities. Circ Res. 1975 Jan;36(1):8–17. doi: 10.1161/01.res.36.1.8. [DOI] [PubMed] [Google Scholar]


