Abstract
An Artificial Placenta (AP) utilizing extracorporeal life support (ECLS) could protect premature lungs from injury and promote continued development.
Preterm lambs at estimated gestational age (EGA) 114–128d (term=145) were delivered by Caesarian section and managed in one of 3 groups: AP, mechanical ventilation (MV), or tissue control (TC). AP lambs (114d EGA, n=3; 121d, n=5) underwent venovenous (VV)-ECLS with jugular drainage and umbilical vein reinfusion for 7 days, with a fluid-filled, occluded airway. MV lambs (121d, n=5; 128d, n=5) underwent conventional MV until failure or maximum 48 hours. TC lambs (114d, n=3; 121d, n=5; 128d, n=5) were sacrificed at delivery. At the conclusion of each experiment, lungs were procured and sectioned. H&E slides were scored 0–4 in 7 injury categories, which were summed for a total injury score. Slides were also immunostained for platelet-derived growth factor receptor (PDGFR)-α and α-actin; lung development was quantified by the area fraction of double-positive tips of secondary alveolar septa.
Support duration of AP lambs was 163±9 (mean±SD) hours, 4±3 for early MV lambs and 40±6 for late MV lambs. Total injury scores at 121d were 1.7±2.1 for AP vs. 5.5±1.6 for MV (p=0.02). Using immunofluorescence, double-positive tip area fraction at 121d was 0.017±0.011 in AP lungs compared to 0.003±0.003 in MV lungs (p<0.001) and 0.009±0.005 in TC lungs. At 128d, double-positive tip area fraction was 0.012±0.007 in AP lungs compared to 0.004±0.004 in MV lungs (p<0.001) and 0.016±0.009 in TC lungs.
The artificial placenta is protective against lung injury and promotes lung development compared to mechanical ventilation in premature lambs.
Keywords: Artificial Placenta, Lung Injury, Lung Development, Prematurity
Introduction
The Centers for Disease Control reports nearly 500,000 premature births in the US each year. More infants die of preterm-related problems than from any other single cause.(1) Approximately 40,000 of these are extremely low gestational age newborns (ELGANs) defined as <28 weeks estimated gestational age. These infants are at the greatest risk of death and poor long-term outcomes.(2–6)
Complications of prematurity involve virtually every organ system. In particular, respiratory failure and chronic lung disease in ELGANs remain significant problems. Lung disease is the leading cause of early death, long-term hospitalization, and recurrent respiratory disorders in this population.(7) Several strategies have been developed to minimize this trauma including surfactant replacement, inhaled nitric oxide to decrease pulmonary vascular resistance, steroid administration and advanced modes of ventilation.(8, 9) Treatment has recently focused on noninvasive positive pressure ventilation, but the use of invasive mechanical ventilation is often unavoidable and can cause injury to the underdeveloped lungs.
A novel solution to this problem would be to maintain the intrauterine environment, particularly placental gas exchange and fetal circulation, by an extracorporeal artificial placenta (AP). By maintaining the fetal circulation and avoiding mechanical ventilation, an AP would minimize injuries associated with oxygen toxicity and barotrauma. Several investigators have studied this possibility beginning as early as the 1960s and although many of these studies appeared promising, the primary outcome variable was survival. The physiologic effects of the AP on lung development have not been well examined.
We hypothesized that an artificial placenta (AP) based on venovenous extracorporeal life support (VV-ECLS) without mechanical ventilation (MV) would protect the lungs of premature lambs from barotrauma and allow for ongoing lung development compared to MV controls.
Methods
Humane treatment of animal subjects
The sheep in this experiment were treated in compliance with the Guide for Care and Use of Laboratory Animals (US National Institutes of Health publication No. 85–23, National Academy Press, Washington D.C., revised 1996) and all methods were approved by the University of Michigan Institutional Animal Care and Use Committee (protocol 00007211). All animals were monitored continuously over the course of the experiment by laboratory staff trained in the care of both neonatal and adult sheep. All efforts were made to minimize animal suffering and distress, including appropriate administration of narcotics and benzodiazepines.
In addition to the pre-defined endpoints discussed below, humane endpoints were used as well. Criteria for euthanasia were hypotension (defined as mean arterial pressure < 30mmHg) refractory to two vasopressors, or multiple signs of distress (tachycardia, kicking legs, straining to lift head, attempted vocalization) despite exhaustive efforts to correct physiologic derangements and minimize distress. If criteria for humane euthanasia were met, euthanasia was performed within 1–2 minutes. All animals were euthanized using IV injection of 2ml Fatal-Plus (Vortech Pharmaceuticals: Dearborn, MI).
Animal model
Premature lambs of estimated gestational age (EGA) of 114–128 days (term = 145 days) were delivered via Cesarean section. Confirmed pregnant sheep were placed under inhaled isoflurane general anesthesia and a midline incision was used to expose the uterus. The uterus was delivered and a hysterotomy performed to expose the fetus. One of the legs was withdrawn and an intramuscular dose of buprenorphine given for pain. AP lambs also received a dose of atropine to blunt the vagal response caused by neck dissection.
Cannulation and Artificial Placenta support
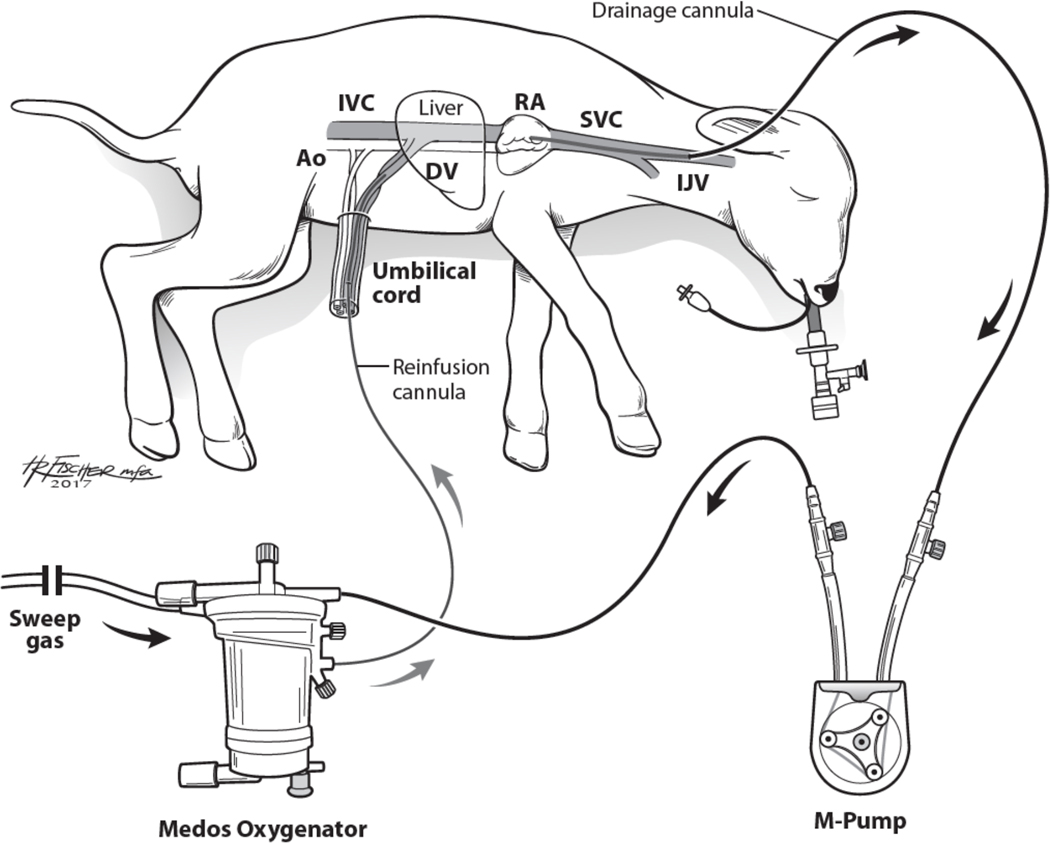
AP lambs were delivered at either 114 (“Early”; n=3) or 121 (“Late”; n=5) days EGA (term=145) and cannulated for VV-ECLS while still on maternal placental support. The internal jugular vein was exposed via open cutdown and cannulated for drainage. A reinfusion cannula was placed in the umbilical vein. Arterial and venous lines were placed in the umbilical artery and the second umbilical vein for hemodynamic monitoring and IV fluid and medication administration. The animals were then intubated, the lungs filled with either amniotic fluid (n=4) or crystalloid (n=4) and the endotracheal tube capped. Earlier experiments used amniotic fluid harvested at the time of delivery, however due to concerns regarding sterility and the amount needed to refill periodically as the experiment duration was consistently longer, we transitioned to using crystalloid. The circuit consisted of a collapsible-tubing roller pump and oxygenator/heat exchanger (either Capiox Baby Rx, Terumo: Ann Arbor MI, or Medos HiLite, Xenios: Heilbronn, Germany), connected by ¼” Tygon tubing, and was primed with crystalloid and maternal red blood cells (Fig 1). Flow was initiated and increased to approximately 100ml/kg/min, then titrated based on gas exchange and hemodynamics. Goal arterial blood gas (ABG) values were pH 7.35–7.45, pO2 25–35mmHg, SaO2 60–75%, and pCO2 35–45mmHg. These values were chosen in an effort to mimic intrauterine blood gas values. Maintaining a low pO2 was also used to help keep the ductus arteriosus patent throughout the experiment. Adequate perfusion was assessed by maintaining hemodynamic stability with lactate < 2mmol/L. Lambs received prostaglandin infusions to maintain a patent ductus arteriosus. All lambs also received IV fluids, parenteral nutrition, prophylactic antibiotics and methylprednisolone at scheduled intervals. Heparin was administered IV to maintain an activated clotting time of 180–220 seconds. Vasopressors were used sparingly and temporarily as needed to maintain adequate perfusion. Lambs were supported for a goal of 7 days while resting on clean foam padding. After 7 days of support, they obtained post conceptual ages of 121 and 128 days, which was then comparable to the “early” and “late” mechanical ventilation and tissue control groups, described below.
Fig 1: Artificial Placenta Setup.
Blood is drained from the right jugular vein by a collapsible-tubing roller pump (M-pump, MC3: Ann Arbor, MI) and propelled to an oxygenator/heat exchanger (Medos HiLite, Xenios: Heilbronn, Germany), then returned via an umbilical vein. The second umbilical vein is accessed for IV fluid and medication administration, and an umbilical arterial line is placed for hemodynamic monitoring and blood gas sampling. The lamb is intubated and the lungs remain filled with amniotic fluid by clamping the endotracheal tube. Ao – aorta; DV – Ductus venosus; IJV – internal jugular vein; IVC – inferior vena cava; RA – right atrium; SVC – superior vena cava
Mechanical Ventilation (MV) lambs
Mechanically ventilated (MV) controls were divided into two groups based on gestational age; Early (121 days EGA; n=5) and Late (128 days EGA; n=5). Umbilical arterial and venous catheters were placed for monitoring and medication infusion. They were then intubated, conventional mechanical ventilation (CMV) was initiated, and lambs were placed in an incubated padded isolette. All animals were given exogenous surfactant (Survanta®, AbbVie, North Chicago, IL) via the endotracheal tube and transitioned to high-frequency oscillatory ventilation in the case of refractory hypoxemia. Goal ABG values were pH 7.30–7.45, pO2 40–60mmHg, SaO2 >85% and pCO2 35–45mmHg. Failure of standard mechanical ventilation was defined as the inability to achieve SaO2 >80%. At this point the lambs were changed to more advanced modes of rescue ventilation using high frequency oscillatory ventilation. Permissive hypercapnia was employed as needed to limit peak airway pressures to < 30cmH2O. These values were chosen with the help of a neonatologist in an attempt to provide these lambs with the standard of care used in the Neonatal ICU for premature infants with respiratory failure on mechanical ventilation. Lambs were supported for up to 48 hours, or until lung failure.
Tissue Control (TC) lambs
Tissue controls were divided into Early (121 days, n= 5) and Late (128 days, n=5) groups. They were immediately sacrificed upon delivery without intervention for tissue procurement.
Lung procurement and processing
At the conclusion of each experiment, necropsy was performed. The lungs of animals in all groups were flushed via the pulmonary artery with phosphate buffered saline and ethylenediaminetetraacetic acid (EDTA), followed by 10% formalin. Formalin was then instilled into the trachea to a pressure of 30cm in order to fix the tissue with standard inflation.
A section was taken from the left lower lobe of each animal and placed face down in a tissue cassette using random orientation. Hematoxylin and eosin (H&E) stained slides were analyzed by a single pathologist who was blinded to experimental groups and assigned injury severity scores (10). Injury variables scored were alveolar and interstitial inflammation, alveolar and interstitial hemorrhage, edema, atelectasis, and necrosis. Each variable was scored using a 0- to 4-point scale. No injury scored 0, and a point was added for every 25% of the field identified as injured, up to a score of 4 for 100%. Injury scores in each category were then summed to create a total injury score.
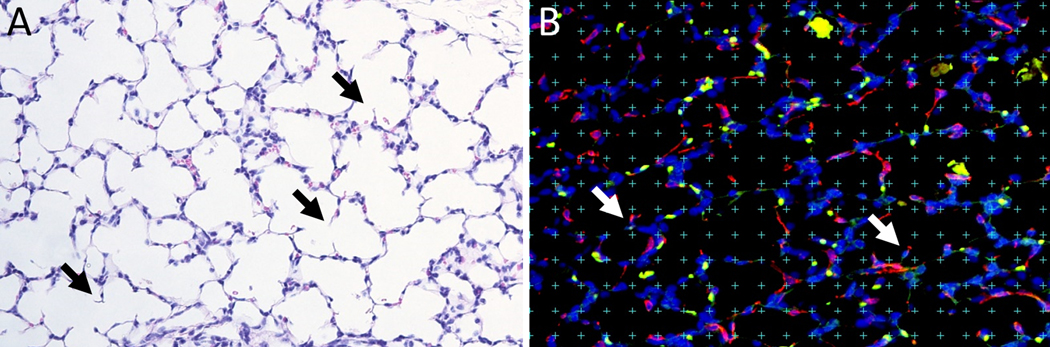
Five-micron sections from each sample were then stained using immunofluorescence for α-smooth muscle actin (anti smooth muscle alpha actin–Cy3 conjugate, Sigma Aldrich, St. Louis, MO) and platelet-derived growth factor receptor-α (PDGFRα; Santa Cruz Biotechnology, Santa Cruz, CA) conjugated to AlexaFluor 488 N-hydroxy succinimidyl ester (ThermoFisher, Waltham, MA). Nuclei were visualized with Hoechst 33342 (Sigma-Aldrich). In normal developing lungs, PDGFRα-expressing cells migrate to the tips of secondary alveolar septa and differentiate into α-actin- and elastin-producing myofibroblasts that are required for alveolar development and gas exchange.(11) Alveolar tips of secondary crests were identified by their characteristic structure as small ridges that extend out from both sides of the primary alveolar septa (Fig 2A). Identification was confirmed using α-actin- and PDGFRα staining.
Fig 2: Methods of assessment of lung alveolarization.
(A) Examples of alveolar secondary crests on 20X H&E-stained slides are identified as ridges extending out from primary alveolar septae (black arrows). (B) Slides were immunostained for α-actin and PDGFRα and viewed at 20X magnification. Tips of alveolar secondary septal crests are again identified (white arrows), and a defined grid was laid over the slide as shown. The total tip area fraction was determined by dividing the number of tips falling on defined grid points by total grid points, whereas the positive tip area fraction was similarly determined by identifying only those tips double-positive for α-actin and PDGFRα.
Alveolarization was quantified using a morphometric technique.(12–14) A 18×13 point grid was randomly overlaid on immunostained lung micrographs using the public domain NIH Image program (developed at the U.S. National Institutes of Health, available at http://rsb.info.nih.gov/nih-image). Five images were obtained at 20x magnification from each slide for counting. The ratio of points falling on the alveolar tips of secondary crests compared to the total number of reference points (area fraction) was calculated. In addition, to verify the identification of alveolar tips, we calculated the area fraction of α-actin- and PDGFRα-positive tips (Fig 2B).
Statistical Analysis
Statistics were performed using GraphPad Prism 7.0. Data were described as mean ± SD. Statistical significance was assessed by one-way analysis of variance (ANOVA). Group differences were pinpointed by Tukey’s multiple comparison compared to MV and TC control lungs using t-test. P values of <0.05 were considered significant.
Results
Support duration and physiologic variables
Average support duration for AP lambs at both gestational ages was 163±9 hours, 4±3 hours for Early MV and 40±6 hours for Late MV lambs (p<0.001). Table 1 displays average hemodynamic and arterial blood gas values. All Early MV lambs (n=5), and one Late MV lamb were euthanized due to meeting humane endpoints (hypotension refractory to multiple pressors). Two AP lambs died suddenly and unexpectedly after six days of support, one due to accidental umbilical cannula dislodgement and the other to cardiac arrest while on vasopressor support. All other AP lambs (n=6) were supported for a planned 7 days and then euthanized, all other Late MV lambs (n=4) were euthanized after 48 hours of MV support, and all Early and Late TC lambs (n=10) were euthanized as planned immediately after delivery.
Table 1:
Average hemodynamic and blood gas values in supported lambs.
| Early Artificial Placenta | Late Artificial Placenta | Early Mechanical Ventilation | Late Mechanical Ventilation | |
|---|---|---|---|---|
|
| ||||
| Support duration (hours) | 161.3±10 | 164.2±9.7 | 4.2±3.0 | 40.2±15.8 |
| MAP (mmHg) | 47.2±5 | 47.8±6.3 | 44.3±6.4 | 46.4±7.0 |
| HR (bpm) | 199.2±28.8 | 199±21.4 | 130.4±6.7 | 182.1±17.1 |
| pH | 7.32±.0.2 | 7.34±0.06 | 7.06±0.18 | 7.25±0.17 |
| pCO2 (mmHg) | 43.7±5.3 | 44.3±3.7 | 135.7±57.4 | 50.0±20.3 |
| pO2 (mmHg) | 38±8.7 | 38±8.7 | 53.8±28.7 | 100.2±41.2 |
| SaO2 (%) | 73.2±1 | 79.4±7.7 | 77.0±12.9 | 88.4±14.5 |
| Lactate (mmol/L) | 2±0.6 | 4.1±2.7 | 5.5±1.0 | 5.4±3.5 |
MAP – mean arterial pressure; HR – heart rate
Lung injury
Early AP lambs displayed better lung inflation and less hemorrhage than Early MV lambs, with lungs appearing similar to Early TC lambs (Fig 3A-C). Similar morphology was observed on H&E-stained lung tissue in Late groups, though the differences were qualitatively less obvious (Fig 3D-F). The average total injury score of Early AP lambs (supported from 114–121 days PCA) was 1.7±2.1, compared to 0±0 for Early TC and 5.5±1.6 for MV (p=0.02). Late AP lambs (supported 121–128 days PCA) had an average total injury score of 6.2±3.5, versus 1.0±1.0 for Late TC and 4.6±2.3 for Late MV. The greatest differences were seen in the categories of alveolar hemorrhage and atelectasis in Early animals. Alveolar hemorrhage scores were 0.3±0.6 for Early AP lambs, versus 1.5±0.5 for Early MV (p=0.006) and 0±0 for Early TC (Fig 3G). Atelectasis scores were 0.3±0.6 for Early AP lambs, compared to 1.0±0.0 for Early MV (p=0.03) and 0±0 for Early TC (Fig 3H). There were no differences in any injury scores between late groups. One Late AP lamb did manifest alveolar and interstitial inflammation scores of 4 and 3, respectively, the result of septic thrombi. The total injury score for this lamb was 11. All other lambs in all groups scored 0 for both inflammation scores.
Fig 3: Histologic assessment of lung injury.
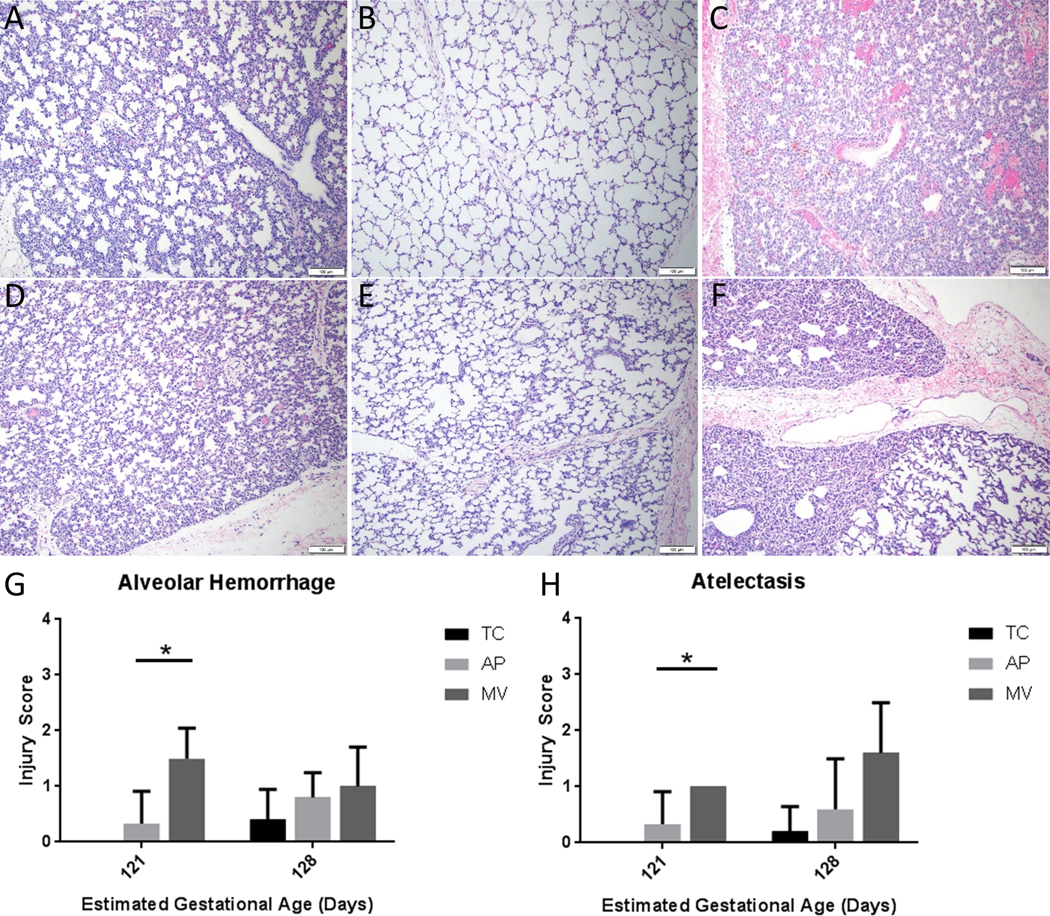
Representative H&E slides of lungs at 10x magnification. Early TC lungs (A) appear qualitatively similar to Early AP lungs, (B) while Early MV lungs, (C) display obvious alveolar hemorrhage and atelectasis. Similar qualitative comparisons can be made between Late TC (D) Late AP, (E) and Late MV, (F) lungs, though the differences do not appear as stark. Other areas of the MV lungs showed larger than normal air spaces (not shown). Early AP lungs displayed lower scores than Early MV lungs in alveolar hemorrhage (G), as well as atelectasis (H) N=5 for all groups, except n=3 for Early AP. Mean ± SD, *p<0.05, ANOVA.
Lung development and immunofluorescence
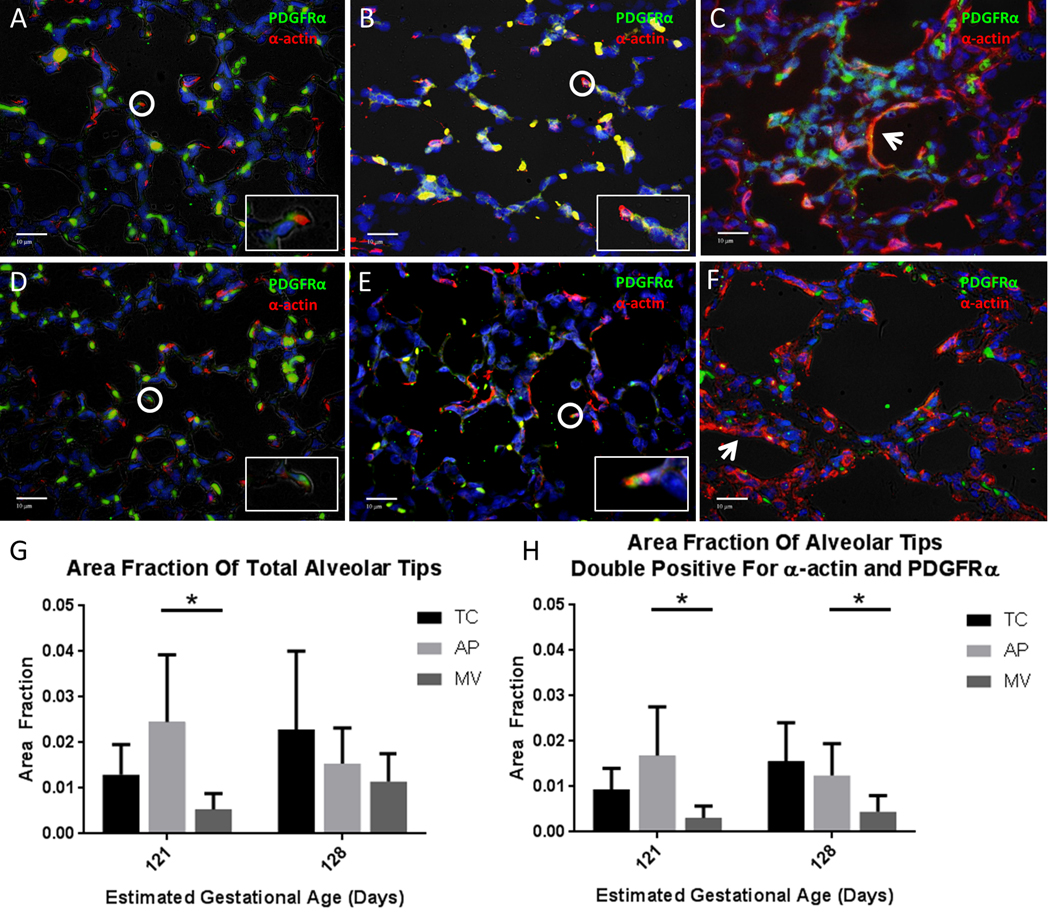
The lungs of AP lambs, both Early and Late, displayed lung saccules with multiple secondary crests indicating alveolarization. Secondary crest alveolar tips showed colocalization of α-actin and PDGFRα, as did Early and Late TC lambs. However, Early MV lambs displayed thickened lung interstitia with α-actin accumulation. In addition, there were fewer, larger air spaces and few secondary alveolar crests. Alveolar crests that were present showed a loss of PDGFRα positive myofibroblasts, resulting in a lack of colocalization of α-actin and PDGFRα in the tips. Similar findings were present in Late MV lambs, though they were less pronounced (Fig 4A-F).
Fig 4: Immunostaining for markers of lung development.
Representative slides of lungs at 40x magnification, immunostained for α-smooth muscle actin (red) and platelet-derived growth factor-α (green). (A) Early TC, (B) Early AP, (C) Early MV, (D) Late TC, (E) Late AP, (F) Late MV. Circles highlight alveolar tips with adjacent α-actin and PDGFRα staining or colocalization of a-smooth muscle actin and PDGFR (orange or yellow), which are enlarged for detail in inset images. Arrows indicate interstitial α-smooth muscle actin. In panels A and B, red blood cells also autofluoresce yellow. (G) Total tip area fractions identified by histologic appearance, and (H) Positive tip area fractions, defined as the ratio of reference points falling on total or double-positive alveolar tips, respectively, divided by the total number of reference points. Area fractions were determined for 5 slides from each sample and then averaged to produce an area fraction for that sample. N=5 for all groups, except n=3 for Early AP. Mean ± SD, *p < 0.05, ANOVA.
In Early groups, the average area fraction of alveolar tips identified by histologic appearance (i.e. “Total area fraction”) was 0.024±0.015 for AP, compared to 0.013±0.007 for TC and 0.005±0.004 for MV (p<0.001). In Late groups, the total tip area fraction was 0.015±0.008 for AP lambs versus 0.023±0.017 for TC lambs and 0.011±0.006 for MV lambs (Fig 4G). In quantifying area fractions of only alveolar tips double-positive for α-actin and PDGFRα, Early AP lungs had an area fraction of 0.017±0.011, compared to 0.009±0.005 for Early TC and 0.003±0.003 for Early MV (p<0.001). In Late groups, AP lungs had a double-positive area fraction of 0.012±0.007, versus 0.016±0.009 for TC and 0.004±0.004 for MV (p<0.001; Fig 3H).
Discussion
Mortality for extremely low gestational age newborns (ELGANs) is still high despite significant improvements in neonatal care. Overall morbidity for those born at less than 28 weeks is 30–50%.(15) Extremely premature infants are especially susceptible to pulmonary morbidity. These complications are caused in part by using the same treatment to sustain premature infants as those born at term. Immature lungs and exposure to mechanical ventilation can lead to long-term lung injury and bronchopulmonary dysplasia (BPD). More than half of ELGANs will have BPD (15, 16), and many develop long-term sequelae. Over 40% of those with BPD as infants report asthma later in life, leading to one of the most common reasons for hospitalization in older survivors.(17) A novel approach to avoiding these pulmonary complications is through the development of an artificial placenta (AP) that utilizes ECLS for gas exchange instead of mechanical ventilation (MV) and maintaining fetal circulation with low pO2 levels. The aim of this study was to evaluate lung protection and development with AP support.
Our results show a clear survival advantage with the use of the AP when compared to MV for lambs of earlier gestational ages. Despite surfactant and advanced ventilator and critical care management, there were no early MV lambs that survived beyond 7 hours. The lung histology shows that even brief exposure to MV results in significant injury. MV lungs show a lack of alveolar maturation and increased injury, especially when evaluating for hemorrhage and atelectasis. Lambs of later gestational ages placed on MV show less damage. It is encouraging to hypothesize that if left on the AP, extremely premature lambs would continue to develop and be better able to tolerate MV at a later gestational age. This is supported by the pattern of alveolar branching and colocalization of α-smooth muscle actin and PDGFRα in the AP lungs that is similar to later-aged gestational controls. Our findings suggest the lungs are continuing to develop while on AP support.
The idea of an artificial placenta utilizing ECLS is not new, with reports of support of premature animals as early as 1969.(18) Work on the AP at our institution has undergone an evolution. The first attempts used the umbilical vessels for cannulation; however, this led to cardiac failure due to high resistance and vasospasm.(19) The current VV approach has demonstrated reproducible long-term survival.(20–22)
When looking specifically at lung development, our choice of the lamb model is well-supported in the literature. Lamb lungs reach the canalicular stage of development between 80 and 120 days gestation.(23) This is when type I pneumocytes form the first thin air-blood barriers and surfactant production begins, making gas exchange possible. In humans this correlates with 17–26 weeks estimated gestational age. Multiple studies on lambs have shown injury to lung parenchyma after even brief exposure to MV, (24, 25) similar to our results with early MV animals. Longer-term studies in lambs placed on MV have shown dysregulation of elastin and development of BPD and chronic lung disease.(26, 27)
Platelet derived growth factor receptor-α (PDGFRα) and α-smooth muscle actin (α-actin) are used as makers of lung development. In normally developing lungs, PDGFRα-expressing cells migrate to the tips of secondary alveolar septa and differentiate into α-actin- and elastin-producing myofibroblasts.(28) These are required for alveolar development and gas exchange. Lungs suffering from BPD or acute lung injury have shown a paucity of PDGFRα and dysregulated α-actin.(29) Our results in MV animals show similar patterns of dysregulated proteins where the AP lungs show normal distribution as well as alveolar branching similar to later aged gestational controls.
We were also able to quantify injury on H&E stained sections of lung. Early MV lungs showed significantly higher scores for atelectasis and hemorrhage than AP lungs. Although all lungs were formalin-distended to a standard pressure at the time of necropsy, distention in MV lungs was heterogenous, with regions of profound atelectasis, potentially due to airway obstruction from hemorrhage. AP lungs were similar to gestational age controls in all categories. It should also be noted that only one AP lamb displayed lung inflammation, which was the result of septic thrombi. This resulted in a total injury score of 11 in this lamb (scores of 4 and 3 for alveolar and interstitial inflammation, respectively), which influenced the mean total injury score of the Late AP group. While this infection was unfortunate, the fact that no other lamb manifested signs of pulmonary infection or inflammation during 7 days of AP support is highly encouraging.
The ideal airway and cannulation strategy for the AP is an area of continued investigation. The EXTEND pumpless model more closely resembles normal fetal development using the umbilical vessels and normal fetal breathing movements while submerged. (30) Our laboratory attempted this technique in the past, but abandoned its pursuit due to infection, poor survival, and the challenges it would pose for clinical translation. Although Partridge et al showed good results in a lamb model, we believe there are significant barriers to clinical translation using this approach. An Ex Utero Intrapartum Treatment (EXIT) procedure would be required to transition the fetus from native placental support to the EXTEND system with no fetal risk stratification apart from gestational age. Premature birth is often precipitous and this approach would require a classical cesarian section incision and would engender significant maternal risk. Our AP system is intended as a rescute therapy for premature infants that are stratified postnatally. As such, we believe our AP has a high potential for clinical translation. Lastly, while there are potential physiologic advantages of a fluid bath, infection, clinical access and family bonding would be a challenge.
The animals in this study underwent a version of tracheal occlusion. Human and animal studies suggest such occlusion requires optimal timing for both initiation and release to promote growth and development of lungs, potentially at a rate even greater than normal development.(31, 32) We acknowledge that the development of the lamb lungs may not be normal using this technique, but potentially accelerated, although this could not be proven on our histology given the relatively short period of time the lambs were supported on the AP. Perfluorocarbons have also been trialed in neonates with congenital diaphragmatic hernia in an attempt to accelerate lung growth.(33) As we move forward with these experiments, alternate methods of airway management are being tested with promising results using perfluorodecalin as the fluid medium.
The fetal lamb model we have created is ideal for studying lung development. Using the knowledge we have gained in these experiments, we expect to be able to transition lambs from the AP to conventional ventilation and finally to air breathing with relative ease after lung development has occurred to a degree that will allow these transitions.
A limitation of this study is the variability in animal gestational age. Although measured as close as possible to conception, we allow for a deviation of three days plus or minus the stated gestational age. At necropsy, inflation of the lungs with formalin is standardized; however, there is the possibility that inter-user variability may have contributed to variability in inflation and atelectasis scores on histologic analysis.
Conclusion
The artificial placenta has the unique ability to allow premature lungs to continue developing in a protective environment. Our data show that with a clamped endotracheal tube and the avoidance of mechanical ventilation, the lungs of seven-day survivors appear to be developing normally when compared to gestational controls. This is in contrast to even brief exposure to mechanical ventilation, which causes a traumatic change in the architecture of the lung parenchyma, potentially leading to long-term pulmonary sequela.
Acknowledgements
The authors would like to thank Lucinda Cooke for her editorial assistance.
Conflicts of Interest and Source of Funding
This work was supported by National Institutes of Health Grant # 1R01HD073475–01A1.
Footnotes
The authors do not have any conflict of interest to disclose.
References
- 1.Sharma S, Abubakar KM, Keszler M. Tidal volume in infants with congenital diaphragmatic hernia supported with conventional mechanical ventilation. Am J Perinatol. 2015;32(6):577–82. [DOI] [PubMed] [Google Scholar]
- 2.Markestad T, Kaaresen PI, Ronnestad A, Reigstad H, Lossius K, Medbo S, et al. Early death, morbidity, and need of treatment among extremely premature infants. Pediatrics. 2005;115(5):1289–98. [DOI] [PubMed] [Google Scholar]
- 3.Preterm Birth: Causes, Consequences, and Prevention. Behrman RE, Butler AS, editors. Washington DC: National Academy of Sciences; 2007. [PubMed] [Google Scholar]
- 4.Laughon M, O’Shea MT, Allred EN, Bose C, Kuban K, Van Marter LJ, et al. Chronic lung disease and developmental delay at 2 years of age in children born before 28 weeks’ gestation. Pediatrics. 2009;124(2):637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early human development. 2009;85(11):719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Washburn LK, Dillard RG, Goldstein DJ, Klinepeter KL, deRegnier RA, O’Shea TM. Survival and major neurodevelopmental impairment in extremely low gestational age newborns born 1990–2000: a retrospective cohort study. BMC pediatrics. 2007;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. The New England journal of medicine. 2015;372(4):331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rimensberger PC. Neonatal respiratory failure. Current opinion in pediatrics. 2002;14(3):315–21. [DOI] [PubMed] [Google Scholar]
- 9.Ventre KM, Arnold JH. High frequency oscillatory ventilation in acute respiratory failure. Paediatric respiratory reviews. 2004;5(4):323–32. [DOI] [PubMed] [Google Scholar]
- 10.Mrozek JD, Smith KM, Bing DR, Meyers PA, Simonton SC, Connett JE, et al. Exogenous surfactant and partial liquid ventilation: physiologic and pathologic effects. American journal of respiratory and critical care medicine. 1997;156(4 Pt 1):1058–65. [DOI] [PubMed] [Google Scholar]
- 11.McGowan SE, Grossmann RE, Kimani PW, Holmes AJ. Platelet-derived growth factor receptor-alpha-expressing cells localize to the alveolar entry ring and have characteristics of myofibroblasts during pulmonary alveolar septal formation. Anatomical record (Hoboken, NJ : 2007). 2008;291(12):1649–61. [DOI] [PubMed] [Google Scholar]
- 12.Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. American journal of respiratory and critical care medicine. 2010;181(4):394–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knudsen L, Weibel ER, Gundersen HJ, Weinstein FV, Ochs M. Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. Journal of applied physiology (Bethesda, Md : 1985). 2010;108(2):412–21. [DOI] [PubMed] [Google Scholar]
- 14.Bolender RP, Hyde DM, Dehoff RT. Lung morphometry: a new generation of tools and experiments for organ, tissue, cell, and molecular biology. The American journal of physiology. 1993;265(6 Pt 1):L521–48. [DOI] [PubMed] [Google Scholar]
- 15.Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesthesia and analgesia. 2015;120(6):1337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ (Clinical research ed). 2012;344:e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zapol WM, Kolobow T, Pierce JG, Bowman RL. Artificial placenta: two days of total extrauterine support of the isolated premature lamb fetus. Science (New York, NY). 1969;166(3905):617–8. [DOI] [PubMed] [Google Scholar]
- 19.Reoma JL, Rojas A, Kim AC, Khouri JS, Boothman E, Brown K, et al. Development of an artificial placenta I: pumpless arterio-venous extracorporeal life support in a neonatal sheep model. Journal of pediatric surgery. 2009;44(1):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray BW, El-Sabbagh A, Rojas-Pena A, Kim AC, Gadepali S, Koch KL, et al. Development of an artificial placenta IV: 24 hour venovenous extracorporeal life support in premature lambs. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2012;58(2):148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray BW, El-Sabbagh A, Zakem SJ, Koch KL, Rojas-Pena A, Owens GE, et al. Development of an artificial placenta V: 70 h veno-venous extracorporeal life support after ventilatory failure in premature lambs. Journal of pediatric surgery. 2013;48(1):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryner B, Gray B, Perkins E, Davis R, Hoffman H, Barks J, et al. An extracorporeal artificial placenta supports extremely premature lambs for 1 week. Journal of pediatric surgery. 2015;50(1):44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinkerton KE, Joad JP. The mammalian respiratory system and critical windows of exposure for children’s health. Environmental health perspectives. 2000;108 Suppl 3:457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinsella JP, Parker TA, Galan H, Sheridan BC, Abman SH. Independent and combined effects of inhaled nitric oxide, liquid perfluorochemical, and high-frequency oscillatory ventilation in premature lambs with respiratory distress syndrome. Chest. 1999;116(1 Suppl):15S–6S. [DOI] [PubMed] [Google Scholar]
- 25.Hillman NH, Nitsos I, Berry C, Pillow JJ, Kallapur SG, Jobe AH. Positive end-expiratory pressure and surfactant decrease lung injury during initiation of ventilation in fetal sheep. American journal of physiology Lung cellular and molecular physiology. 2011;301(5):L712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, et al. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. American journal of physiology Lung cellular and molecular physiology. 2007;292(6):L1370–84. [DOI] [PubMed] [Google Scholar]
- 27.Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, Cho SC, et al. Chronic lung injury in preterm lambs. Disordered respiratory tract development. American journal of respiratory and critical care medicine. 1999;159(3):945–58. [DOI] [PubMed] [Google Scholar]
- 28.Popova AP, Bentley JK, Cui TX, Richardson MN, Linn MJ, Lei J, et al. Reduced platelet-derived growth factor receptor expression is a primary feature of human bronchopulmonary dysplasia. American journal of physiology Lung cellular and molecular physiology. 2014;307(3):L231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leslie KO, Mitchell JJ, Woodcock-Mitchell JL, Low RB. Alpha smooth muscle actin expression in developing and adult human lung. Differentiation; research in biological diversity. 1990;44(2):143–9. [DOI] [PubMed] [Google Scholar]
- 30.Partridge EA, Davey MG, Hornick MA, Flake AW. An EXTrauterine environment for neonatal development: EXTENDING fetal physiology beyond the womb. Seminars in fetal & neonatal medicine. 2017;22(6):404–9. [DOI] [PubMed] [Google Scholar]
- 31.Jelin EB, Etemadi M, Encinas J, Schecter SC, Chapin C, Wu J, et al. Dynamic tracheal occlusion improves lung morphometrics and function in the fetal lamb model of congenital diaphragmatic hernia. Journal of pediatric surgery. 2011;46(6):1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan PA, Cloutier M, Piedboeuf B. Tracheal occlusion: a review of obstructing fetal lungs to make them grow and mature. American journal of medical genetics Part C, Seminars in medical genetics. 2007;145C(2):125–38. [DOI] [PubMed] [Google Scholar]
- 33.Hirschl RB, Philip WF, Glick L, Greenspan J, Smith K, Thompson A, et al. A prospective, randomized pilot trial of perfluorocarbon-induced lung growth in newborns with congenital diaphragmatic hernia. Journal of pediatric surgery. 2003;38(3):283–9; discussion −9. [DOI] [PubMed] [Google Scholar]