Abstract
Background
Achondroplasia, the most prevalent form of skeletal dysplasia involving short stature, necessitates a multidisciplinary approach that includes otology and auditory rehabilitation. Despite this, the clinical characteristics of hearing loss and otologic manifestations in achondroplasia patients remain poorly defined. This study aimed to explore the prevalence and treatment outcomes of otologic disease in individuals with achondroplasia.
Methods
A retrospective review of medical records was conducted for 70 patients who visited the otolaryngology clinic at 3 institutions in South Korea from 1999 to 2023. Demographic and clinical characteristics, including audiometric findings, imaging studies, treatment modalities, and outcomes, were analyzed.
Results
Among 53 patients who underwent audiometry, 26 showed conductive hearing loss, 2 had mixed-type hearing loss, and 4 had sensorineural hearing loss. Fifty-one patients (72.9%) had middle ear effusion at least once. Myringotomy or ventilation tube insertion was performed on 33 patients (47.1%), and 16 patients (22.9%) required multiple insertions. Eighteen patients (25.7%) had adenoid hypertrophy, and 16 (22.9%) underwent adenoidectomy. Temporal bone computed tomography (TBCT) scans were taken in 9 patients (12.9%) for middle ear evaluation. Computed tomography (CT) scans showed a high jugular bulb and rotated inner ear structures. Chronic otitis media with cholesteatoma was diagnosed in 2 patients (2.9%), in whom tympanomastoidectomy was performed.
Conclusion
Half of the children with achondroplasia experienced hearing loss, most commonly due to conductive hearing loss. Three-quarters of these children exhibited otitis media with effusions, often necessitating the insertion of a ventilation tube and adenoidectomy. Given the anatomical variations present in these children, such as a high jugular bulb and rotated structures of the inner ear and facial nerve, a cautious approach is essential when performing middle ear surgery.
Keywords: Achondroplasia, hearing loss, otitis media, temporal bone, middle ear
Main Points
Achondroplasia is a widely known skeletal dysplasia disease that accompanies numerous otologic complications, such as hearing impairment and otitis media, that frequently require interventions.
Temporal bone CT findings of patients with achondroplasia exhibit multiple notable anatomic anomalies, including hypoplastic and broad-shaped incus body, towering petrous ridges, and abnormal facial nerve course.
With knowledge of otologic and anatomic characteristics of achondroplasia, physicians may treat such patients more accurately and provide high quality medical care by implementing safe surgeries.
Introduction
Achondroplasia is the most prevalent form of short-stature dysplasia, with an estimated occurrence of 1 in every 20 000-30 000 births.1 This condition follows an autosomal dominant inheritance pattern, and its pathogenicity is primarily due to a gain-of-function mutation in the FGFR3 gene.1,2 Individuals with achondroplasia often experience not only issues related to height but also a range of complications resulting from impaired bone maturation. These complications can be quite severe, including neurological issues such as foramen magnum compression and orthopedic symptoms. Furthermore, patients with achondroplasia may also suffer from otorhinolaryngologic complications, such as recurrent otitis media, hearing loss, and sleep-related airway problems. These complications occur substantially more frequently in patients with achondroplasia than in the general population, necessitating a multidisciplinary approach to treatment.3
Achondroplasia is a relatively common disorder, with many patients undergoing otologic treatment or surgery at least once in their lifetime. However, the otologic manifestations and hearing characteristics of achondroplasia patients are not well documented, particularly in Asian countries where research is still insufficient. Otologic issues often include otitis media with effusion and conductive hearing loss, both of which are believed to be caused by Eustachian tube dysfunction. This dysfunction can lead to disturbances in middle ear ventilation. The recurrence rate of otitis media is nearly 26% in a study conducted on South Indian children aged 1-6 years old;4 thus, otitis media is a significant medical issue among the pediatric population in developing countries. Consequently, a substantial number of patients with achondroplasia undergo repeated treatments involving ventilation tube insertion and otitis media surgeries over an extended period of time.5 Skeletal anomalies in patients with achondroplasia are due to problems in endochondral ossification. In the temporal bone region, the ossicles, petrous bone, and mastoid bone are affected. A previous study analyzing temporal bone CT scans of achondroplasia patients revealed findings such as underdeveloped mastoid air cells, upward tilting of the internal acoustic canal, and anomalies in the facial nerve and ossicles.6-8 Hearing loss is one of the most common medical complications of achondroplasia, affecting 61% of subjects in a retrospective single-clinic study.9 A comprehensive literature review on the conditions accompanying achondroplasia also highlighted a high prevalence of hearing loss in achondroplastic children.10 However, studies focusing on the otologic problems of achondroplasia patients are limited, with the largest single-center population pilot study to date involving only 22 subjects.
The aim of this study was to examine the prevalence and treatment trajectory of otologic diseases in patients with achondroplasia through a comprehensive review of clinical data. Furthermore, we will analyze the types of treatments administered and provide a suitable surgical treatment guide by investigating the common CT findings in patients with achondroplasia.
Methods
Study Population
This retrospective case series included patients diagnosed with achondroplasia by genetic specialists or pediatric orthopedists between March 1999 and May 2023. These patients received otorhinolaryngological treatment and underwent physical examinations or audiologic diagnostic tests at 3 hospitals in South Korea. Patients with systemic or developmental diseases other than achondroplasia, which could potentially cause neurological impairment including auditory dysfunction, were excluded from the study.
Data Collection
We reviewed the medical records of each patient, which included their age, sex, underlying disease, date and findings of their first otology examination, history of otitis media and ventilation tube insertion, and results from otologic diagnostic tests. The following tests were analyzed: tympanic endoscopy, tympanometry, pure tone audiometry (PTA)/speech audiometry, auditory brainstem response threshold (ABR) test, and temporal bone computed tomography (TBCT).
Hearing Evaluation Protocol
Hearing outcomes were assessed using the mean thresholds of 500, 1000, 2000, and 4000 Hz frequencies of the air conduction hearing threshold PTA. A PTA average exceeding 25 dB was classified as poor hearing. The near coincidence of 2 lines, representing air and bone conduction, with an air-bone conduction gap of ≤10 dB on the audiogram, was interpreted as suggesting sensory neural hearing loss. Conversely, hearing loss with audiograms showing air-bone gaps of >10 dB at a specific frequency indicated a conductive nature. Mixed-type hearing loss was characterized as a combination of sensory neural and conductive types. Subjects younger than 2 years underwent ABR. For ABR, the hearing threshold was not evaluated beyond 25 dB, which was considered normal.
Otitis Media with Effusion Diagnosis and Ventilation Tube Insertion Indication Protocol
Otitis media with effusion (OME) is diagnosed by identifying the presence of fluid in the middle ear via otoscopy or tympanometry in accordance with clinical practice guidelines.11 The diagnostic findings from pneumatic otoscopy and otomicroscopy may include dullness of the tympanic membrane, impaired mobility, and the presence of an air-fluid level or bubble. Furthermore, a type B or C tympanogram was considered indicative of OME. Ventilation tube insertion (VTI) was deemed to be indicated in cases where there was a history of OME lasting 3 months or longer, structural damage to the tympanic membrane or middle ear, or a risk of speech and language learning difficulties. If adenoid hypertrophy was discovered during a preoperative assessment, an adenoidectomy could also be performed. Children were typically followed up every 3-6 months until 12 months after the tube insertion. If OME persisted for more than 3 months, re-insertion of the ventilation tube was recommended.
Temporal Bone Computed Tomography Protocol
In this study, 10 patients underwent TBCT without contrast. Two independent otologists retrospectively reviewed 9 of these scans, excluding 1 case where the imaging was conducted during infancy. The otologists meticulously examined the coronal and axial views, analyzing each slice to identify temporal bone anatomy features specific to achondroplasia. The TBCT scanning parameters used were as follows: a slice thickness of 0.625-1.0 mm, a field of view of 140-170 × 140-170, a current of 122-200 mA, and a voltage of 100-120 kVp.
Information of Ethics Committee Approval
Informed consent for the collection and review of medical records of subjects was obtained, and this study was approved on September 18, 2020, by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No: 2004-244-1121).
Results
In total, 65 patients, 7 patients, and 1 patient were enrolled from SNUH, SNUBH, and BH, respectively, with a total number of 73 subjects. The average age of patients at their first visit to the otolaryngology clinic was 6.5 years, although there was a large SD. The majority of subjects were diagnosed with otitis media or tonsil/adenoid hypertrophy between the ages of 1 and 3 at a different hospital, or they were referred for screening purposes from the pediatrics department. The sex ratio was approximately 1:1, with 37 males and 36 females (Table 1).
Table 1.
Demographic Characteristics, and Distribution of Hearing Loss Types Based on Audiologic Exams (Pure Tone Audiometry and Auditory Brainstem Response Threshold Test)
| Characteristics | Total Subjects (n = 73) |
|---|---|
| Age at first visit (year ± SD) | 6.58 ± 11.44 |
| Sex (M: F) | 37:36 |
| Audiologic exam results | n = 55 |
| Normal | 23 (41.8%) |
| Conductive hearing loss | 18 (32.7%; 10 unilateral, 8 bilateral) |
| Sensory neural hearing loss | 2 (3.6%; 1 unilateral, 1 bilateral) |
| Mixed-type hearing loss | 3 (5.5%; 3 bilateral) |
| Inadequate hearing loss | 9 (16.3%; 2 unilateral, 7 bilateral) |
Hearing Loss
Of the 55 patients with initial audiologic exam results, 32 patients (58.2%) exhibited hearing impairment. In accordance with previous studies, of the 32 cases, conductive hearing loss was most common (18 cases, 32.7%), followed by mixed-type hearing loss (3 cases, 5.5%), and sensorineural hearing loss (2 cases, 3.6%). Nine patients underwent PTA without bone conduction or play audiogram due to poor compliance or young age, thereby classified as inadequate hearing loss. Among these 9 cases that displayed insufficient audiograms with limited evidence of an air-bone gap, 6 cases showed accompanying middle ear effusion, thereby falling under the conductive hearing loss category (Table 1).
Otitis Media
Approximately 70% of the total subjects (51 cases, 69.9%) exhibited at least unilateral middle ear effusion (MEE) as observed under tympanic endoscopy. Of these, 32 cases underwent VTI. This procedure was typically performed at an average age of 4 years. In 50% of these cases, the MEE was resolved, but in the remaining half, the MEE recurred, necessitating multiple reinsertions. A review of the audiograms of 12 patients who underwent postoperative hearing evaluations following the resolution of MEE revealed a persistent air-bone gap in 8 cases (66.7%) (Table 2). This suggests that the hearing impairment cannot be solely attributed to MEE. Instead, concurrent anatomic anomalies may also play a role.
Table 2.
Physical Exam Results and Ventilation Tube Insertion History of Subjects with Follow-up Audiogram (If Available)
| Characteristics | Total Subjects (n = 73) |
|---|---|
| Physical examination | n = 73 |
| Normal | 22 (30.1%) |
| Unilateral MEE or retraction | 9 (12.3%) |
| Bilateral MEE or retraction | 42 (57.5%) |
| Adenoid hypertrophy | 16 (21.9%) |
| Ventilation tube insertion | n = 32 |
| Age of first tube placement (year ± SD) | 4.06 ± 5.37 years |
| Single insertion | 16 (50.0%) |
| Multiple insertion | 16 (50.0%) |
| Postoperative hearing evaluation | n = 12 |
| Resolved conductive hearing loss | 4 (33.3%) |
| Remained air-bone gap (≥10 dB) | 8 (66.7%) |
MEE, middle ear effusion.
Middle Ear Anomaly
Ten patients underwent a TBCT scan to evaluate chronic otitis media or cholesteatoma. However, 1 case was excluded because the CT scan was taken during infancy (less than 1 year of age). The TBCT scans of the remaining 9 cases displayed characteristics such as a hypoplastic and broad-based incus, an anomalous course of the facial nerve, poor development of the mastoid air cells, and a shortened carotid canal. These findings are consistent with those of previous studies.
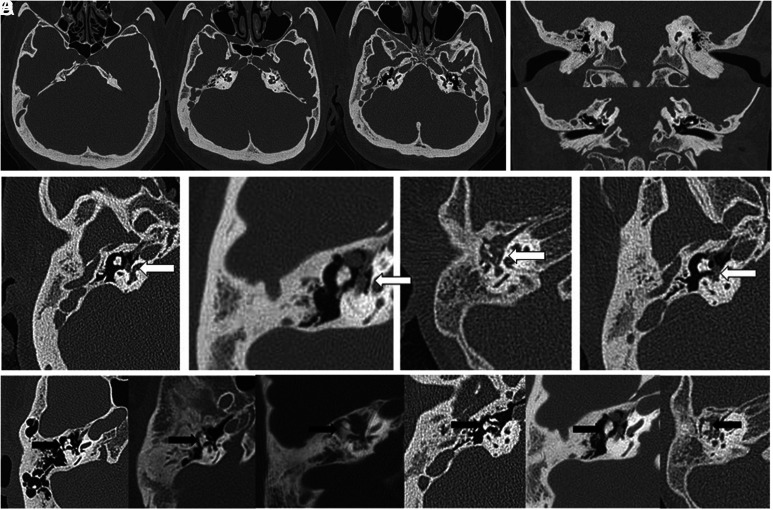
A typical TBCT scan of a patient with achondroplasia often reveals prominent emissary veins, underdeveloped mastoid air cells, and a shortened carotid canal when viewed axially. Additionally, an upward tilt of both the external auditory canal and internal auditory canal, a downward-facing oval window, and a horizontally positioned scutum can be observed in the coronal view. Ossicular anomalies were identified in all 9 cases examined, making this the most common feature of achondroplasia observed in TBCT scans. Eight of the cases displayed towering petrous ridges, a downward-facing oval window, and a horizontally positioned scutum. An underdeveloped mastoid and a shortened carotid canal were found in 7 scans, while a prominent mastoid emissary vein and an anomalous course of the facial nerve were observed in nearly half (4/9) of the cases. These findings are all relatively common in the TBCT scans analyzed in our study. Therefore, otologic surgeons should familiarize themselves with these anatomical anomalies before performing middle ear surgery on patients with achondroplasia.
A detailed review of the abnormal facial nerve course, identified in 4 cases, reveals that the facial nerve overhangs the oval window (Table 3). This positioning obscures the incudo-stapedial joint and stapes suprastructure in the axial view. A hypoplastic and broad-shaped incus body, which is presumed to have developed due to complications in endochondral ossification, can be observed in Figure 1D. The residual air-bone conduction gap in post-VTI audiograms may be attributed to such ossicular anomalies.
Table 3.
Temporal Bone Computed Tomography Findings
| Case | Ossicular Anomaly | Towering of the Petrous Ridge | Downward Oval Window | Horizontal Position of the Scutum | Poor Development of Mastoid Air Cells | Foreshortening Carotid Canal | Prominent Mastoid Emissary Vein | Anomalous Facial n. Course |
|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | + | + | + |
| 2 | + | + | + | + | + | + | + | − |
| 3 | + | + | + | + | + | + | + | − |
| 4 | + | + | + | + | + | + | − | + |
| 5 | + | + | + | + | + | + | − | − |
| 6 | + | + | + | + | + | + | − | − |
| 7 | + | + | + | + | − | + | + | − |
| 8 | + | + | + | + | − | − | − | + |
| 9 | + | − | − | − | + | − | − | + |
Figure 1.
Axial (A) view of a typical temporal bone computed tomography scan of an achondroplastic patient demonstrating prominent emissary veins, poor mastoid pneumatization, and carotid canal shortening. Coronal (B) view of a typical temporal bone computed tomography scan of an achondroplastic patient displaying upward tilting of the external and internal auditory canals, downward-facing oval window, and horizontal placement of scutum. (C) Aberrant facial nerve course (white arrow) observed in 4 temporal bone computed tomography scans. The facial nerve overhangs the oval window and eclipses the incudostapedial joint and stapes suprastructure in the axial view. (D) Six temporal bone computed tomography scans showing a hypoplastic and broad-based incus body (black arrow), presumably caused by dysfunction in endochondral ossification.
Inner Ear Anomaly
All 9 TBCT scans exhibited no definite abnormalities of the vestibular structure upon examination of the inner ear. Due to the limitation in the number of available TBCT scans in this study, it is inappropriate to generalize the intact inner ear structures of all achondroplastic patients. Through subsequent research on a larger achondroplastic population with imaging studies, the possibility of vestibular structural anomalies remains open.
Discussion
In this retrospective case series study, approximately 60% of achondroplasia patients who visited the otolaryngology clinics at 3 different hospitals exhibited hearing impairment. In 70% of these cases, MEE or a retracted tympanic membrane was observed during endoscopic tympanoscopy. The majority of the hearing loss was conductive, and VTI was required for symptom resolution in 62.7% of otitis media cases. Notably, at least 2 reinsertions were necessary due to MEE recurrence in half of the patients who underwent VTI. Generally, larger silicone tubes, such as Paparella type II and T-tubes, have been reported to have a lower extrusion rate compared to Paparella type I or titanium tubes.12 Therefore, type II tubes were the primary material used for VTI in our study. Despite these interventions, conductive hearing loss still partially remained in 66.7% of patients after VTI, suggesting a potential association with anatomical anomalies.
The mechanism that generates middle ear dysfunction in patients with achondroplasia is rooted in the atypical structure and subpar function of the eustachian tubes.13 Hence, patients with achondroplasia typically experience at least 1 episode of otitis media before they reach the age of one. Furthermore, approximately 90% of these patients will have an episode before they turn 2. It has also been confirmed that nearly 50% of patients with achondroplasia undergo a VTI procedure during infancy. Given these facts, it is crucial for pediatricians to be cognizant of these issues when treating children with achondroplasia. They should also ensure they consult with otologists for accurate assessments of hearing impairment at the appropriate times.
An Australian study investigating potential medical complications in 108 achondroplastic participants found that 66 (61.1%) had hearing impairment, 55 (50.9%) underwent tympanostomy tube (grommet) insertion, and an additional 48 patients (44.4%) received non-invasive ventilation. This resulted in the majority of subjects undergoing some form of otologic intervention.9
Another study reported that, of 45 Norwegian adults diagnosed with achondroplasia, at least unilateral hearing loss was observed in 53% (24/45) of cases. The majority of these cases (15/24) exhibited conductive hearing loss, and VTI was conducted in 44% (20/45) of cases.14 Although a comprehensive audiologic evaluation was performed and the classification pattern aligns with the results of our study, the subject population of the aforementioned study consisted solely of Caucasian achondroplasia patients. Furthermore, a review of imaging scans was not conducted.
Dorney et al15 proposed a correlation between Eustachian tube dysfunction and achondroplasia, demonstrating a risk approximately 8.65 times higher than that of the general population, and its role in the development of middle ear diseases. Both this study and the one conducted by Glass et al 15 attribute the cause of Eustachian tube dysfunction and the high incidence of hearing loss to the unusual craniofacial anatomy of children with achondroplasia.,16 However, a limitation of these studies is that they did not provide a detailed examination of the specific anatomical characteristics.
Our study’s findings align with previous pilot studies and literature reviews regarding the otologic manifestations in patients with achondroplasia. We observed morphological abnormalities in the temporal bone anatomy of these patients, including poor mastoid pneumatization, shortened carotid canal, and towering petrous ridges.7,8,17 These findings require careful review before planning any middle ear surgeries on patients with achondroplasia. A previous case report analyzing a high-resolution TBCT scan of a 50-year-old woman with achondroplasia revealed characteristics that align with our study's findings.17 These shared features include a downward to horizontally positioned scutum, towering petrous ridge, ossicular anomaly (broad-shaped ice cream cone), a downward-facing oval window, and a foreshortened carotid canal. However, this case report did not identify the anomalous facial nerve course in the TBCT scan, a feature we described in 4 cases in our study. Furthermore, our paper aimed to generalize the TBCT properties of patients with achondroplasia through the analysis of multiple CT scans.
Since the average age of the subjects was 6.5 years, it was challenging to implement a study on the pediatric patients’ vestibular function. However, we performed an additional analysis on the given TBCT scans to explore the vestibular structure. Through this supplementary investigation, we speculated on the potential for balancing difficulties among our achondroplastic subjects. Temporal bone computed tomography scan analysis of the inner ear demonstrated no significant abnormalities in the vestibular structures, suggesting that achondroplastic patients in this study have a low chance of experiencing balancing issues or dizziness. Regardless of vestibular anatomical variations, vestibular compensation generally prevents patients from experiencing such symptoms.
Patients with achondroplasia who have middle ear diseases or hearing impairments may require more invasive treatments than VTI, but these can yield excellent outcomes. A case was reported of a 25-year-old patient with achondroplasia and bilateral mixed hearing loss who received bone conduction implants. This patient had a significantly favorable prognosis over a 5-year follow-up period.18 Given that a high proportion of patients with achondroplasia experience conductive hearing loss and middle ear anomalies, the use of a bone conduction device could be beneficial. Understanding the TBCT anatomy of patients with achondroplasia allows for the safe execution of invasive procedures, including middle ear surgeries, with positive results.
Achondroplasia is a rare disease with a relatively low prevalence, which results in a limited number of subjects. This factor, along with selection bias and incomplete electronic medical records, restricted the scope of analysis in this retrospective study. Despite these limitations, the study's significance is underscored by the fact that it is the largest multi-center study to date, examining various otologic manifestations in patients with achondroplasia. Moreover, this study is groundbreaking in that it represents the first investigation of its kind conducted on Asian patients with achondroplasia. Further advancement of this paper may include topics such as a 5-to-10-year step-wise follow-up of subjects’ hearing levels, middle ear state, and vestibular function. In addition, a long-term examination and prognosis of achondroplastic patients who underwent middle ear surgeries may be of interest to numerous otologists.
Conclusion
Achondroplasia is a prevalent skeletal dysplasia disorder that can lead to various medical complications, including otologic diseases such as hearing impairment and otitis media. Our study, based on a retrospective review of medical records from patients with achondroplasia at 3 different hospitals, provides data on the incidence rate and treatment approaches for otologic manifestations in achondroplasia. Additionally, we have identified and described commonly observed anatomic anomalies in the temporal bone CT findings of patients with achondroplasia. This information offers valuable insights and precautions for surgeons to consider before performing middle ear surgeries on patients with achondroplasia.
Acknowledgements
This research was supported and funded by SNUH Kun-hee Lee Child Cancer & Rare Disease Project, Republic of Korea (grant number: FP-2022-00004).
Funding Statement
The authors declare that this study received no financial support.
Footnotes
Ethics Committee Approval: This study was approved by the Ethics Committee of Seoul National University Hospital (Approval No: 2004-244-1121, Date: September 18th, 2020).
Informed Consent: Verbal informed consent was obtained from the patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – D.K., J.Y., M.P.; Design – D.K., J.Y., M.P.; Supervision – M.P.; Resources – M.S., J.L., M.P.; Materials – M.S., J.L., M.P.; Data Collection and/or Processing – D.K., J.Y.; Analysis and/or Interpretation – D.K., J.Y.; Literature Search – D.K.; Writing – D.K.; Critical Review – D.K., M.P.
Declaration of Interests: The authors have no conflicts of interest to declare.
Data Availability Statement
The data presented in this article can be obtained by contacting the corresponding author at aseptic@snu.ac.kr.
References
- 1. Hoover-Fong JE, Alade AY, Hashmi SS, et al. Achondroplasia Natural History Study (CLARITY): a multicenter retrospective cohort study of achondroplasia in the United States. Genet Med. 2021;23(8):1498 1505. ( 10.1038/s41436-021-01165-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370(9582):162 172. ( 10.1016/S0140-6736(07)61090-3) [DOI] [PubMed] [Google Scholar]
- 3. Collins WO, Choi SS. Otolaryngologic manifestations of achondroplasia. Arch Otolaryngol Head Neck Surg. 2007;133(3):237 244. ( 10.1001/archotol.133.3.237) [DOI] [PubMed] [Google Scholar]
- 4. Harinath S, Lakshmanan S, James S, Maruthy S. Recovery from otitis media and associated factors among 1- to 6-year-old children in South India: a longitudinal study. J Audiol Otol. 2023;27(3):139 144. ( 10.7874/jao.2022.00542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tunkel DE, Gough E, Bober MB, et al. Otolaryngology utilization in patients with achondroplasia: results from the CLARITY study. Laryngoscope. 2022;132(8):1548 1554. ( 10.1002/lary.29915) [DOI] [PubMed] [Google Scholar]
- 6. Sarioglu FC, Sarioglu O, Guleryuz H. Neuroimaging and calvarial findings in achondroplasia. Pediatr Radiol. 2020;50(12):1669 1679. ( 10.1007/s00247-020-04841-8) [DOI] [PubMed] [Google Scholar]
- 7. Cobb SR, Shohat M, Mehringer CM, Lachman R. CT of the temporal bone in achondroplasia. AJNR Am J Neuroradiol. 1988;9(6):1195 1199. [PMC free article] [PubMed] [Google Scholar]
- 8. Shohat M, Flaum E, Cobb SR, et al. Hearing loss and temporal bone structure in achondroplasia. Am J Med Genet. 1993;45(5):548 551. ( 10.1002/ajmg.1320450504) [DOI] [PubMed] [Google Scholar]
- 9. Armstrong JA, Pacey V, Tofts LJ. Medical complications in children with achondroplasia. Dev Med Child Neurol. 2022;64(8):989 997. ( 10.1111/dmcn.15194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stender M, Pimenta JM, Cheung M, Irving M, Mukherjee S. Comprehensive literature review on the prevalence of comorbid conditions in patients with achondroplasia. Bone. 2022;162:116472. ( 10.1016/j.bone.2022.116472) [DOI] [PubMed] [Google Scholar]
- 11. Yoo MH, Cho YS, Choi J, et al. Factors affecting the extrusion rate and complications after ventilation tube insertion: a multicenter registry study on the effectiveness of ventilation tube insertion in pediatric patients with chronic otitis media with effusion-part II. Clin Exp Otorhinolaryngol. 2022;15(4):326 334. ( 10.21053/ceo.2022.00934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Llerena J, Kim CA, Fano V, et al. Achondroplasia in Latin America: practical recommendations for the multidisciplinary care of pediatric patients. BMC Pediatr. 2022;22(1):492. ( 10.1186/s12887-022-03505-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fredwall SO, Åberg B, Berdal H, Savarirayan R, Solheim J. Hearing loss in Norwegian adults with achondroplasia. Orphanet J Rare Dis. 2021;16(1):468. ( 10.1186/s13023-021-02095-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dorney I, Otteson T, Kaelber DC. Epidemiology of Eustachian tube dysfunction and related otologic diagnoses among children with achondroplasia. Int J Pediatr Otorhinolaryngol. 2022;163:111339. ( 10.1016/j.ijporl.2022.111339) [DOI] [PubMed] [Google Scholar]
- 15. Glass L, Shapiro I, Hodge SE, Bergstrom L, Rimoin DL. Audiological findings of patients with achondroplasia. Int J Pediatr Otorhinolaryngol. 1981;3(2):129 135. ( 10.1016/0165-5876(81)90028-8) [DOI] [PubMed] [Google Scholar]
- 16. Lyford-Pike S, Hoover-Fong J, Tunkel DE. Otolaryngologic manifestations of skeletal dysplasias in children. Otolaryngol Clin North Am. 2012;45(3):579 598, vii. ( 10.1016/j.otc.2012.03.002) [DOI] [PubMed] [Google Scholar]
- 17. Kochar PS, Soin P, Megahed A. High-resolution computed tomography temporal bone imaging in achondroplasia. Proc (Bayl Univ Med Cent). 2021;34(3):419 421. ( 10.1080/08998280.2020.1868245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siegel L, Araslanova R, Stepniak C, Zimmerman K, Agrawal SK. Achondroplasia and severe sensorineural hearing loss: the role of active bone conduction implants. Cochlear Implants Int. 2022;23(5):291 299. ( 10.1080/14670100.2022.2045073) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this article can be obtained by contacting the corresponding author at aseptic@snu.ac.kr.



 Content of this journal is licensed under a
Content of this journal is licensed under a