Abstract
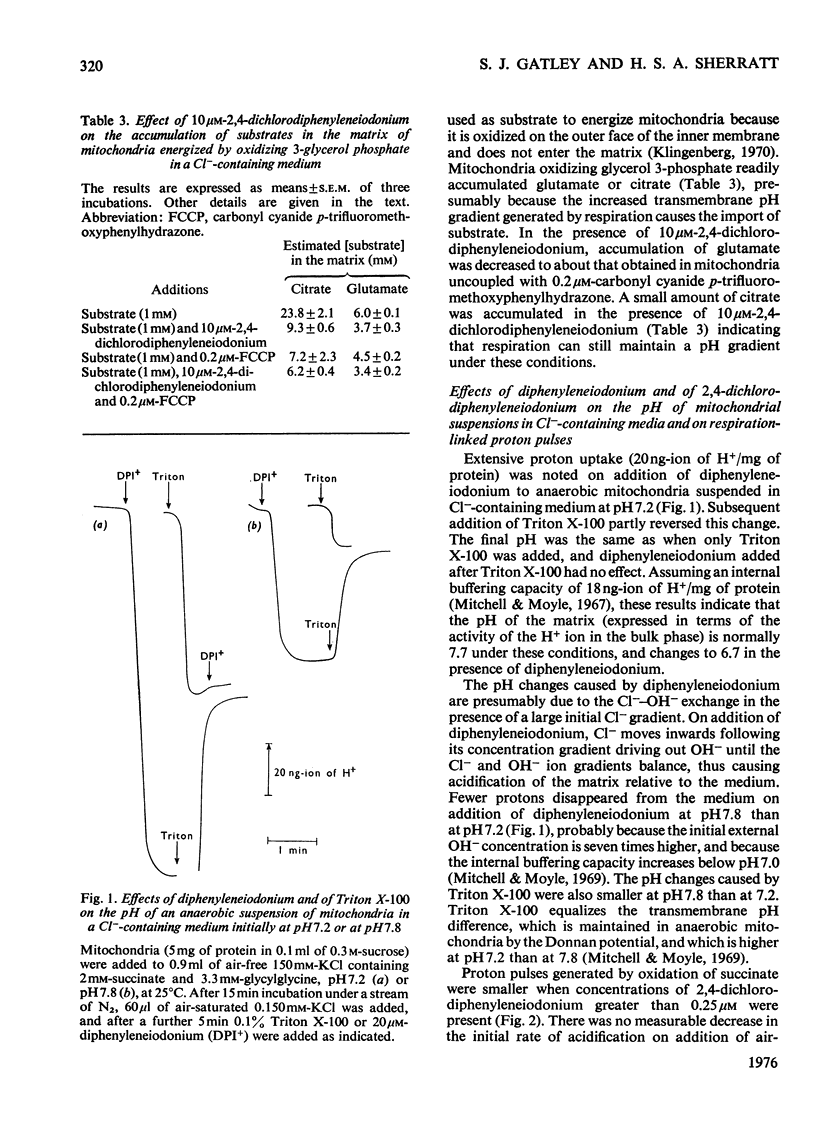
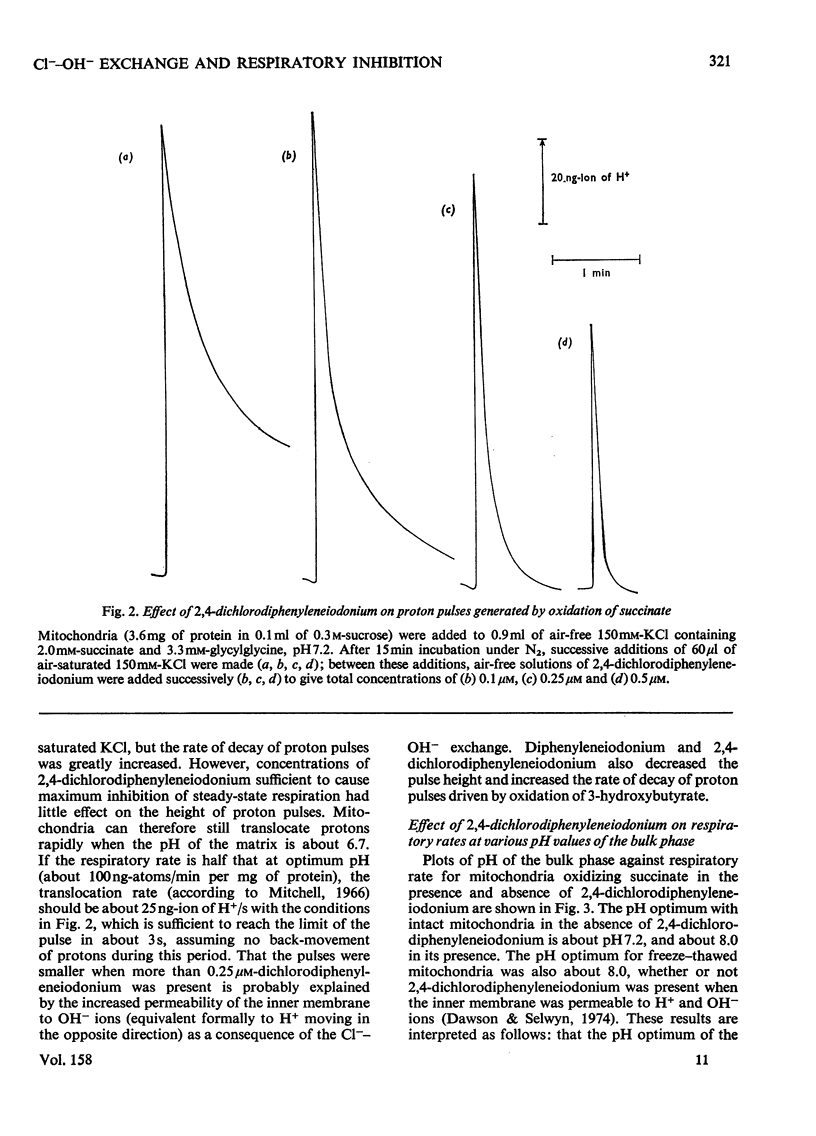
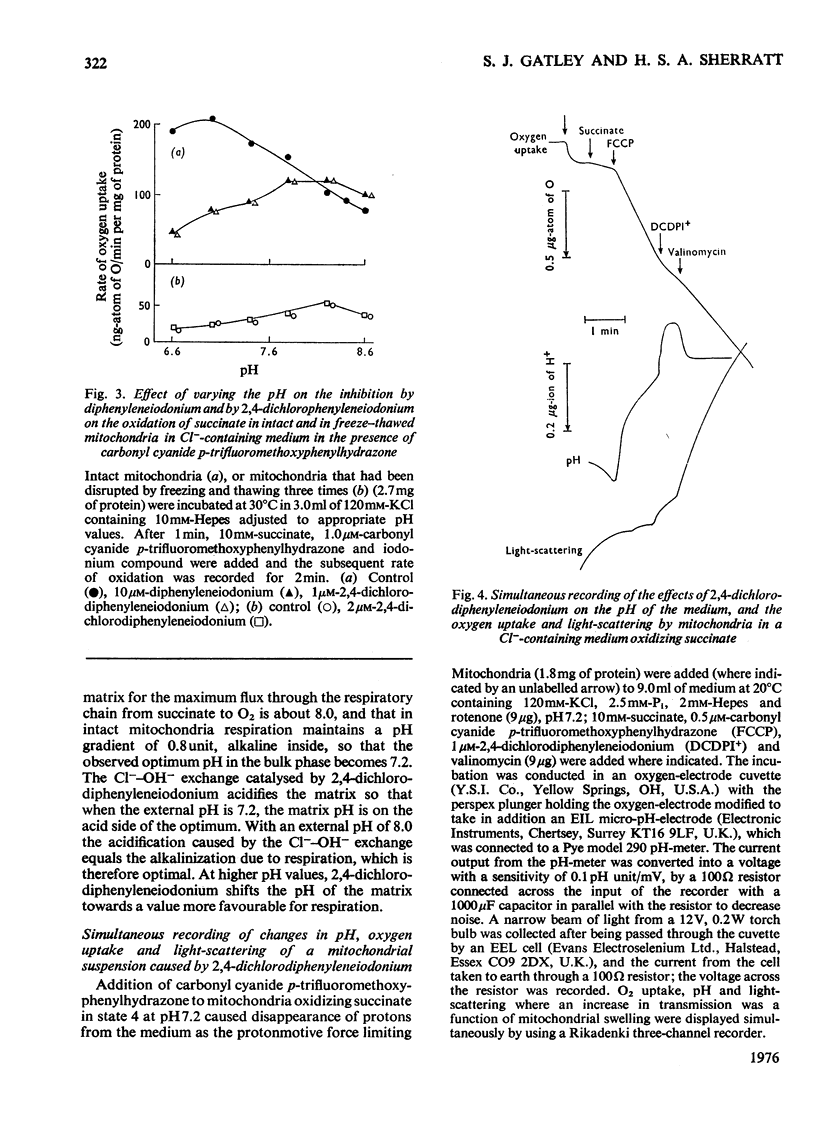
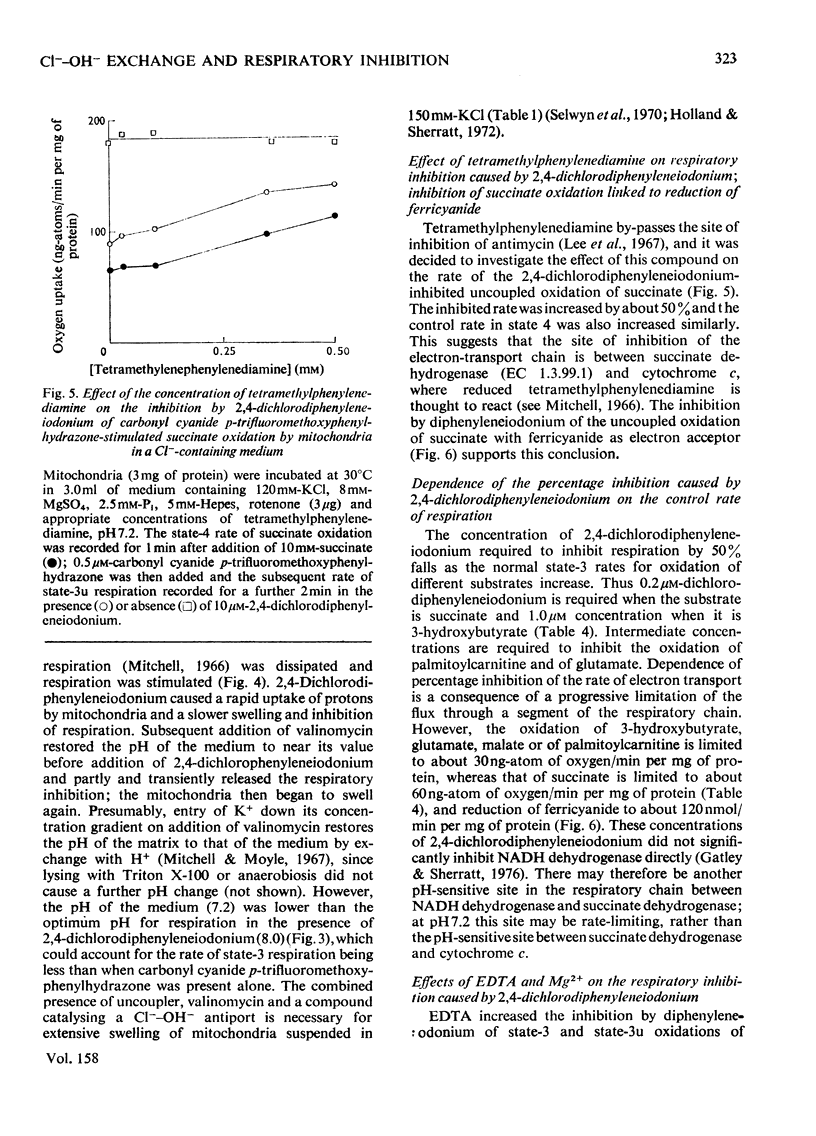
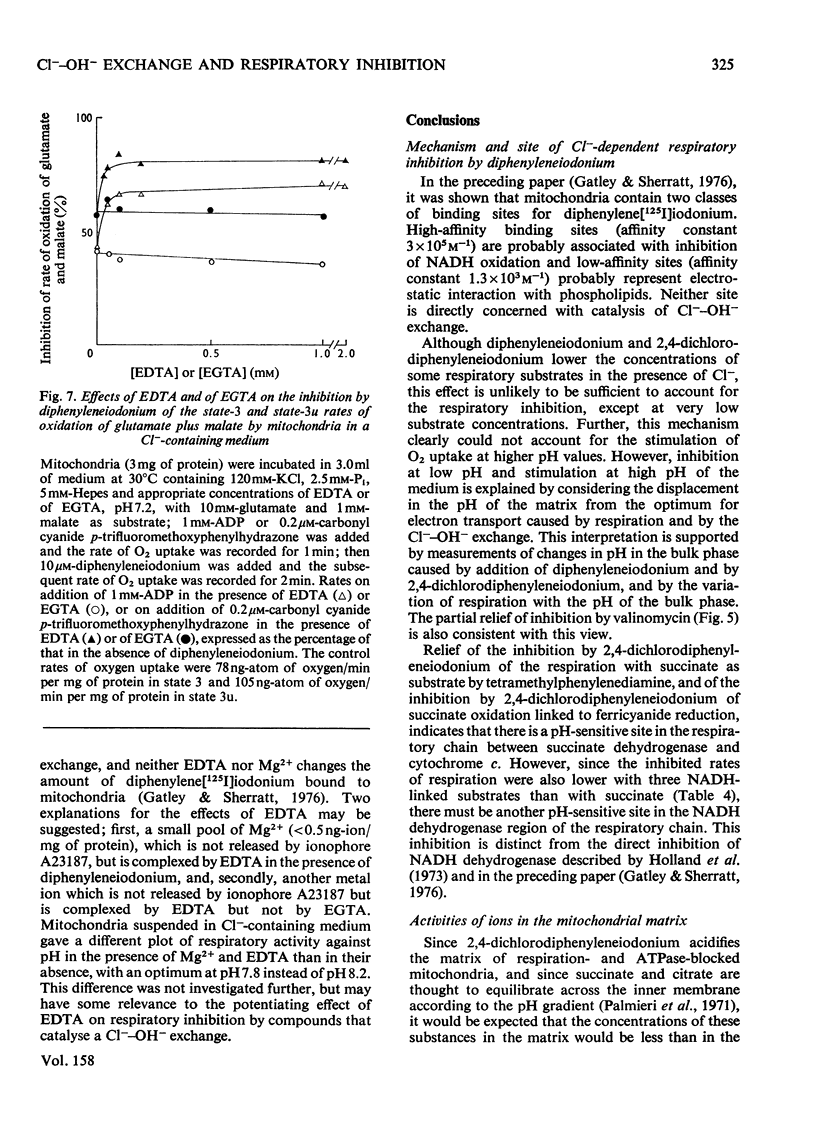
1. Increasing the substrate concentration only decreased the inhibition of mitochondrial oxidations by diphenyleneiodonium or by 2,4-dichlorophenyleneiodonium by a small amount. 2. Diphenyleneiodonium and 2,4-dichlorodiphenyleneiodonium lowered the amounts of succinate, citrate and glutamate accumulated in the matrix of mitochondria in the presence of Cl-, but not in its absences. 2,4-Dichlorodiphenyleneiodonium decreased the accumulation of substrates by mitochondria oxidizing glycerol 3-phosphate. 3. Diphenyleneiodonium caused an alkalinization of the medium with an anaerobic suspension of mitochondria, which was only partly reversed by Triton X-100. 4. The rate of proton extrusion by mitochondria oxidizing succinate was not altered by diphenyleneiodonium or by 2,4-dichlorodiphenyleneiodium, although the rate of decay of proton pulses was increased. 5. 2,4-Dichlorodiphenyleneiodonium shifted the pH optimum for succinate oxidation by intact mitochondria from pH 7.2 to 8.0, whereas there was no effect on that of freeze-thawed mitochondria, which was pH 8.0. 6. The concentration of 2,4-dichlorophenyleneiodonium required to inhibit respiration by 50% is less the higher the absolute rate of oxygen uptake. 7. EDTA, but not EGTA [ethanedioxybis(ethylamine)-tetra-acetic acid] increased the inhibition of respiration by diphenyleneiodonium, 2,4-dichlorodiphenyleneiodonium and by tri-n-propyltin. 8. It is concluded that diphenyleneiodonium and 2,4-dichlorodiphenyleneiodonium limit respiration in Cl--containing medium by causing an acidification of the matrix, and that there are pH-sensitive sites in the respiratory chain between NADH and succinate, and between succinate and cytochrome c.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawson A. P., Selwyn M. J. The action of trialkyltin compounds on mitochondrial respiration. The effect of pH. Biochem J. 1974 Mar;138(3):349–357. doi: 10.1042/bj1380349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley S. J., Sherratt S. A. The effects of diphenyleneiodonium on mitochondrial reactions. Relation of binding of diphenylene[125I]iodonium to mitochondria to the extent of inhibition of oxygen uptake. Biochem J. 1976 Aug 15;158(2):307–315. doi: 10.1042/bj1580307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Bangham J. A., Zukovic B. Equilibration of chloride and pyruvate distributions between liver mitochondria and medium mediated by organo-tin salts. FEBS Lett. 1973 Feb 1;29(3):339–344. doi: 10.1016/0014-5793(73)80054-7. [DOI] [PubMed] [Google Scholar]
- Holland P. C., Clark M. G., Bloxham D. P., Lardy H. A. Mechanism of action of the hypoglycemic agent diphenyleneiodonium. J Biol Chem. 1973 Sep 10;248(17):6050–6056. [PubMed] [Google Scholar]
- Holland P. C., Sherratt H. S. Biochemical effects of the hypoglycaemic compound diphenyleneiodonnium. Catalysis of anion-hydroxyl ion exchange across the inner membrane of rat liver mitochondria and effects on oxygen uptake. Biochem J. 1972 Aug;129(1):39–54. doi: 10.1042/bj1290039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Klingenberg M., Palmieri F., Quagliariello E. Quantitative correlation between the distribution of anions and the pH difference across the mitochondrial membrane. Eur J Biochem. 1970 Dec;17(2):230–238. doi: 10.1111/j.1432-1033.1970.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Manger J. R. The effect of triethyltin on mitochondrial ion accumulation. FEBS Lett. 1969 Dec 30;5(5):331–334. doi: 10.1016/0014-5793(69)80349-2. [DOI] [PubMed] [Google Scholar]
- McGivan J. D., Klingenberg M. Correlation between H+ and anion movement in mitochondria and the key role of the phosphate carrier. Eur J Biochem. 1971 Jun 11;20(3):392–399. doi: 10.1111/j.1432-1033.1971.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Prezioso G., Quagliariello E., Klingenberg M. Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1971 Sep 13;22(1):66–74. doi: 10.1111/j.1432-1033.1971.tb01515.x. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Skilleter D. N. The decrease of mitochondrial substrate uptake caused by trialkyltin and trialkyl-lead compounds in chloride media and its relevance to inhibition of oxidative phosphorylation. Biochem J. 1975 Feb;146(2):465–471. doi: 10.1042/bj1460465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERKHEISER W. C., BARTLEY W. The study of steady-state concentrations of internal solutes of mitochondria by rapid centrifugal transfer to a fixation medium. Biochem J. 1957 May;66(1):79–91. doi: 10.1042/bj0660079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. T., Wilkinson J. R., Hamill R. L., Horng J. S. Effects of antibiotic ionophore, A23187, on oxidative phosphorylation and calcium transport of liver mitochondria. Arch Biochem Biophys. 1973 Jun;156(2):578–585. doi: 10.1016/0003-9861(73)90308-1. [DOI] [PubMed] [Google Scholar]


