Abstract
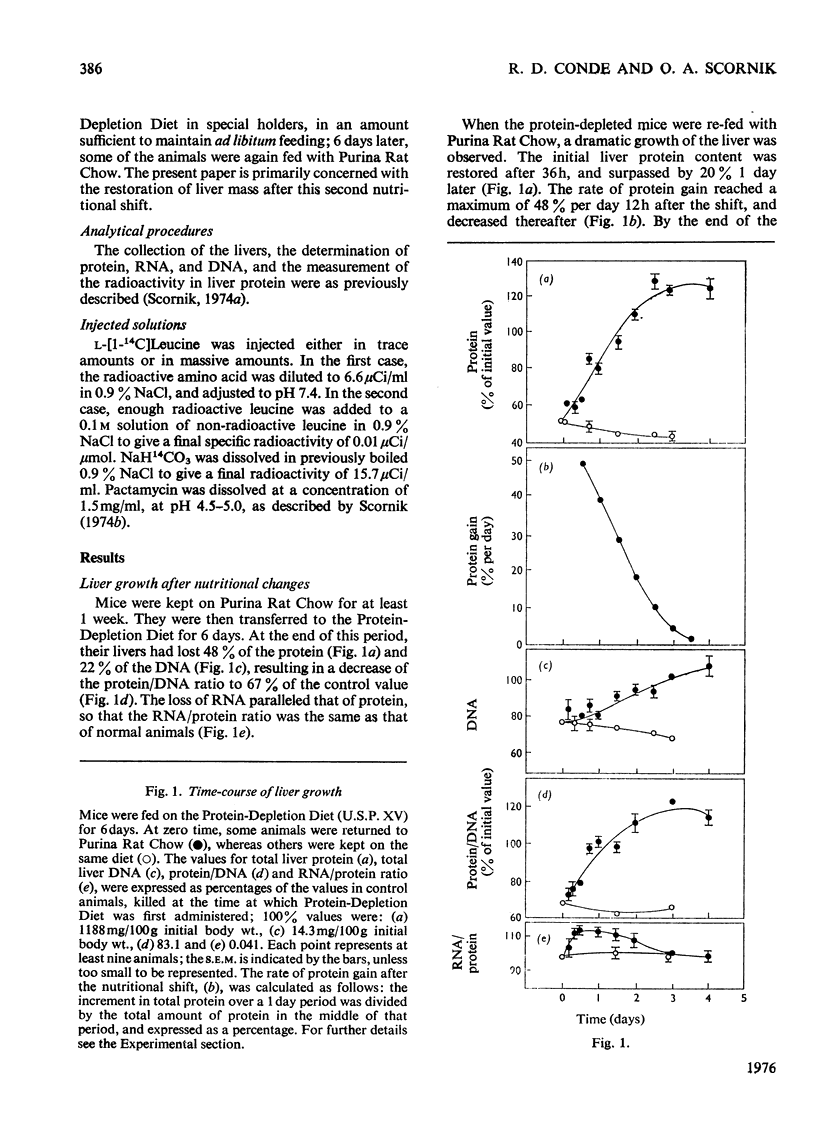
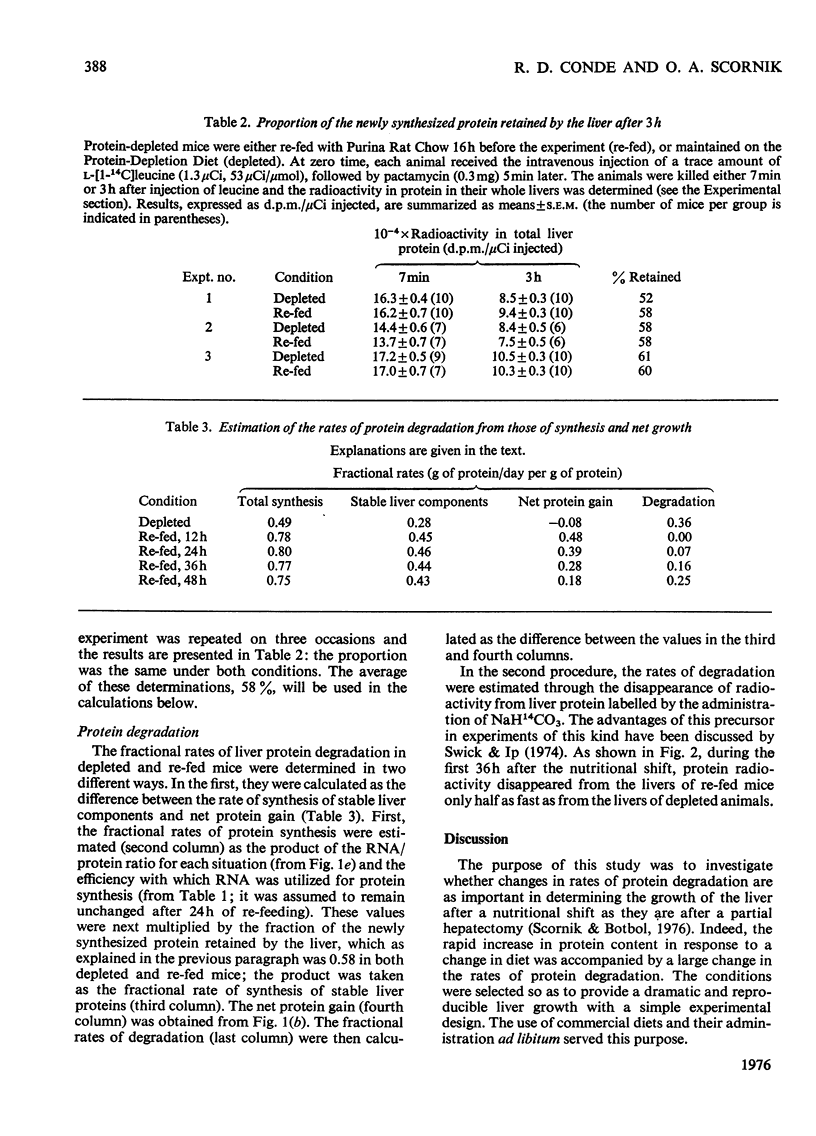
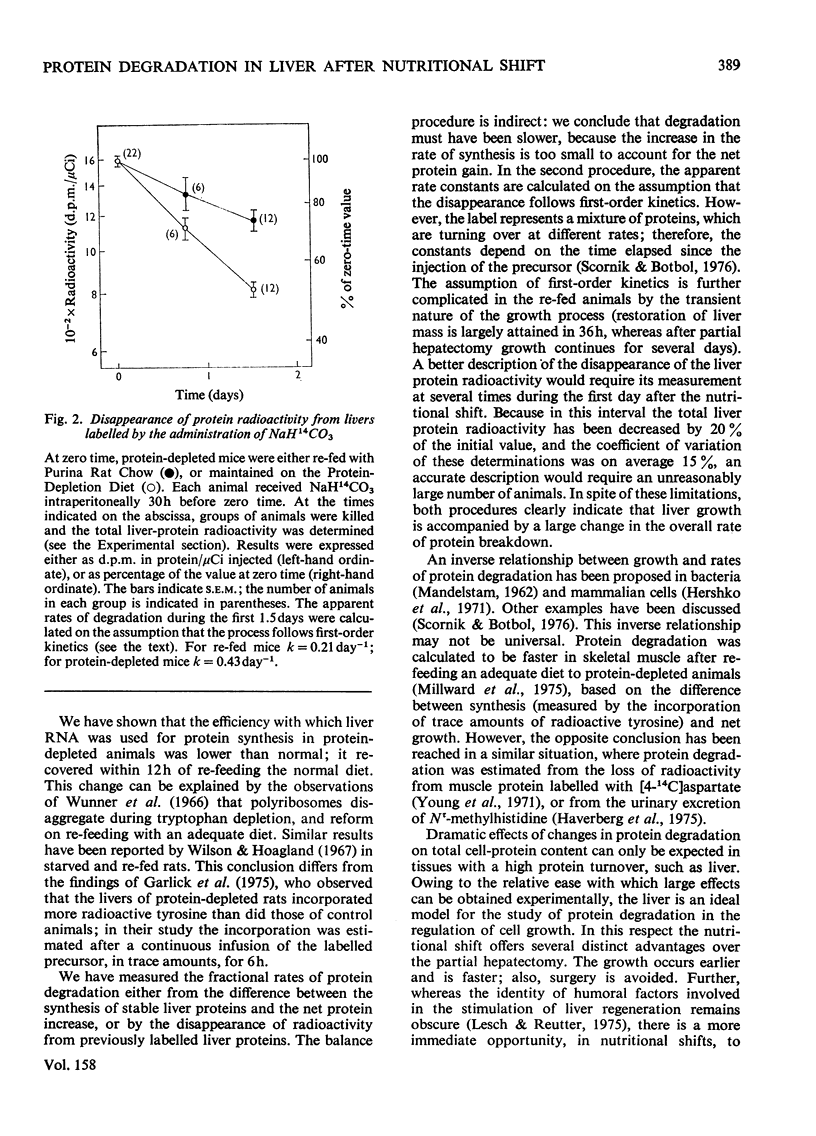
Fractional rates of synthesis and degradation of liver porteins were estimated during the rapid restoration of liver mass observed in protein-depleted mice when they are fed with an adequate diet. 1. Net protein gain was fastest 12h after the nutritional shift, when it reached a rate of 48% per day. 2. The RNA/protein ratio in livers of protein-depleted animals was essentially the same as in normal livers; it increased by a maximum of 13% 12h after the nutritional shift. 3. Rates of protein synthesis in vivo were measured by the incorporation into liver protein of massive amounts of L-[1-14C]leucine. In protein-depleted animals, the rate of synthesis per mg of RNA was 72% of that in normal livers. Normal rates were recovered within 12h of the nutritional shift. 4. The fraction of newly synthesized protein retained by the liver was studied after they were pulse-labelled by the intravenous injection of radioactive leucine, and, 5 min later, pactamycin (an inhibitor of the initiation of protein synthesis); 3h later the livers in both experimental situations retained 58% of the newly synthesized protein. 5. Fractional rates of protein degradation were estimated either from the difference between the synthesis of stable liver proteins and the net protein increase, or by the disappearance of radioactivity from the liver protein previously labelled by the administration to the mice of NaH14CO3. Both procedures demonstrated a large decrease in the rate of protein degradation during liver growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fern E. B., Garlick P. J. The specific radioactivity of the precursor pool for estimates of the rate of protein synthesis. Biochem J. 1973 Aug;134(4):1127–1130. doi: 10.1042/bj1341127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P. The diurnal response of muscle and liver protein synthesis in vivo in meal-fed rats. Biochem J. 1973 Dec;136(4):935–945. doi: 10.1042/bj1360935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P., Waterlow J. C. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochim Biophys Acta. 1975 Nov 18;414(1):71–84. doi: 10.1016/0005-2787(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Haverberg L. N., Deckelbaum L., Bilmazes C., Munro H. N., Young V. R. Myofibrillar protein turnover and urinary N-tau-methylhistidine output. Response to dietary supply of protein and energy. Biochem J. 1975 Dec;152(3):503–510. doi: 10.1042/bj1520503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Hider R. C., Fern E. B., London D. R. Identification in skeletal muscle of a distinct extracellular pool of amino acids, and its role in protein synthesis. Biochem J. 1971 Mar;121(5):817–827. doi: 10.1042/bj1210817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Stewart R. J., Nnanyelugo D. O., Waterlow J. C. Skeletal-muscle growth and protein turnover. Biochem J. 1975 Aug;150(2):235–243. doi: 10.1042/bj1500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J. Protein turnover in skeletal muscle. II. The effect of starvation and a protein-free diet on the synthesis and catabolism of skeletal muscle proteins in comparison to liver. Clin Sci. 1970 Nov;39(5):591–603. doi: 10.1042/cs0390591. [DOI] [PubMed] [Google Scholar]
- Richmond J. E., Shoemaker W. C., Elwyn D. H. Rates of biosynthesis of plasma and liver proteins. Am J Physiol. 1963 Nov;205(5):848–856. doi: 10.1152/ajplegacy.1963.205.5.848. [DOI] [PubMed] [Google Scholar]
- Scornik O. A., Botbol V. Role of changes in protein degradation in the growth of regenerating livers. J Biol Chem. 1976 May 25;251(10):2891–2897. [PubMed] [Google Scholar]
- Scornik O. A. In vivo rate of translation by ribosomes of normal and regenerating liver. J Biol Chem. 1974 Jun 25;249(12):3876–3883. [PubMed] [Google Scholar]
- Scornik O. A. In vivo rates of deaggregation of polyribosomes in normal and regenerating liver after the injection of pactamycin. Biochim Biophys Acta. 1974 Nov 20;374(1):76–81. doi: 10.1016/0005-2787(74)90200-7. [DOI] [PubMed] [Google Scholar]
- Short J., Brown R. F., Husakova A., Gilbertson J. R., Zemel R., Lieberman I. Induction of deoxyribonucleic acid synthesis in the liver of the intact animal. J Biol Chem. 1972 Mar 25;247(6):1757–1766. [PubMed] [Google Scholar]
- Swick R. W., Ip M. M. Measurement of protein turnover in rat liver with (14C)carbonate. Protein turnover during liver regeneration. J Biol Chem. 1974 Nov 10;249(21):6836–6841. [PubMed] [Google Scholar]
- Waterlow J. C., Stephen J. M. The effect of low protein diets on the turn-over rates of serums, liver and muscle proteins in the rat, measured by continuous infusion of L-[14C]lysine. Clin Sci. 1968 Oct;35(2):287–305. [PubMed] [Google Scholar]
- Wilson S. H., Hoagland M. B. Physiology of rat-liver polysomes. The stability of messenger ribonucleic acid and ribosomes. Biochem J. 1967 May;103(2):556–566. doi: 10.1042/bj1030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside K. H., Mortimore G. E. Suppression of protein turnover by amino acids in the perfused rat liver. J Biol Chem. 1972 Oct 25;247(20):6474–6481. [PubMed] [Google Scholar]
- Wunner W. H., Bell J., Munro H. N. The effect of feeding with a tryptophan-free amino acid mixture on rat-liver polysomes and ribosomal ribonucleic acid. Biochem J. 1966 Nov;101(2):417–428. doi: 10.1042/bj1010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. R., Stothers S. C., Vilaire G. Synthesis and degradation of mixed proteins, and composition changes in skeletal muscle of malnourished and refed rats. J Nutr. 1971 Oct;101(10):1379–1390. doi: 10.1093/jn/101.10.1379. [DOI] [PubMed] [Google Scholar]


