Abstract
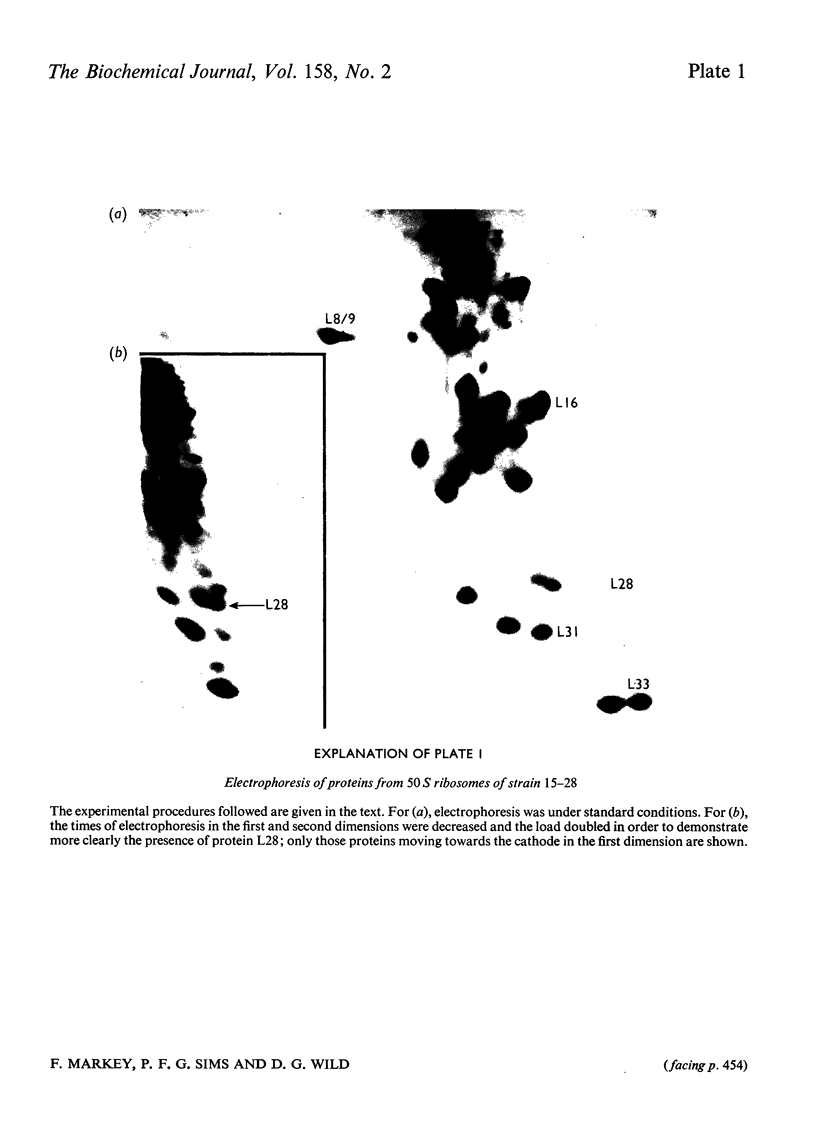
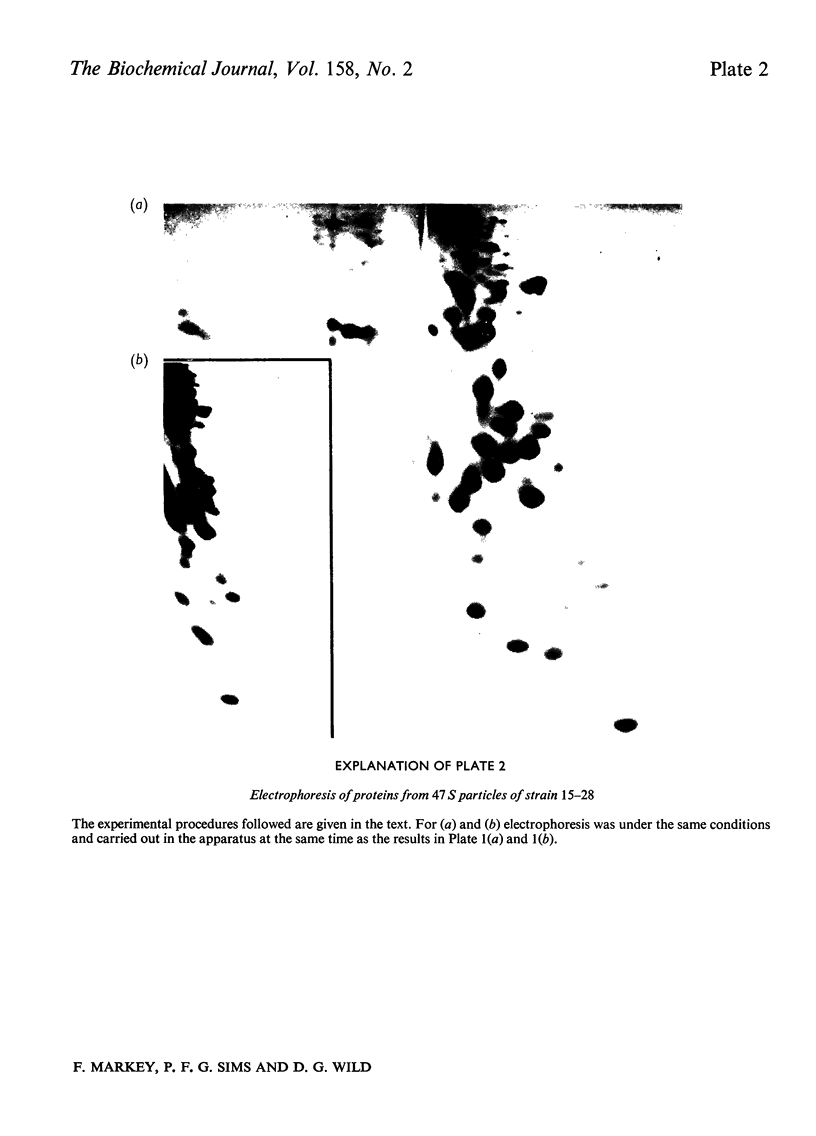
Escherichia coli strain 15--28 is a mutant which during exponential growth contains large amounts of a '47S' ribonucleoprotein precursor to 50S ribosomes. The '47S particles' are more sensitive to ribonuclease than are 50S ribosomes. The 23 S RNA of 47S particles may be slightly undermethylated, but cannot be distinguished from the 23S RNA of 50S ribosomes by sedimentation or electrophoresis. Isolated particles have 10--15% less protein than do 50S ribosomes; proteins L16, L28 and L33 are absent. Comparison with precursor particles studied by other workers in wild-type strains of E. coli suggests that the assembly of 50S ribosomes in strain 15--28 is atypical.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blundell M. R., Wild D. G. Inhibition of bacterial growth by metal salts. The accumulation of ribonucleic acid during inhibition of Escherichia coli by cobalt chloride. Biochem J. 1969 Nov;115(2):213–223. doi: 10.1042/bj1150213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzionara M., Kaltschmidt E., Wittmann H. G. Ribosomal proteins. 8. Molecular weights of isolated ribosomal proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1909–1913. doi: 10.1073/pnas.67.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner P. Nucleotide sequences from specific areas of the 16S and 23S ribosomal RNAs of E. coli. Eur J Biochem. 1969 Nov;11(1):12–27. doi: 10.1111/j.1432-1033.1969.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Varricchio F. A naturally occurring 43 s ribosomal precursor particle in Escherichia coli: nature and ribonucleic acid composition. J Mol Biol. 1970 Mar;48(3):409–419. doi: 10.1016/0022-2836(70)90054-9. [DOI] [PubMed] [Google Scholar]
- Hardy S. J. The stoichiometry of the ribosomal proteins of Escherichia coli. Mol Gen Genet. 1975 Oct 3;140(3):253–274. doi: 10.1007/BF00334270. [DOI] [PubMed] [Google Scholar]
- Hayes F., Hayes D. H. Biosynthesis of ribosomes in E. coli. I. Properties of ribosomal precursor particles and their RNA components. Biochimie. 1971;53(3):369–382. doi: 10.1016/s0300-9084(71)80104-9. [DOI] [PubMed] [Google Scholar]
- Hecht N. B., Woese C. R. Separation of bacterial ribosomal ribonucleic acid from its macromolecular precursors by polyacrylamide gel electrophoresis. J Bacteriol. 1968 Mar;95(3):986–990. doi: 10.1128/jb.95.3.986-990.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann H. E., Nierhaus K. H. Ribosomal proteins. Protein compositions of biosynthetic precursors and artifical subparticles from ribosomal subunits in Escherichia coli K 12. Eur J Biochem. 1971 May 28;20(2):249–257. doi: 10.1111/j.1432-1033.1971.tb01388.x. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Traut R. R. Separation and radioautography of microgram quantities of ribosomal proteins by two-dimensional polyacrylamide gel electrophoresis. FEBS Lett. 1973 Jan 15;29(2):177–180. doi: 10.1016/0014-5793(73)80555-1. [DOI] [PubMed] [Google Scholar]
- KONO M., OSAWA S. INTERMEDIARY STEPS OF RIBOSOME FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Jun 22;87:326–334. doi: 10.1016/0926-6550(64)90228-2. [DOI] [PubMed] [Google Scholar]
- KONO M., OTAKA E., OSAWA S. CHANGES IN SEDIMENTATION PROPERTIES OF RIBOSOMAL RIBONUCLEIC ACIDS DURING THE COURSE OF RIBOSOME FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Dec 16;91:612–618. doi: 10.1016/0926-6550(64)90009-x. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. XII. Number of proteins in small and large ribosomal subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1276–1282. doi: 10.1073/pnas.67.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. XXXII. Comparison of several extraction methods for proteins from Escherichia coli ribosomes. Biochimie. 1972;54(2):167–175. doi: 10.1016/s0300-9084(72)80101-9. [DOI] [PubMed] [Google Scholar]
- Knopf U. C., Sommer A., Kenny J., Traut R. R. A new two-dimensional gel electrophoresis system for the analysis of complex protein mixtures: application to the ribosome of E. coli. Mol Biol Rep. 1975 Mar;2(1):35–40. doi: 10.1007/BF00357295. [DOI] [PubMed] [Google Scholar]
- Lindahl L. Intermediates and time kinetics of the in vivo assembly of Escherichia coli ribosomes. J Mol Biol. 1975 Feb 15;92(1):15–37. doi: 10.1016/0022-2836(75)90089-3. [DOI] [PubMed] [Google Scholar]
- Lindahl L. Two new ribosomal precursor particles in E. coli. Nat New Biol. 1973 Jun 6;243(127):170–172. doi: 10.1038/newbio243170a0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Turnock G., Forchhammer J. The synthesis and function of ribosomes in a new mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Jan;57(1):141–147. doi: 10.1073/pnas.57.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Markey F., Wild D. G. An unusual precursor of 50S ribosomes in a mutant of Escherichia coli. Biochem J. 1976 Feb 15;154(2):311–318. doi: 10.1042/bj1540311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore V. G., Atchison R. E., Thomas G., Moran M., Noller H. F. Identification of a ribosomal protein essential for peptidyl transferase activity. Proc Natl Acad Sci U S A. 1975 Mar;72(3):844–848. doi: 10.1073/pnas.72.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus D., Nierhaus K. H. Identification of the chloramphenicol-binding protein in Escherichia coli ribosomes by partial reconstitution. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2224–2228. doi: 10.1073/pnas.70.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus K. H., Bordasch K., Homann H. E. Ribosomal proteins. 43. In vivo assembly of Escherichia coli ribosomal proteins. J Mol Biol. 1973 Mar 15;74(4):587–597. doi: 10.1016/0022-2836(73)90049-1. [DOI] [PubMed] [Google Scholar]
- Nomura M. Bacterial ribosome. Bacteriol Rev. 1970 Sep;34(3):228–277. doi: 10.1128/br.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S., Otaka E., Itoh T., Fukui T. Biosynthesis of 50 s ribosomal subunit in Escherichia coli. J Mol Biol. 1969 Mar 28;40(3):321–351. doi: 10.1016/0022-2836(69)90158-2. [DOI] [PubMed] [Google Scholar]
- Pace B., Peterson R. L., Pace N. R. Formation of all stable RNA species in Escherichia coli by posttranscriptional modification. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1097–1104. doi: 10.1073/pnas.65.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Pongs O., Bald R., Erdmann V. A. Identification of chloramphenicol-binding protein in Escherichia coli ribosomes by affinity labeling. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2229–2233. doi: 10.1073/pnas.70.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O., Nierhaus K. H., ERDMANN V. A., Wittmann H. G. Active sites in Escherichia coli ribosomes. FEBS Lett. 1974 Mar 23;40(0):suppl–suppl:S37. [PubMed] [Google Scholar]
- Stöffler G., Daya L., Rak K. H., Garrett R. A. Ribosomal proteins. XXVI. The number of specific protein binding sites on 16 s and 23 s RNA of Escherichia coli. J Mol Biol. 1971 Dec 14;62(2):411–414. doi: 10.1016/0022-2836(71)90437-2. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Parker J., Fiil N. P., Flaks J. G., Friesen J. D. New chromosomal location for structural genes of ribosomal proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2765–2769. doi: 10.1073/pnas.72.7.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H. J. Stoichiometric measurements of 30S and 50S ribosomal proteins from Escherichia coli. Mol Gen Genet. 1972;119(3):233–248. doi: 10.1007/BF00333861. [DOI] [PubMed] [Google Scholar]