Abstract
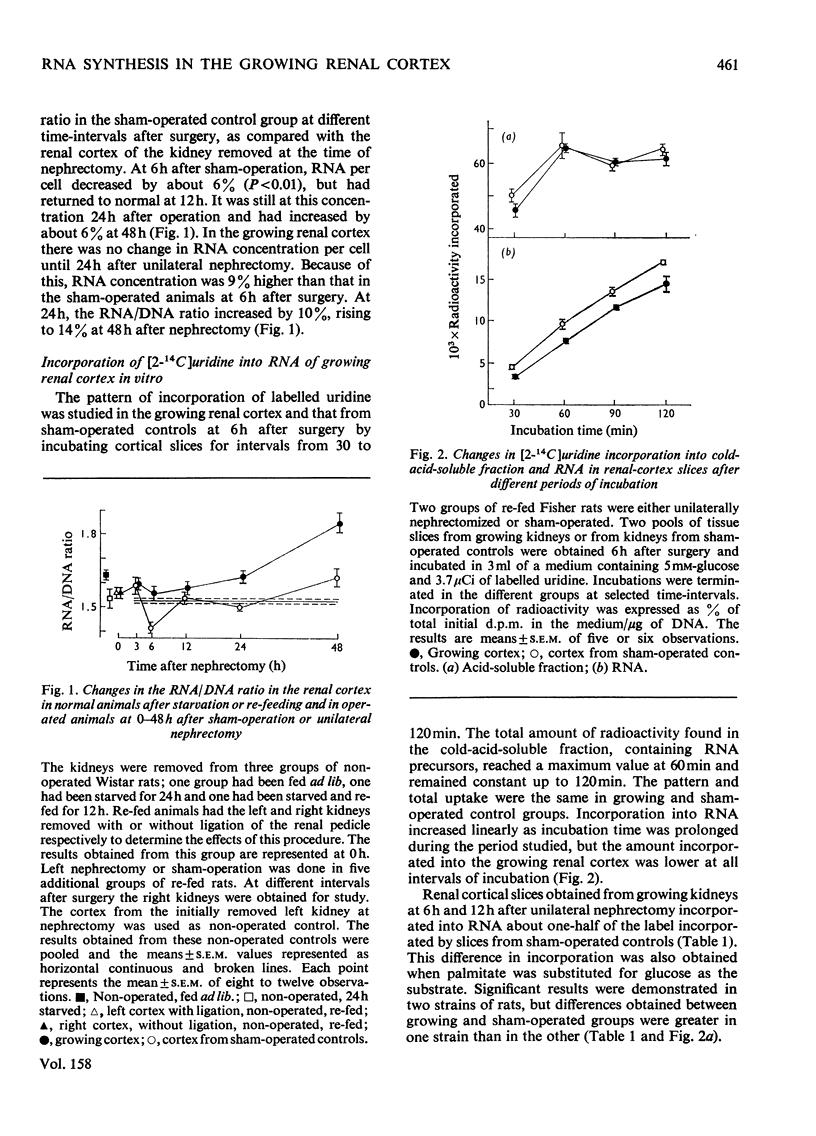
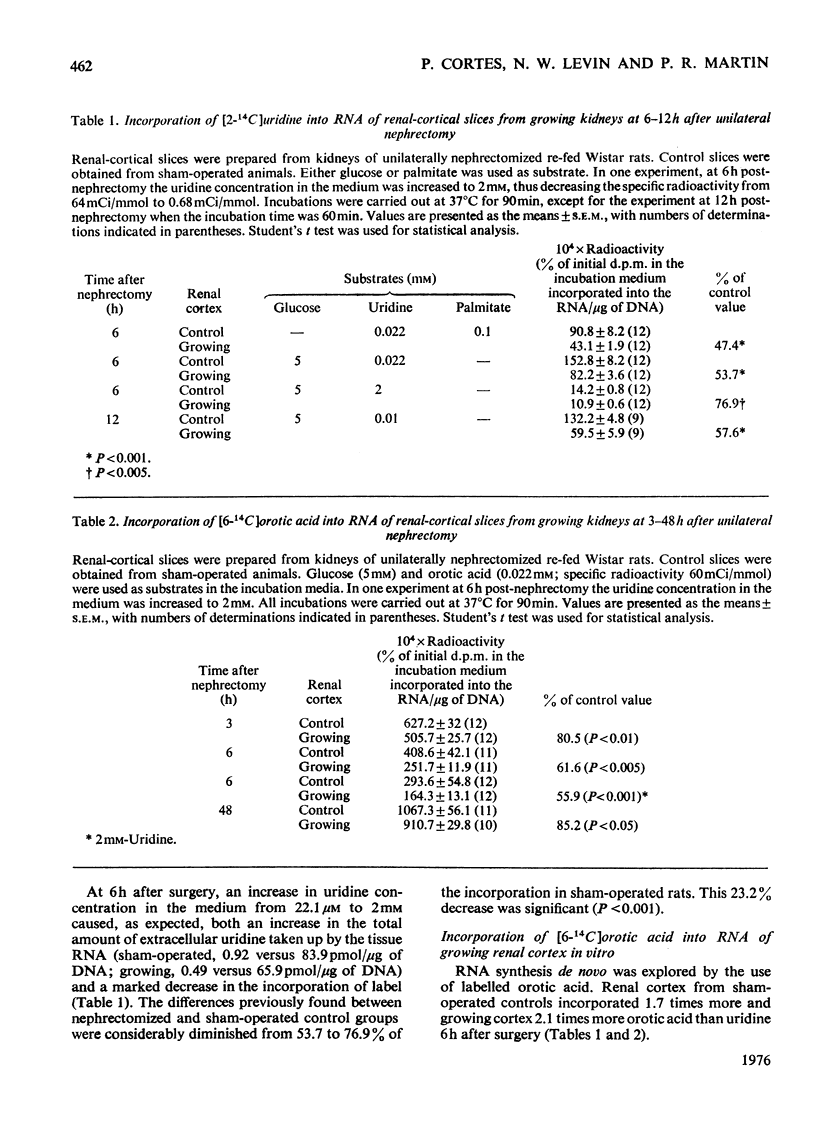
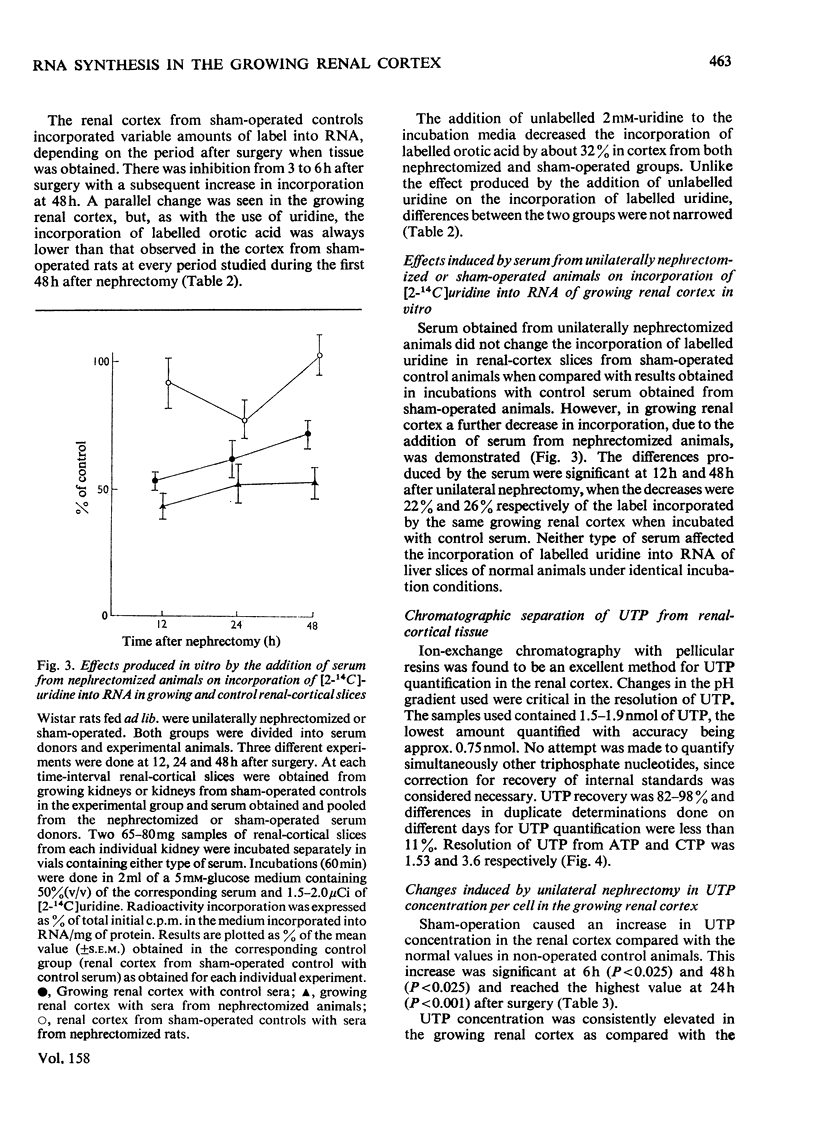
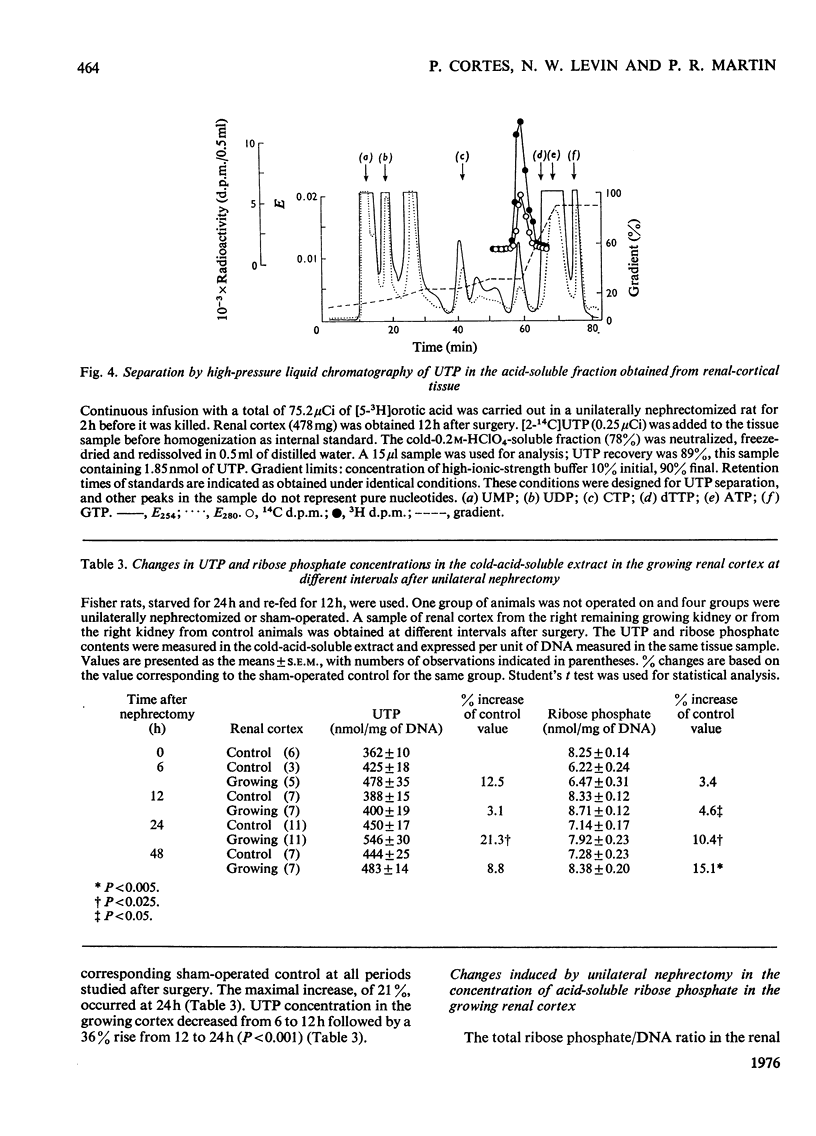
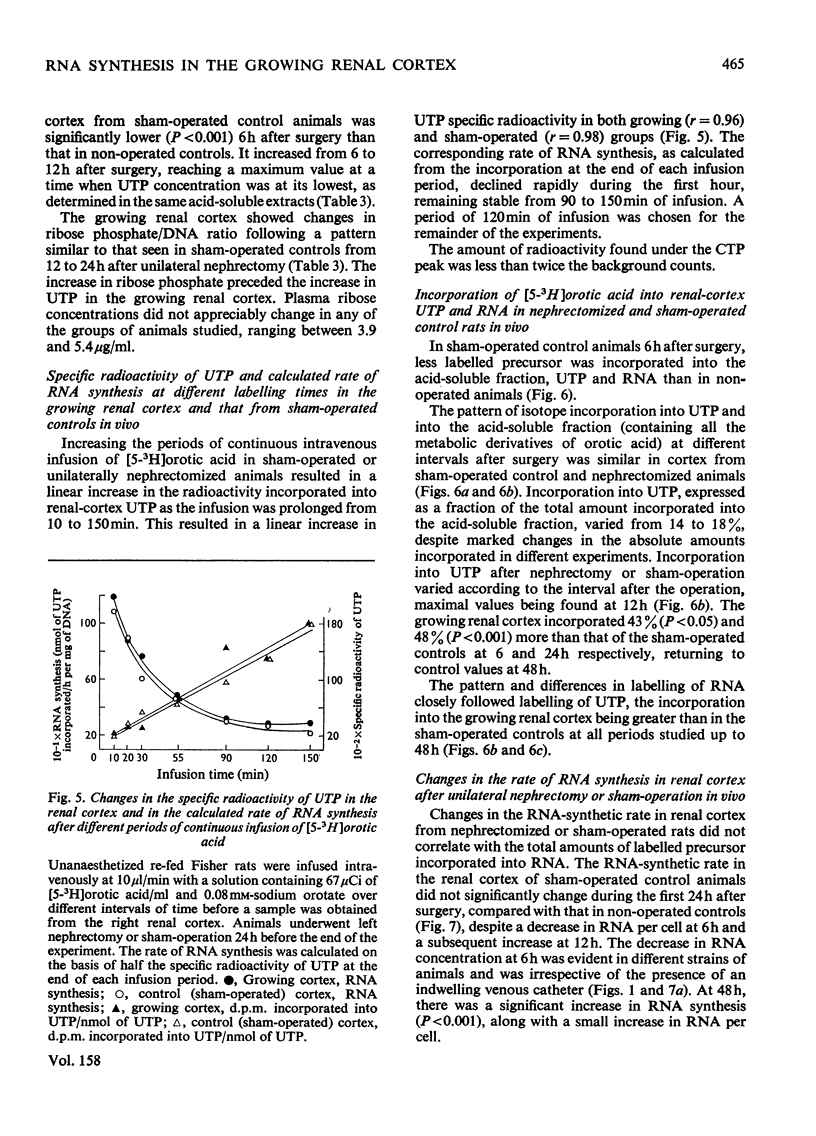
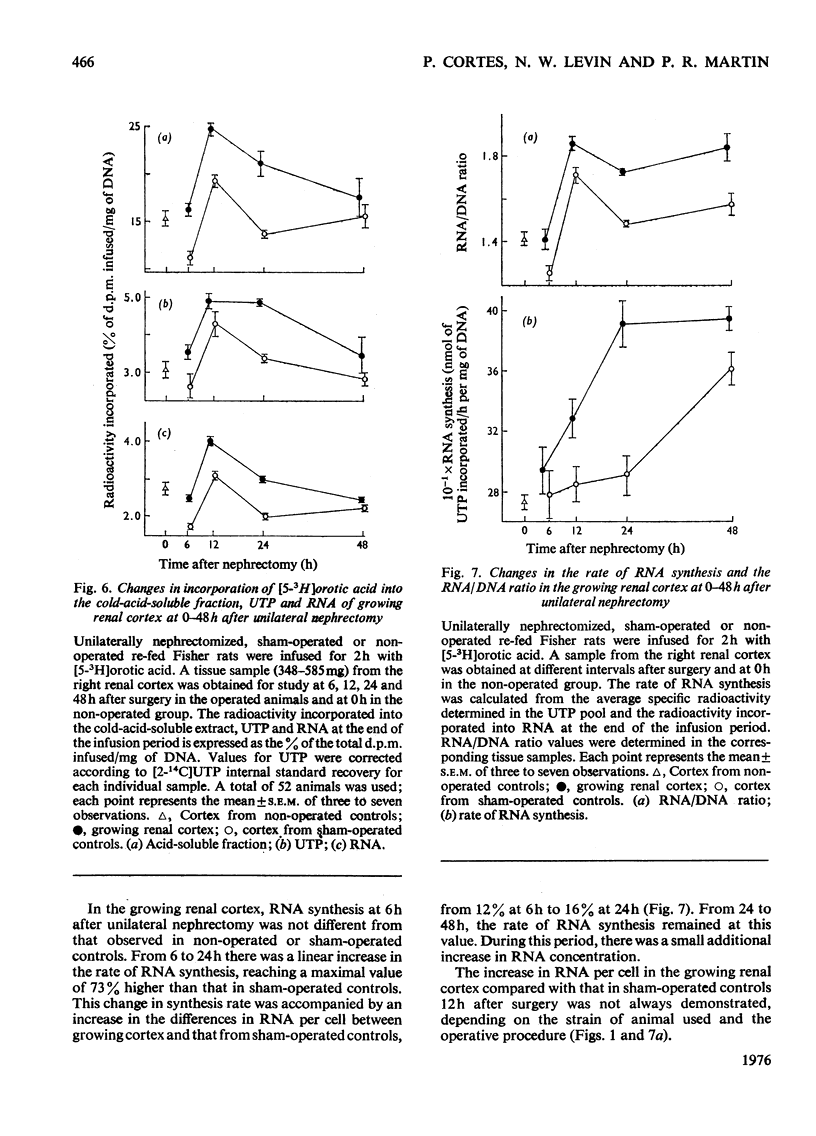
The mechanisms responsible for the increase in RNA per cell during the first 48h of renal compensatory growth were studied in the renal cortex. Unilaterally nephrectomized, sham-operated or non-operated rats were used. Incorporation into RNA of labelled precursors was studied in vivo and in vitro. Sham-operation produced significant changes in precursor incorporation, absolute amounts of UTP and RNA, and the rate of RNA synthesis. At 6h after surgery, the amount of RNA decreased in sham-operated controls, whereas that in growing cortex remained unchanged. Incorporation into RNA in vivo was greater in the growing cortex, although the rate of RNA synthesis was not increased. At 24h, precursor incorporation into RNA and UTP and RNA synthesis were all increased in the growing cortex. In contrast with results obtained in vivo, slices of growing cortex incorporated less labelled precursor into RNA than did cortex slices from sham-operated controls, from 3 to 48h. Maximal differences were found from 6 to 24h. An attempt was made to equalize endogenous precursor pool sizes by increasing the concentration of unlabelled uridine in the media; incorporation differences were narrowed significantly. Serum from nephrectomized animals did not increase precursor incorporation into RNA in vitro. An increase in RNA synthesis is an important factor in RNA accretion in the renal cortex beyond 12h of compensatory growth. This is accompanied by increased UTP content and preceded by expansion of other pools. The amount of labelled precursor incorporated into RNA is greatly influenced by its delivery rate to the growing kidney in vivo and by intracellular dilution of expanded precursor pools in vitro.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNANKE D., EPSTEIN F. H. METABOLISM OF THE RENAL MEDULLA. Am J Physiol. 1965 Mar;208:541–545. doi: 10.1152/ajplegacy.1965.208.3.541. [DOI] [PubMed] [Google Scholar]
- BRESNICK E. EARLY CHANGES IN PYRIMIDINE BIOSYNTHESIS AFTER PARTIAL HEPATECTOMY. J Biol Chem. 1965 Jun;240:2550–2556. [PubMed] [Google Scholar]
- Brown P. R., Miech R. P. Comparison of cell extraction procedures for use with high pressure liquid chromatography. Anal Chem. 1972 May;44(6):1072–1073. doi: 10.1021/ac60314a033. [DOI] [PubMed] [Google Scholar]
- Brown P. R. Stability of nucleotide solutions on storage as determined by high-pressure liquid chromatography. Anal Biochem. 1971 Sep;43(1):305–306. doi: 10.1016/0003-2697(71)90138-2. [DOI] [PubMed] [Google Scholar]
- Brown P. R. The rapid separation of nucleotides in cell extracts using high-pressure liquid chromatography. J Chromatogr. 1970 Oct 21;52(2):257–272. doi: 10.1016/s0021-9673(01)96573-2. [DOI] [PubMed] [Google Scholar]
- Bucher N. L., Swaffield M. N. Ribonucleic acid synthesis in relation to precursor pools in regenerating rat liver. Biochim Biophys Acta. 1969 Feb 18;174(2):491–502. doi: 10.1016/0005-2787(69)90278-0. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- DOHI S. R., TERZIAN J. F., WIDMAN A., BRENTANI R., FAUSTO N., LIBERMAN B., RABINOVITCH M. Inactivation of serum ribonuclease by the kidney. Am J Physiol. 1959 Apr;196(4):924–926. doi: 10.1152/ajplegacy.1959.196.4.924. [DOI] [PubMed] [Google Scholar]
- Daugharty T. M., Ueki I. F., Nicholas D. P., Brenner B. M. Renal response to chronic intravenous salt loading in the rat. J Clin Invest. 1973 Jan;52(1):21–31. doi: 10.1172/JCI107167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N. The control of RNA synthesis during liver regeneration. OMP pyrophosphorylase and decarboxylase activities in normal and regenerating liver. Biochim Biophys Acta. 1969 May 20;182(1):66–75. doi: 10.1016/0005-2787(69)90521-8. [DOI] [PubMed] [Google Scholar]
- Fields T., Brox L. Specific activities of the UTP pools of human lymphocytes after uridine- 3 H labeling. Can J Biochem. 1973 Jun;51(6):954–957. doi: 10.1139/o73-121. [DOI] [PubMed] [Google Scholar]
- Forsdyke D. R. Application of the isotope-dilution principle to the analysis of factors affecting the incorporation of (3H) uridine and (3H) cytidine into cultured lymphocytes. Evaluation of pools in serum and culture media. Biochem J. 1971 Dec;125(3):721–732. doi: 10.1042/bj1250721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody H. E., Ellem K. A. Nutritional effects on precursor uptake and compartmentalization of intracellular pools in relation to RNA synthesis. Biochim Biophys Acta. 1975 Feb 24;383(1):30–39. doi: 10.1016/0005-2787(75)90243-9. [DOI] [PubMed] [Google Scholar]
- HARTMAN M. E., BONFILIO A. C. Effect of nephrectomy on the minute renal circulation. Am J Physiol. 1959 May;196(5):1119–1121. doi: 10.1152/ajplegacy.1959.196.5.1119. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Thomson R. Y. Chemical aspects of compensatory renal hypertrophy. Cancer Res. 1965 Dec;25(11):1882–1887. [PubMed] [Google Scholar]
- Halliburton I. W., Thomson R. Y. The effect of diet and of unilateral nephrectomy on the composition of the kidney. Cancer Res. 1967 Sep;27(9):1632–1638. [PubMed] [Google Scholar]
- Hill J. M., Ab G., Malt R. A. Ribonucleic acid labelling and nucleotide pools during compensatory renal hypertrophy. Biochem J. 1974 Dec;144(3):447–453. doi: 10.1042/bj1440447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. M. Ribosomal RNA metabolism during renal hypertrophy. Evidence of decreased degradation of newly synthesized ribosomal RNA. J Cell Biol. 1975 Jan;64(1):260–265. doi: 10.1083/jcb.64.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki R., Lingis J. Unabated renal hypertrophy in uninephrectomized rats treated with hydroxyurea. Am J Physiol. 1970 Nov;219(5):1188–1191. doi: 10.1152/ajplegacy.1970.219.5.1188. [DOI] [PubMed] [Google Scholar]
- Johnson H. A., Vera Roman J. M. Compensatory renal enlargement. Hypertrophy versus hyperplasia. Am J Pathol. 1966 Jul;49(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Kessler J. I., Demeny M., Sobotka H. Rates of tissue uptake of palmitic acid-1-14C complexed with albumin by two different procedures. J Lipid Res. 1967 May;8(3):185–190. [PubMed] [Google Scholar]
- Koide T., Rabinowitz M. Biochemical correlates of cardiac hypertrophy. II. Increased rate of RNA synthesis in experimental cardiac hypertrophy in the rat. Circ Res. 1969 Jan;24(1):9–18. doi: 10.1161/01.res.24.1.9. [DOI] [PubMed] [Google Scholar]
- Kramer G., Wiegers U., Hilz H. mRNA turnover studies applying labeled uridine require an evaluation of specific radioactivities of UTP and RNA-U. Biochem Biophys Res Commun. 1973 Nov 16;55(2):273–281. doi: 10.1016/0006-291x(73)91084-x. [DOI] [PubMed] [Google Scholar]
- Kurnick N. B., Lindsay P. A. Nucleic acids in compensatory renal hypertrophy. Lab Invest. 1968 Jun;18(6):700–708. [PubMed] [Google Scholar]
- LEE J. B., VANCE V. K., CAHILL G. F., Jr Metabolism of C14-labeled substrates by rabbit kidney cortex and medulla. Am J Physiol. 1962 Jul;203:27–36. doi: 10.1152/ajplegacy.1962.203.1.27. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN L. M., STERN A. SERUM FACTOR IN RENAL COMPENSATORY HYPERPLASIA. Science. 1963 Dec 13;142(3598):1479–1480. doi: 10.1126/science.142.3598.1479. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee M. J., Vaughan M. H., Abrams R. Nature of the ribonucleic acid formed prior to deoxyribonucleic acid synthesis in kidney cortex cells cultured directly from the rabbit. J Biol Chem. 1970 Sep 10;245(17):4525–4533. [PubMed] [Google Scholar]
- Liberti J. P., Kline E. S. Differential protein and RNA synthesis of rat kidney cortex and medulla. Life Sci. 1974 Nov 15;15(10):1815–1826. doi: 10.1016/0024-3205(74)90183-0. [DOI] [PubMed] [Google Scholar]
- Lyons H. J., Evan A. P., McLaren L. C., Solomon S. In vitro evidence for a renotrophic factor in renal compensatory hypertrophy. Nephron. 1974;13(3):198–211. doi: 10.1159/000180394. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- MCKAY E. PENTOSE ESTIMATION BY THE ORCINOL METHOD, WITH PARTICULAR REFERENCE TO PLASMA PENTOSE. Clin Chim Acta. 1964 Oct;10:320–329. doi: 10.1016/0009-8981(64)90062-2. [DOI] [PubMed] [Google Scholar]
- Malt R. A. Compensatory growth of the kidney. N Engl J Med. 1969 Jun 26;280(26):1446–1459. doi: 10.1056/NEJM196906262802606. [DOI] [PubMed] [Google Scholar]
- Malt R. A., Lemaitre D. A. Accretion and turnover of RNA in the renoprival kidney. Am J Physiol. 1968 May;214(5):1041–1047. doi: 10.1152/ajplegacy.1968.214.5.1041. [DOI] [PubMed] [Google Scholar]
- Malt R. A., Stoddard S. K. Synthesis of ribosomal RNA and of microsomal membranes in the renoprival kidney. Biochim Biophys Acta. 1966 Apr 18;119(1):207–210. doi: 10.1016/0005-2787(66)90055-4. [DOI] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Novello F., Gumaa J. A., McLean P. The pentose phosphate pathway of glucose metabolism. Hormonal and dietary control of the oxidative and non-oxidative reactions of the cycle in liver. Biochem J. 1969 Mar;111(5):713–725. doi: 10.1042/bj1110713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGAWA K., SINCLAIR J. G. Study of mitosis in the compensatory hypertrophic kidney following unilateral nephrectomy in rat. Tex Rep Biol Med. 1958;16(2):215–218. [PubMed] [Google Scholar]
- Oertel J., Herken H. Ribonucleotides during the regeneration of the rat kidney after lesions by folic acid, HgCl2 and ligation of the ureter. Naunyn Schmiedebergs Arch Pharmacol. 1974;284(4):383–393. doi: 10.1007/BF00504707. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools in Novikoff rat hepatoma cells growing in suspension culture. 3. Effects of nucleosides in medium on levels of nucleotides in separate nucleotide pools for nuclear and cytoplasmic RNA synthesis. J Cell Biol. 1972 Jan;52(1):131–146. doi: 10.1083/jcb.52.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools of Novikoff rat hepatoma cells growing in suspension culture. II. Independent nucleotide pools for nucleic acid synthesis. J Cell Physiol. 1971 Apr;77(2):241–248. doi: 10.1002/jcp.1040770213. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Richey D. P. Transport of nucleosides, nucleic acid bases, choline and glucose by animal cells in culture. Biochim Biophys Acta. 1974 Dec 16;344(3-4):263–305. doi: 10.1016/0304-4157(74)90010-0. [DOI] [PubMed] [Google Scholar]
- Preuss H. G., Goldin H. A renotropic system in rats. J Clin Invest. 1976 Jan;57(1):94–101. doi: 10.1172/JCI108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss H. G., Terryi E. F., Keller A. I. Renotropic factor(s) in plasma from uninephrectomized rats. Nephron. 1970;7(5):459–470. doi: 10.1159/000179845. [DOI] [PubMed] [Google Scholar]
- Reiter R. J. Cellular proliferation and deoxyribonucleic acid synthesis in compensating kidneys of mice and the effect of food and water restriction. Lab Invest. 1965 Sep;14(9):1636–1643. [PubMed] [Google Scholar]
- Rickwood D., Klemperer H. G. Decreased ribonucleic acid synthesis in isolated rat liver nuclei during starvation. Biochem J. 1970 Nov;120(2):381–384. doi: 10.1042/bj1200381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. S., Malamud D., Caulfield J. A., Malt R. A. Differential labeling with orotic acid and uridine in compensatroy renal hypertrophy. Am J Physiol. 1975 Oct;229(4):952–954. doi: 10.1152/ajplegacy.1975.229.4.952. [DOI] [PubMed] [Google Scholar]
- Rous S. N., Wakim K. G. Kidney function before, during and after compensatory hypertrophy. J Urol. 1967 Jul;98(1):30–35. doi: 10.1016/S0022-5347(17)62817-9. [DOI] [PubMed] [Google Scholar]
- Royce P. C. Role of renal uptake of plasma protein in compensatory renal hypertrophy. Am J Physiol. 1967 Apr;212(4):924–930. doi: 10.1152/ajplegacy.1967.212.4.924. [DOI] [PubMed] [Google Scholar]
- Seifert J., Vácha J. Differences in the turnover of uridylic and cytidylic acids of rat liver cytoplasmic ribosomes. Arch Biochem Biophys. 1974 Jan;160(1):285–288. doi: 10.1016/s0003-9861(74)80036-6. [DOI] [PubMed] [Google Scholar]
- Sendecki W., Ombach M., Kwiatkowska-Patzer B. Isolation of polysomes from normal and hypertrophic rat kidney. Bull Acad Pol Sci Biol. 1972;20(5):273–278. [PubMed] [Google Scholar]
- Simpson D. P. Effect of nephrectomy on citrate metabolism in the rat. Am J Physiol. 1963 Nov;205(5):1049–1052. doi: 10.1152/ajplegacy.1963.205.5.1049. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Ehrenfeld E. Cytoplasmic and nuclear pyrimidine ribonucleotide pools in HeLa cells. J Mol Biol. 1973 Jun 15;77(1):177–187. doi: 10.1016/0022-2836(73)90371-9. [DOI] [PubMed] [Google Scholar]
- Threlfall G., Taylor D. M., Buck A. T. Studies of the changes in growth and DNA synthesis in the rat kidney during experimentally induced renal hypertrophy. Am J Pathol. 1967 Jan;50(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- Toback F. G., Lowenstein L. M. Uridine metabolism during normal and compensatory renal growth. Growth. 1974 Mar;38(1):17–34. [PubMed] [Google Scholar]
- Toback F. G., Mayers A. M., Lowenstein L. M. Alterations in renal and plasma amino acid concentrations during renal compensatory growth. Am J Physiol. 1973 Nov;225(5):1247–1251. doi: 10.1152/ajplegacy.1973.225.5.1247. [DOI] [PubMed] [Google Scholar]
- Toback F. G., Smith P. D., Lowenstein L. M. Effect of hyperosmolality on renal uridine metabolism. Am J Physiol. 1974 Jun;226(6):1474–1479. doi: 10.1152/ajplegacy.1974.226.6.1474. [DOI] [PubMed] [Google Scholar]
- Toback F. G., Smith P. D., Lowenstein L. M. Phospholipid metabolism in the initiation of renal compensatory growth after acute reduction of renal mass. J Clin Invest. 1974 Jul;54(1):91–97. doi: 10.1172/JCI107754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichi F. L., Earle D. P. Renal hypertrophy factor in serum of nephrectomized rats, with observations on species specificity. Proc Soc Exp Biol Med. 1970 Oct;135(1):38–41. doi: 10.3181/00379727-135-34982. [DOI] [PubMed] [Google Scholar]
- Vácha J., Seifert J. Turnover of cytidine and uridine components of acid-soluble pool and RNA of cytoplasmic ribosomes after repeated phenobarbital administration. Biochem Pharmacol. 1975 Feb 1;24(3):401–405. doi: 10.1016/0006-2952(75)90225-7. [DOI] [PubMed] [Google Scholar]
- WILLIAMS G. E. Some aspects of compensatory hyperplasia of the kidney. Br J Exp Pathol. 1961 Aug;42:386–396. [PMC free article] [PubMed] [Google Scholar]
- Willems M., Musilova H. A., Malt R. A. Giant nucleoplasmic RNA in the switch-on of compensatory renal growth. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1189–1194. doi: 10.1073/pnas.62.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Soeiro R. Turnover of nuclear RNA in HeLa cells: evidence for a single ribonucleotide pool. J Mol Biol. 1971 Jun 14;58(2):481–487. doi: 10.1016/0022-2836(71)90365-2. [DOI] [PubMed] [Google Scholar]


