Simple Summary
The odd-scaled snake genus Achalinus Peters, 1869 is widely distributed in northern Vietnam, China, and Japan, but is a group of snakes about which there is meager information. Achalinu ningshanensis was first described in 2022 and is only known from Ningshan County, Shaanxi Province, China. However, we detected a clear contradiction in the phylogenetic position between the type series and our newly collected topotypes. To solve this, we combined a mitochondrial phylogenetic analysis and morphological comparisons to revise the taxonomic status of A. ningshanensis in this study. Based on four mitochondrial combined gene fragments, molecular phylogenetic analyses indicated that A. ningshanensis is nested within a highly supported monophyletic group, forming a sister taxon to A. spinalis, which also revealed two well-supported lineages of A. ningshanensis. Based on morphology and phylogenetic methods, the lineage composed of the population from western Sichuan and southwestern Shaanxi represents a new subspecies, Achalinus ningshanensis occidentalis ssp. nov., and the other lineage represents the original species from southern Shaanxi and northeastern Sichuan, which we allocated as Achalinus ningshanensis ningshanensis. Finally, we provide a further discussion of the phylogenetic and taxonomic issues among the genus Achalinus.
Keywords: Achalinus ningshanensis occidentalis ssp. nov., Achalinus ningshanensis ningshanensis, mitochondrial DNA, morphological characters, taxonomy, molecular phylogeny
Abstract
Achalinu ningshanensis (Yang, Huang, Jiang, Burbrink, and Huang, 2022) was first described in Ningshan County, Shaanxi Province, China in 2022, based on seven female specimens. In this study, based on phylogenetic analyses using mitochondrial 12S ribosomal RNA (12S), 16S ribosomal RNA (16S), cytochrome c oxidase subunit 1 (CO1), cytochrome b (cyt b) gene fragments, and morphological examinations of specimens, we revise the taxonomic status of A. ningshanensis, and provide additional data on this species. The molecular phylogeny indicated that A. ningshanensis is nested in a highly supported monophyletic group, forming a sister taxon to A. spinalis, and is divided into two well-supported lineages, A and B, with an uncorrected p-distance between lineages from 3.6 to 4.3% for CO1. Therefore, we proposed that Lineage B from western Sichuan and southwestern Shaanxi is a new subspecies, Achalinus ningshanensis occidentalis ssp. nov., and Lineage A from southern Shaanxi and northeastern Sichuan is allocated as Achalinus ningshanensis ningshanensis. Morphologically, the new subspecies can be distinguished from its congeners, especially from Achalinus ningshanensis ningshanensis, by the following characteristics: (1) the tail is relatively short, with a TAL/TL ratio of 0.202–0.226 in males, and 0.155–0.178 in females; (2) there are two pairs of chin-shields; (3) there are 21–22 maxillary teeth; (4) the length of the suture between internasals is significantly shorter than that between prefrontals, with an LSBI/LSBP ratio of 0.502–0.773; (5) there are six supralabials, with the fourth and fifth in contact with the eye; (6) there are five to six infralabials, and the first to third or fourth touches the first pair of chin-shields; (7) there is one hexagonal loreal, with an LorH/LorL ratio of 0.612–1.040; (8) the two anterior temporals are in contact with the eye; (9) there are 155–160 ventrals in males, and 165–174 in females; (10) there are 60–65 subcaudals in males, and 49–53 in females, which are not paired; and (11) the dorsum is iridescent and uniformly charcoal black, lacks a longitudinal vertebral line, and has a dark brown or dark gray ventral area.
1. Introduction
The genus Achalinus Peters, 1869, commonly named odd-scaled snakes due to their unique scutellation, represents a small-sized, cave-dwelling genus widely distributed across eastern and southeastern Asia, including northern Vietnam, China, and Japan [1,2,3,4,5]. Owing to their cryptic lifestyle, Achalinus species are difficult to detect in the wild. Consequently, for a considerable period, limited studies addressed the taxonomy, ecology, and natural history of Achalinus, and until 2019, this genus included only nine species [2,5]. With more comprehensive field sampling and DNA-barcoding deep efforts, the previously underestimated biodiversity of Achalinus has gradually been revealed, and in the past five years alone, at least 20 new species have been described [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Currently, this genus contains twenty-eight known species and two subspecies, with the majority found in China (twenty-one of twenty-eight) [15,16].
The Ningshan odd-scaled snake, Achalinus ningshanensis Yang, Huang, Jiang, Burbrink and Huang, 2022, was described based on seven female specimens from Ningshan County, Shaanxi Province, China [12]. It is mainly distinguished from its congeners by a combination of the following morphological characteristics: (1) the dorsum is uniformly dark brown and lacks a longitudinal vertebral line; (2) the venter surface is brown; (3) a dotted black streak in the middle of the subcaudals is lacking; (4) the tail length is relatively short, with a TAL/TL ratio of 0.12–0.16 in females; (5) there are fewer subcaudals, 41–46, in females; (6) 23 rows of dorsal scales throughout, which are strongly keeled, and the outer-most rows on both sides of the body are also keeled and slightly enlarged; (7) one loreal; (8) the internasal is not fused to prefrontal area; (9) the length of the suture between the internasals almost equal to that between the prefrontals; (10) preocular and postocular absent; (11) there are six supralabials; (12) there are five infralabials, and the first three (rarely, two) touch the first pair of chin-shields; (13) there are three pairs of chin-shields. During previous herpetological surveys, we collected six Achalinus specimens from Ningshan County, Shaanxi Province, and one specimen from Wanyuan City, Sichuan Province, China (Figure 1). Through morphological examinations, these specimens can undoubtedly be identified as A. ningshanensis. A phylogenetic analysis showed that the specimens from Ningshan County and Wanyuan City are nested within a highly supported monophyletic group, forming a sister taxon to A. spinalis (Peters, 1869). However, the molecular phylogenetic analysis provided by Yang et al. [12] showed that A. ningshanensis formed a sister group to A. yangdatongi Hou, Wang, Guo, Chen, Yuan and Che, 2021, which is clustered together with A. juliani Ziegler, Nguyen, Pham, Nguyen, Pham, Van Schingen, Nguyen and Le, 2019 and A. ater Bourret, 1937. The contradiction between the morphological and molecular results is undoubtedly confusing.
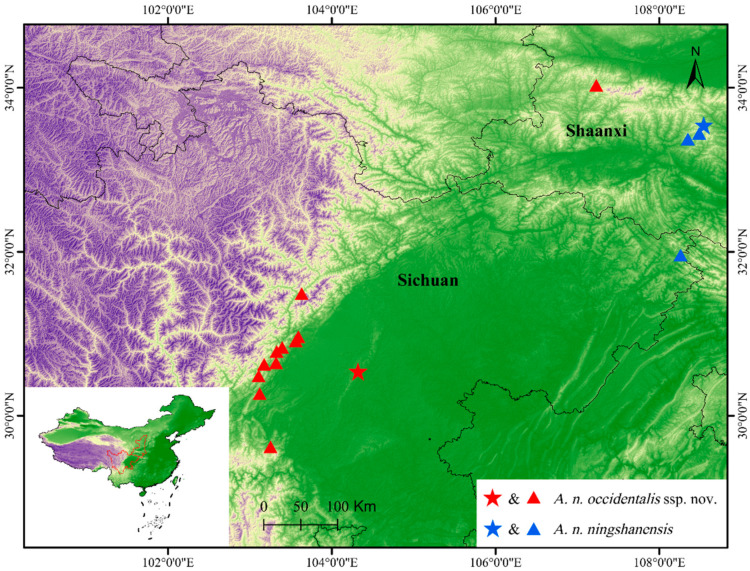
Figure 1.
Known distribution of two subspecies of Achalinus ningshanensis: Achalinus ningshanensis occidentalis ssp. nov. (red star and red triangles) and A. n. ningshanensis (blue star and blue triangles). Stars represent the type of locality, and triangles represent the other known localities.
To address the phylogenetic divergence observed between the newly collected specimens and A. ningshanensis, we reached out to the original authors to re-sequence the type series of A. ningshanensis. However, due to the poor preservative conditions, we could not obtain high-quality PCR products from the tissues of all types of specimens to sequence and reestablish the phylogenetic relationship. Therefore, we conducted a detailed morphological examination of both the new specimens and the type series of A. ningshanensis. Our findings showed that the newly collected Achalinus specimens from Ningshan County and Wanyuan County matched the morphology of the type series, confirming their identification as A. ningshanensis. The discrepancies in the phylogenetic analysis appear to be sequencing errors in Yang et al. [12].
During the synchronous field work in 2024, we collected a total of 13 Achalinus specimens from western Sichuan Province, China. The phylogenetic analysis showed that these specimens were clustered within the same monophyletic group as the authentic sequences of A. ningshanensis but formed a distinct, well-supported lineage to A. ningshanensis within the clade. Morphologically, the Achalinus specimens from western Sichuan are distinguished from A. ningshanensis. Additionally, during our examination of specimens deposited in the collections of Anhui Normal University Museum (ANU), we found that a specimen collected from Taibai County, which was identified as A. spinalis, exhibited the same morphological characteristics as specimens from western Sichuan. Hence, in this study, based on the examination of museum material and newly collected Achalinus specimens, we reassessed the taxonomic status of A. ningshanensis, which was also supported by the data on molecular differentiation from the analyses of mitochondrial 12S ribosomal RNA (12S), 16S ribosomal RNA (16S), cytochrome c oxidase subunit 1 (CO1), and cytochrome b (cyt b) gene fragments. We provide additional data of this species and describe a new subspecies of A. ningshanensis from western Sichuan and southwestern Shaanxi Province, China.
2. Materials and Methods
2.1. Morphological Comparisons
A total of 32 specimens of the genus Achalinus were collected from 2021 to 2024 (Table 1). Sex was determined by tail dissection and by determining if hemipenes were present. After euthanasia with a lethal injection of a 0.7% tricaine methanesulfonate (MS-222, Changmao Biochemical Engineering Co., Ltd., Changzhou, China) solution, fresh liver tissue was extracted and immediately preserved in 95% ethanol. The specimens were fixed in 10% formaldehyde for one day, then transferred to 75% ethanol for permanent preservation, and deposited in Qinghai University Museum (QHU) and Chengdu Institute of Biology (CIB), Chinese Academy of Sciences. Sampling procedures involving live snakes were in accordance with the Wild Animals Protection Law of China and approved by the Institutional Ethics Committee of Qinghai University (protocol code SL-2023028). In addition, we also examined eight Achalinus specimens (including the type series of A. ningshanensis) deposited in Anhui Normal University Museum (ANU).
The terminology and methods of measurement characteristics and scalation counts followed Zhao [17], Ma et al. [13], and Xu et al. [18]. Bilateral morphological character measurements and scale feature counts were given as left/right. The utilization of abbreviations for morphological characteristics follows the conventions established by Darko et al. [19]. Three measurement characters were measured with a Deli Stainless Ruler (No. 8462) to the nearest 1 mm: SVL (snout–vent length), TAL (tail length), and TL (total length), and all other measurements were performed using Deli digital calipers (DL312200) to the nearest 0.1 mm: HL (head length) was taken from the tip of snout to the posterior margin of mandible; HW (head width) was measured around the widest part of the head in dorsal view; LorH (loreal height) was measured from the highest part to the lowest part of the loreal in the lateral view; LorL (loreal length) was measured from the most anterior loreal to the most posterior loreal in lateral view; LSBI (length of the suture between internasals); LSBP (length of the suture between prefrontals); ED (eye diameter) was taken from the anterior corner of the eye to the posterior corner. The scalation features are listed as follows: SL (supralabials); IL (infralabials); IL-1st Chin (infralabials touching the first pair of chin-shields); Lor (loreals); PRO (preoculars); PO (postoculars); TEMP (temporals); aTEMP-Eye (the number of anterior temporals touching the eye); SPO (supraoculars); DSR (dorsal scale rows), counted at one-head-length behind the head, at midbody, at one-head-length before the cloacal plate; VS (ventrals), counted from the anterior-most wide ventral scale after the chin-shields, following the midline, and concluding before the cloacal plate, CP (cloacal plate), and SC (subcaudals). The keeling states of the outer-most dorsal scale rows (KOD) were measured from the same positions as where we counted the dorsal scales. The number of the maxillary teeth (MT) was confirmed through scanning photography obtained by nano-computerized tomography (CT). Scans were performed using a GE V|tome|X m dual tube 300/180 kV system (developed by the Institute of High Energy Physics (IHEP), Chinese Academy of Sciences) at the Key Laboratory of Vertebrate Evolution and Human Origins, Institute of Vertebrate Paleontology and Paleoanthropology (IVPP), Chinese Academy of Sciences. The specimens were scanned with an energy beam of 80 kV and a flux of 80× μA using a 360° rotation and then reconstructed into the 4096 × 4096 matrices of 1536 slices. The final CT-reconstructed skull images were exported with a minimum resolution of 11.0 μm. Skull images were exported from the virtual 3D model reconstruction using Volume Graphics Studio ver. 3.4.0. Morphological comparison between species of the genus Achalinus were obtained from the specimens examined in this study and many key references [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33].
Given the geographic and phylogenetic proximity between two populations of A. ningshanensis, we conducted statistical analyses on mensural and meristic characteristics using R v.4.3.2. The traits analyzed included HW/HL, LorH/LorL, LSBI/LSBP, TAL/TL, ED/HL, IL, Chin, VS, SC, and MT, which are the common diagnosis traits of Achalinus, with sexes compared separately due to sexual dimorphism in Achalinus. Morpho-spatial clustering and species/population positions were assessed using Principal Component Analysis (PCA) on a dataset of five meristic and five normalized morphometric characters, performed with the prcomp function and visualized using the factoextra package in R v.4.3.2.
2.2. Molecular Phylogeny
Genomic DNA was extracted from preserved liver tissues using QIAamp DNA Mini Kit (QIAGEN, Changsheng Biotechnology Co., Ltd., Changchun, China). Four mitochondrial gene fragments were targeted for phylogenetic analysis, including the 12S ribosomal RNA (12S), 16S ribosomal RNA (16S), cytochrome c oxidase subunit 1 gene (CO1), and cytochrome b (cyt b). PCR conditions followed previously reported protocols; 12S was amplified by primers 12S2LM (5′-ACACACCGCCCGTCACCCT-3′)/16S5H (5′-CTACCTTTGCACGGTTAGGATACCGCGGC-3′) [34], 16S was amplified by primers 16Sar_L (5′-CGCCTGTTTATCAAAAACAT-3′)/16Sbr-H (5′-CCGGTCTGAACTCAGATCACGT-3′) [35], CO1 was amplified by primers Chmf4 (5′-TYTCWACWAAYCAYAAAGAYATCGG-3′)/Chmr4 (5′-ACYTCRGGRTGRCCRAARAATCA-3′) [36], and cyt b was amplified by primers L14910 (5′-GACCTGTGATMTGAAAACCAYCGTTGT-3′)/H16064 (5′-CTTTGGTTTACAAGAACAATGCTTTA-3′) [37]. PCR products were sequenced by Shanghai Map Biotech Co., Ltd. The raw sequences were assembled using SeqMan in the DNASTAR v.11.1 software package [38] and aligned in MEGA X [39]. The newly generated sequences have been submitted to GenBank (Table 1).
To explore the phylogenetic relationships, we used 12S, 16S, CO1, cyt b sequences from 14 species, along with CO1 sequences from 12 additional species of the genus Achalinus. Homologous sequences from Stoliczkia vanhnuailianai Lalronunga, Lalhmangaiha, Zosangliana, Lalhmingliani, Gower, Das and Deepak, 2021 and Xenodermus javanicus Reinhardt, 1836 were used as outgroups. Since considerable species of the genus Achalinus currently only have CO1 sequences available, we generated maximum likelihood (ML) phylogenetic trees based on both the CO1 dataset and the concatenated 12S/16S/CO1/cyt b dataset using IQ-TREE v1.6.12 [40], with node support evaluated through 5000 ultrafast bootstrap replicates (UFB), where UFB ≥ 95% was considered well-supported [41]. Additionally, single-branch support was assessed via the SH-like approximate likelihood ratio test (SH-aLRT) with 1000 replicates, considering nodes with SH ≥ 80% as well-supported [42]. Uncorrected pairwise distances (p-distance) among closely related congeners were calculated in MEGA X software [39]. All sequences were obtained from National Center for Biotechnology Information (NCBI) except the newly generated sequences. Bayesian posterior probabilities (BI, %) ≥ 95 were considered significantly supported.
3. Results
3.1. Phylogenetic Relationship
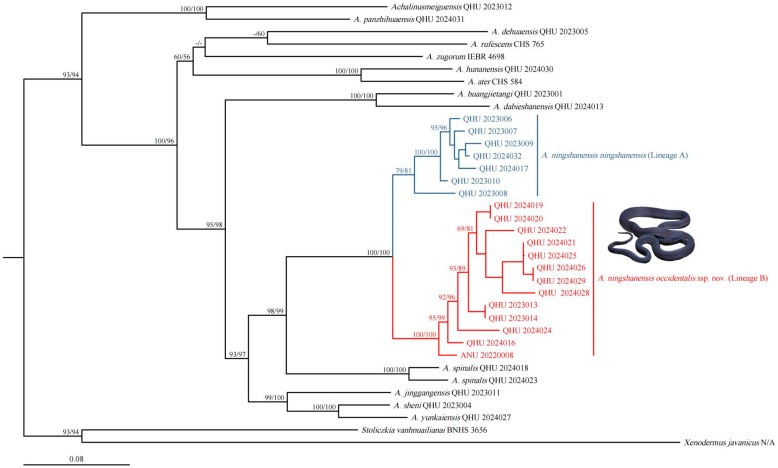
The concatenated sequence alignment was 2088 bp in length (12S = 318 bp; 16S = 492 bp; CO1 = 622 bp; cyt b = 656 bp). The topology obtained by the maximum likelihood analysis is shown in Figure 2: all putative species of Achalinus formed a highly supported lineage (SH 93/UFB 94). The specimens collected from Shaanxi Province and Sichuan Province formed a monophyletic group with well-supported values (SH 98/UFB 99) and were identified as the sister taxon to A. spinalis. The tree result was also consistent with the phylogenic relationship with two well-separated operational taxonomic units (OTUs): Lineage A and Lineage B. Lineage A contains specimens from southern Shaanxi and northeastern Sichuan, representing A. ningshanensis ningshanensis, while Lineage B comprises all samples from western Sichuan Province and southwestern Shaanxi Province, representing a potential subspecies of A. ningshanensis.
Figure 2.
Maximum likelihood tree of the genus Achalinus inferred from four mitochondrial (12S/16S/CO1/cyt b) fragments. The nodes supporting values on branches are presented with the SH-like approximate likelihood ratio test (SH)/Ultrafast Bootstrap Approximation (UFB); the ones lower than 50 are displayed as “–”.
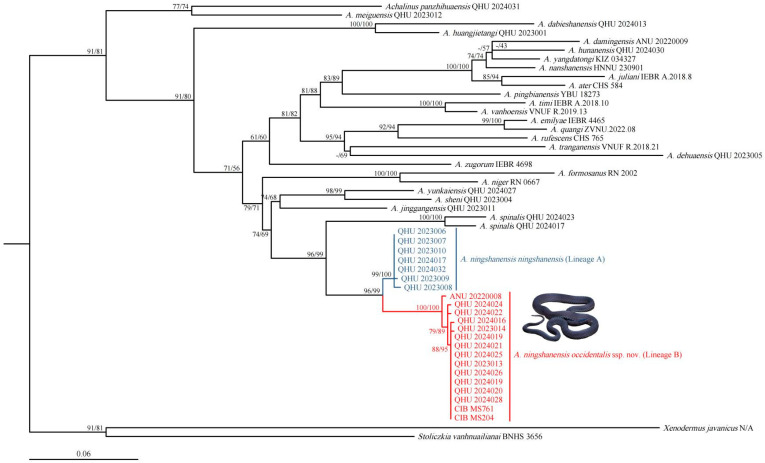
Based on the CO1 gene (622 bp) alone, the phylogenetic tree constructed using maximum likelihood analysis showed that the samples of Achalinus ningshanensis formed a monophyletic group with two well-supported, deeply divergent clades, Lineage A and Lineage B (SH 96/UFB 99, Figure 3). The uncorrected p-distances between A. ningshanensis and other Achalinus species ranged from 8.8% (vs. A. spinalis) to 19.1% (vs. A. panzhihuaensis Hou, Wang, Guo, Chen, Yuan, and Che, 2021) (Table 2). The p-distance between Lineage A and Lineage B was 3.6% to 4.3%, which is close to the distances observed between certain Achalinus species, such as A. nanshanensis Li, Zhu, Xiao, Wu, Yang, Zhang, and Mo, 2024 vs. A. yangdatongi Hou, Wang, Guo, Chen, Yuan, and Che, 2021 (4.5%). The intraspecific genetic distances within Lineage A and Lineage B ranged from 0.0% to 0.5% and 0.0% to 0.7%, respectively.
Figure 3.
Maximum likelihood tree of the genus Achalinus inferred from CO1 fragments. The nodes supporting values on branches are presented with the SH-like approximate likelihood ratio test (SH)/Ultrafast Bootstrap Approximation (UFB); the ones lower than 50 are displayed as “–”.
Table 2.
Uncorrected p-distance (%) among the Achalinus species based on partial mitochondria CO1 gene.
| ID | Species | 1–15 | 16–22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–15 | A. n. occidentalis ssp. nov. | 0.0–0.7 | ||||||||||||
| 16–22 | A. n. ningshanensis | 3.6–4.3 | 0.0–0.5 | |||||||||||
| 23 | A. ater | 16.3–16.4 | 15.1–15.8 | – | ||||||||||
| 24 | A. dabieshanensis | 15.8–16.3 | 15.8–16.5 | 17.6 | – | |||||||||
| 25 | A. damingensis | 16.5–16.9 | 14.3–14.9 | 8.4 | 18.7 | – | ||||||||
| 26 | A. dehuaensis | 17.4–18.0 | 16.7–16.9 | 19.4 | 21.0 | 18.5 | – | |||||||
| 27 | A. emilyae | 14.7–15.3 | 14.6–15.1 | 13.4 | 20.6 | 15.0 | 16.8 | – | ||||||
| 28 | A. meiguensis | 16.8–17.1 | 15.8–16.0 | 17.5 | 20.4 | 19.4 | 20.1 | 17.0 | – | |||||
| 29 | A. formosanus | 15.8–16.0 | 14.3–14.7 | 16.4 | 22.0 | 17.2 | 17.9 | 15.8 | 17.2 | – | ||||
| 30 | A. huangjietangi | 15.6–15.8 | 14.7–15.0 | 15.3 | 9.4 | 17.2 | 17.6 | 16.7 | 16.5 | 19.3 | – | |||
| 31 | A. hunanensis | 17.1–17.5 | 15.1–15.5 | 7.5 | 20.1 | 7.1 | 18.5 | 14.5 | 18.4 | 16.7 | 17.8 | – | ||
| 32 | A. jinggangensis | 11.4–11.6 | 9.4–9.8 | 14.4 | 16.7 | 13.7 | 16.0 | 14.6 | 14.1 | 13.3 | 14.5 | 13.3 | – | |
| 33 | A. juliani | 16.5–17.0 | 14.2–14.8 | 7.2 | 19.0 | 9.1 | 17.2 | 14.4 | 18.6 | 14.0 | 16.8 | 9.5 | 14.0 | – |
| 34 | A. nanshanensis | 16.0–16.0 | 14.9–15.6 | 7.0 | 19.5 | 5.9 | 16.5 | 15.2 | 20.3 | 16.5 | 18.2 | 5.8 | 14.3 | 8.4 |
| 35 | A. niger | 12.5–13.1 | 11.2–11.6 | 15.1 | 17.6 | 16.2 | 19.4 | 13.7 | 14.8 | 9.7 | 16.7 | 15.7 | 12.9 | 13.9 |
| 36 | A. panzhihuaensis | 18.9–19.1 | 17.6–18.0 | 18.4 | 18.3 | 19.8 | 20.8 | 17.7 | 12.5 | 20.2 | 16.3 | 19.1 | 17.6 | 18.6 |
| 37 | A. pingbianensis | 14.4–15.0 | 11.7–11.9 | 12.7 | 17.3 | 11.9 | 17.8 | 14.4 | 18.7 | 16.5 | 16.2 | 12.5 | 12.8 | 13.2 |
| 38 | A. quangi | 14.5–15.1 | 14.4–14.8 | 13.6 | 21.1 | 15.0 | 16.9 | 3.3 | 17.1 | 16.3 | 16.7 | 13.9 | 15.0 | 14.4 |
| 39 | A. rufescens | 16.9–17.4 | 15.5–15.7 | 14.0 | 19.0 | 14.6 | 14.7 | 10.3 | 20.9 | 16.4 | 16.5 | 13.3 | 14.2 | 12.5 |
| 40 | A. sheni | 11.8–12.3 | 10.5–10.7 | 14.8 | 17.8 | 15.9 | 16.4 | 15.5 | 14.7 | 14.6 | 16.7 | 14.6 | 10.4 | 15.9 |
| 41–42 | A. spinalis | 10.6–11.3 | 8.8–9.6 | 16.7–16.9 | 17.2–18.0 | 16.0–16.9 | 16.3–16.5 | 15.5–16.6 | 17.7–17.9 | 15.4 | 14.9–16.9 | 15.9–16.0 | 11.6–11.8 | 15.6–15.8 |
| 43 | A. timi | 14.8–15.1 | 13.6–14.1 | 15.0 | 18.9 | 15.1 | 19.1 | 15.1 | 18.1 | 15.1 | 17.8 | 14.6 | 15.3 | 16.1 |
| 44 | A. tranganensis | 15.2–15.7 | 14.7–15.3 | 14.6 | 17.6 | 16.2 | 16.5 | 13.3 | 17.5 | 19.7 | 15.0 | 15.6 | 16.2 | 15.6 |
| 45 | A. vanhoensis | 15.8–16.0 | 14.1–14.5 | 14.8 | 18.8 | 14.1 | 17.6 | 13.7 | 18.7 | 15.9 | 17.3 | 13.2 | 13.4 | 15.4 |
| 46 | A. yangdatongi | 15.0–15.2 | 13.2–13.9 | 6.5 | 19.6 | 5.9 | 15.6 | 14.4 | 19.2 | 16.6 | 15.8 | 5.7 | 13.0 | 7.7 |
| 47 | A. yunkaiensis | 11.4–12.4 | 11.0–11.4 | 14.1 | 18.0 | 14.5 | 16.1 | 15.1 | 16.9 | 13.3 | 15.6 | 13.9 | 10.2 | 13.9 |
| 48 | A. zugorum | 15.2–15.6 | 13.1–13.5 | 15.7 | 16.9 | 14.4 | 16.3 | 14.1 | 16.6 | 14.9 | 14.9 | 13.7 | 13.0 | 15.0 |
| ID | Species | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41–42 | 43 | 44 | 45 | 46 | 47 |
| 34 | A. nanshanensis | – | ||||||||||||
| 35 | A. niger | 14.7 | – | |||||||||||
| 36 | A. panzhihuaensis | 19.0 | 16.6 | – | ||||||||||
| 37 | A. pingbianensis | 12.9 | 13.2 | 19.2 | – | |||||||||
| 38 | A. quangi | 15.0 | 13.1 | 19.3 | 15.6 | – | ||||||||
| 39 | A. rufescens | 13.1 | 15.9 | 18.2 | 14.1 | 11.1 | – | |||||||
| 40 | A. sheni | 16.0 | 14.1 | 17.1 | 12.9 | 16.6 | 14.9 | – | ||||||
| 41–42 | A. spinalis | 15.9–16.4 | 15.2–15.6 | 18.9–19.6 | 13.8 | 15.0–15.7 | 14.2–14.8 | 11.8–13.0 | 3.2 | |||||
| 43 | A. timi | 15.7 | 13.1 | 18.4 | 12.9 | 15.1 | 16.6 | 15.5 | 15.8–16.6 | – | ||||
| 44 | A. tranganensis | 15.2 | 16.4 | 18.4 | 14.6 | 13.6 | 13.8 | 15.3 | 16.0–16.6 | 15.9 | – | |||
| 45 | A. vanhoensis | 14.3 | 14.3 | 17.5 | 11.8 | 13.9 | 15.6 | 15.6 | 13.9–14.8 | 5.4 | 14.9 | – | ||
| 46 | A. yangdatongi | 4.5 | 15.5 | 17.5 | 12.5 | 14.2 | 12.9 | 15.5 | 15.1–15.3 | 14.8 | 14.4 | 12.5 | – | |
| 47 | A. yunkaiensis | 13.3 | 13.7 | 17.1 | 13.1 | 15.3 | 13.6 | 6.9 | 12.4 | 15.5 | 13.9 | 13.8 | 12.9 | – |
| 48 | A. zugorum | 14.8 | 14.8 | 17.2 | 11.7 | 14.8 | 16.1 | 11.9 | 13.6–14.6 | 15.2 | 13.4 | 13.2 | 13.8 | 12.7 |
Table 1.
GenBank accession numbers, localities, and voucher information for all specimens used in this study.
| Species Name | Locality | Voucher | 12S | 16S | CO1 | cyt b | Reference |
|---|---|---|---|---|---|---|---|
| A. n. occidentalis ssp. nov. | Longquanyi, Sichuan, China | QHU 2023013 | PQ509310 | PQ505725 | PQ507884 | PQ515142 | This study |
| A. n. occidentalis ssp. nov. | Longquanyi, Sichuan, China | QHU 2023014 | PQ509311 | PQ505726 | PQ507885 | PQ515143 | This study |
| A. n. occidentalis ssp. nov. | Hongya, Sichuan, China | QHU 2024016 | PQ509313 | PQ505728 | PQ507887 | PQ515145 | This study |
| A. n. occidentalis ssp. nov. | Aba, Sichuan, China | QHU 2024019 | PQ509316 | PQ505731 | PQ507890 | PQ515148 | This study |
| A. n. occidentalis ssp. nov. | Chongzhou, Sichuan, China | QHU 2024020 | PQ509317 | PQ505732 | PQ507891 | PQ515149 | This study |
| A. n. occidentalis ssp. nov. | Lushan, Sichuan, China | QHU 2024021 | PQ509318 | PQ505733 | PQ507892 | PQ515150 | This study |
| A. n. occidentalis ssp. nov. | Lushan, Sichuan, China | QHU 2024022 | PQ509319 | PQ505734 | PQ507893 | PQ515151 | This study |
| A. n. occidentalis ssp. nov. | Dayi, Sichuan, China | QHU 2024024 | PQ509321 | PQ505736 | PQ507895 | PQ515153 | This study |
| A. n. occidentalis ssp. nov. | Qionglai, Sichuan, China | QHU 2024025 | – | PQ505737 | PQ507896 | PQ515154 | This study |
| A. n. occidentalis ssp. nov. | Qionglai, Sichuan, China | QHU 2024026 | – | PQ505738 | PQ507897 | PQ515155 | This study |
| A. n. occidentalis ssp. nov. | Chongzhou, Sichuan, China | QHU 2024028 | PQ509323 | PQ505740 | PQ507898 | PQ515157 | This study |
| A. n. occidentalis ssp. nov. | Dayi, Sichuan, China | QHU 2024029 | PQ509324 | PQ505741 | PQ507899 | PQ515158 | This study |
| A. n. occidentalis ssp. nov. | Mt. Qingcheng, Sichuan, China | CIB MS204 | – | – | PQ507876 | – | This study |
| A. n. occidentalis ssp. nov. | Dujiangyan, Sichuan, China | CIB MS761 | – | – | PQ507877 | – | This study |
| A. n. occidentalis ssp. nov. | Taibai, Shaanxi, China | ANU 20220008 | NC_032084 | NC_032084 | MK064591 | NC_032084 | Li et al. [43]; Peng et al. [44] |
| A. n. ningshanensis | Ningshan, Shaanxi, China | QHU 2023006 | PQ509303 | PQ505718 | PQ507879 | PQ515135 | This study |
| A. n. ningshanensis | Ningshan, Shaanxi, China | QHU 2023007 | PQ509304 | PQ505719 | PQ507880 | PQ515136 | This study |
| A. n. ningshanensis | Wanyuan, Sichuan, China | QHU 2023008 | PQ509305 | PQ505720 | PQ507881 | PQ515137 | This study |
| A. n. ningshanensis | Ningshan, Shaanxi, China | QHU 2023009 | PQ509306 | PQ505721 | PP725557 | PQ515138 | Xu et al. [18]; This study |
| A. n. ningshanensis | Ningshan, Shaanxi, China | QHU 2023010 | PQ509307 | PQ505722 | PP725558 | PQ515139 | Xu et al. [18]; This study |
| A. n. ningshanensis | Ningshan, Shaanxi, China | QHU 2024017 | PQ509314 | PQ505729 | PQ507888 | PQ515146 | This study |
| A. n. ningshanensis | Ningshan, Shaanxi, China | QHU 2024032 | PQ509328 | PQ505745 | PQ507902 | PQ515162 | This study |
| A. ater | China | CHS 584 | – | MK194076 | MK064760 | MK201421 | Li et al. [43] |
| A. dabieshanensis | Yuexi, Anhui, China | QHU 2024013 | PQ509325 | PQ505742 | PQ507900 | PQ515159 | This study |
| A. damingensis | Nanning, Guangxi, China | ANU 20220009 | – | – | OP644487 | – | Yang et al. [5] |
| A. dehuaensis | Minhou, Fujian, China | QHU 2023005 | PQ509302 | PQ505717 | PQ507878 | PQ515134 | This study |
| A. emilyae | Hoanh Bo, Vietnam | IEBR 4465 | – | – | MK330857 | – | Ziegler et al. [2] |
| A. formosanus | Taiwan, China | RN 2002 | – | – | KU529452 | – | Unpublished |
| A. hunanensis | Guizhou, China | QHU 2024030 | PQ509326 | PQ505743 | PQ281493 | PQ515160 | This study |
| A. huangjietangi | Jinyun, Zhejiang, China | QHU2023001 | PQ509312 | PQ505727 | PQ507886 | PQ515144 | This study |
| A. jinggangensis | Fujian, China | QHU 2023011 | PQ509308 | PQ505723 | PQ507882 | PQ515140 | This study |
| A. juliani | Ha Lang, Cao Bang, Vietnam | IEBR A.2018.8 | – | – | MK330854 | – | Ziegler et al. [2] |
| A. meiguensis | Dayi, Sichuan, China | QHU 2023012 | PQ509309 | PQ505724 | PQ507883 | PQ515141 | This study |
| A. nanshanensis | Huaihua, Hunan, China | HNNU 230901 | – | – | OR523368 | – | Li et al. [15] |
| A. niger | Taiwan, China | RN 0667 | – | – | KU529433 | – | Unpublished |
| A. panzhihuaensis | Panzhihua, Sichuan, China | QHU 2024031 | PQ509327 | PQ505744 | PQ507901 | PQ515161 | This study |
| A. pingbianensis | Honghe, Yunnan, China | YBU 18273 | – | – | MT365521 | – | Li et al. [43] |
| A. quangi | Phu Yen, Son La, Vietnam | ZVNU.2022.08 | – | – | OQ197471 | – | Pham et al., 2023 |
| A. rufescens | China | CHS 765 | – | MK194205 | MK064864 | MK201515 | Li et al. [43] |
| A. sheni | Shaoyang, Hunan, China | QHU 2023004 | PP725556 | Xu et al. [18]; This study | |||
| A. spinalis | Chongqing, China | QHU 2024018 | PQ509315 | PQ505730 | PQ507889 | PQ515147 | This study |
| A. spinalis | Hubei, China | QHU 2024023 | PQ509320 | PQ505735 | PQ507894 | PQ515152 | This study |
| A. timi | Thuan Chau, Son La, Vietnam | IEBR A.2018.10 | – | – | MK330856 | – | Ziegler et al. [2] |
| A. tranganensis | Ninh Binh, Vietnam | VNUF R.2018.21 | – | – | MW023086 | – | Luu et al. [7] |
| A. vanhoensis | Van Ho, Son La, Vietnam | VNUF R.2019.13 | – | – | ON677935 | – | Ha et al. [11] |
| A. yangdatongi | Wenshan, Yunnan, China | KIZ 034327 | – | – | MW664865 | – | Hou et al. [3] |
| A. yunkaiensis | Guizhou, China | QHU 2024027 | PQ509322 | PQ505739 | PQ281492 | PQ515156 | This study |
| A. zugorum | Bac Me, Ha Giang, Vietnam | IEBR 4698 | – | MT503100 | MT502775 | MT513238 | Miller et al. [8] |
| Out group | |||||||
| Stoliczkia vanhnuailianai | Mizoram, India | BNHS 3656 | OL352693 | OL352694 | OL422476 | OL422473 | Deepak et al. [45] |
| Xenodermus javanicus | – | – | AF544781 | AF544810 | – | AF544810 | Vidal and Hedges [46] |
| Xenodermus javanicus | Sumatera Barat, Indonesia | – | – | – | KP410747 | – | Teynié et al. [47] |
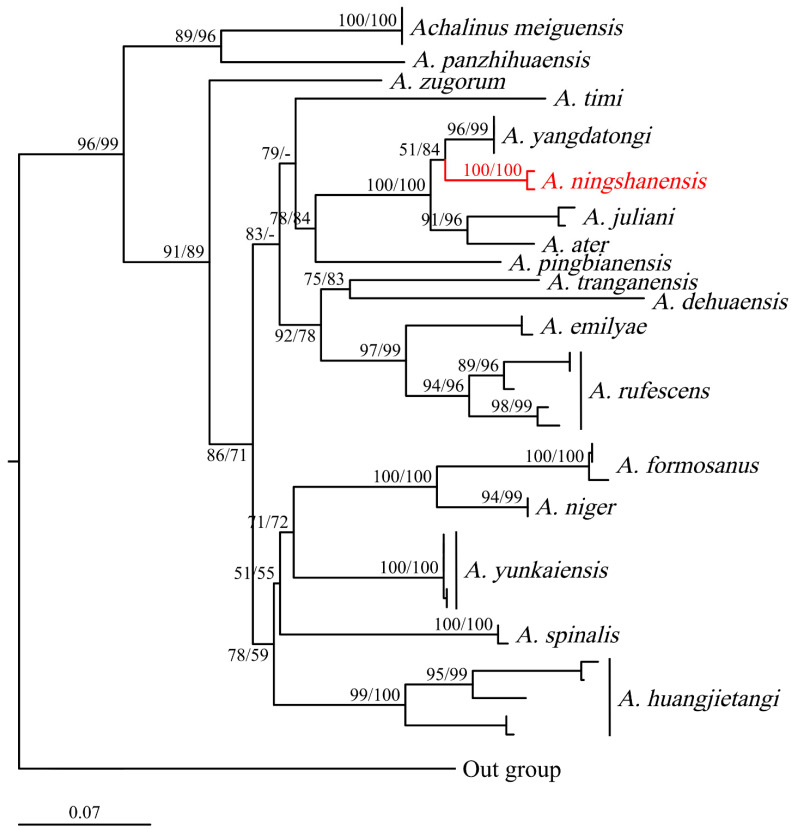
In Yang et al. [12], the molecular phylogenetic analysis showed that A. ningshanensis formed the sister group to A. yangdatongi Hou, Wang, Guo, Chen, Yuan and Che, 2021, and was then clustered together with A. juliani Ziegler, Nguyen, Pham, Nguyen, Pham, Van Schingen, Nguyen and Le, 2019 and A. ater Bourret, 1937 (Figure 4); however, this result appears inconsistent with actual phylogenetic relationships. We propose that the sequences ON548422 and ON548423 in Yang et al. [12] are erroneous. The accurate phylogenetic position and sequences of A. ningshanensis should be based on the findings in this study (Figure 2 and Figure 3; Table 1).
Figure 4.
Reconstructed phylogenetic tree based on data from Yang et al. [12]. The nodes supporting values on branches are presented with the SH-like approximate likelihood ratio test (SH)/Ultrafast Bootstrap Approximation (UFB); the ones lower than 50 are displayed as “–”.
3.2. Morphological Results
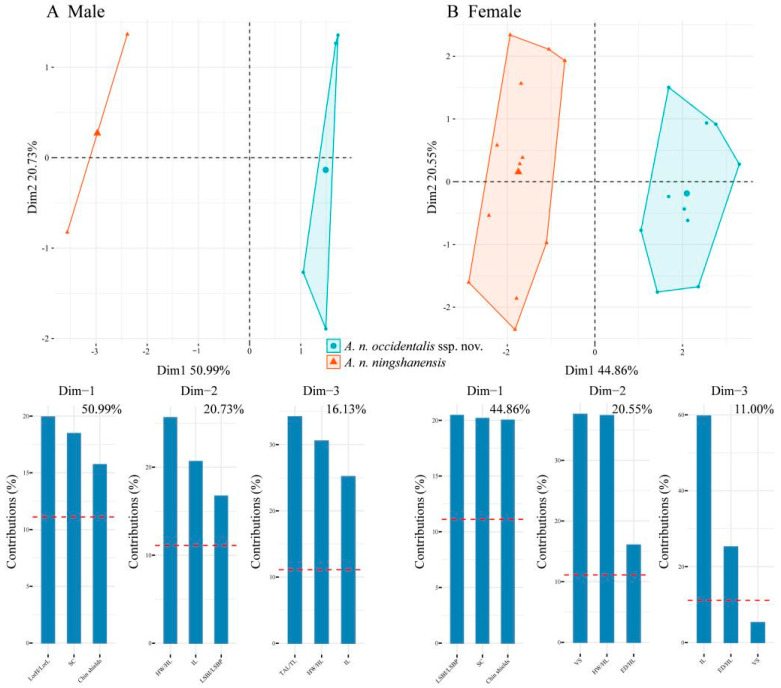
The scatter plots of PC1 and PC2 for the two populations of A. ningshanensis indicate that, regardless of sex, the samples for each subspecies form distinct clusters with no overlap (Figure 5). In the PCA results, the first four principal components explained 95.92% of the total variation in males, with PC1, PC2, PC3, and PC4 accounting for 50.99%, 20.73%, 16.13%, and 8.07% of the variance, respectively. Similarly, for females, the first four components captured a substantial proportion of variation (85.07% in total), with PC1, PC2, PC3, and PC4 contributing 44.86%, 20.55%, 11.00%, and 8.66% of the variance, respectively. Considering these morphological distinctions and the unique phylogenetic position, we propose that the population from western China represents a new subspecies of Achalinus ningshanensis.
Figure 5.
Male (A) and female (B) PCA plots between Achalinus ningshanensis occidentalis ssp. nov. and A. n. ningshanensis and bar plots of the percent contribution of each data type to Dim 1–3 of the PCA. The percentage score at the top of each bar plot is the percent contribution of that dimension to the overall variation in the dataset. The red dotted lines in the bar plots represent the mean percentage values.
3.3. Taxonomy
Achalinus ningshanensis ningshanensis Yang, Huang, Jiang, Burbrink and Huang, 2022
Ningshan odd-scaled Snake/Níng Shǎn Jǐ Shé Zhǐ Míng Yà Zhǒng (宁陕脊蛇指名亚种)
Figure 6, Figure 7, Figure 8, Figure 9 and Figure 15A–C
Figure 6.
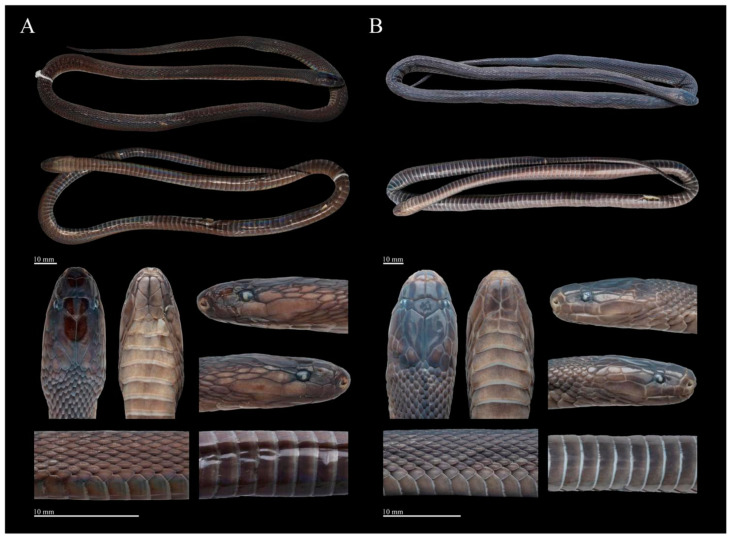
Dorsal (A) and ventral (B) views of living Achalinus ningshanensis ningshanensis. (A1,B1): QHU 2024017, male, from Ningshan County, Shaanxi Province; (A2,B2): QHU 2023009, female, from Ningshan County, Shaanxi Province. Photos by Yuhao Xu. Scale bars are not shown.
Figure 7.
Preserved specimen of the holotype of Achalinus ningshanensis ningshanensis (ANU 20220001, female). Photos by Diancheng Yang and Yuhao Xu. Scale bars: 10 mm.
Figure 8.
Preserved specimen of Achalinus ningshanensis ningshanensis. (A) QHU 2023008, adult male, from Wanyuan City, Sichuan Province; (B) QHU 2024032, adult female, topotype, from Ningshan County, Shaanxi Province. Photos by Yuhao Xu. Scale bars: 10 mm.
Figure 9.
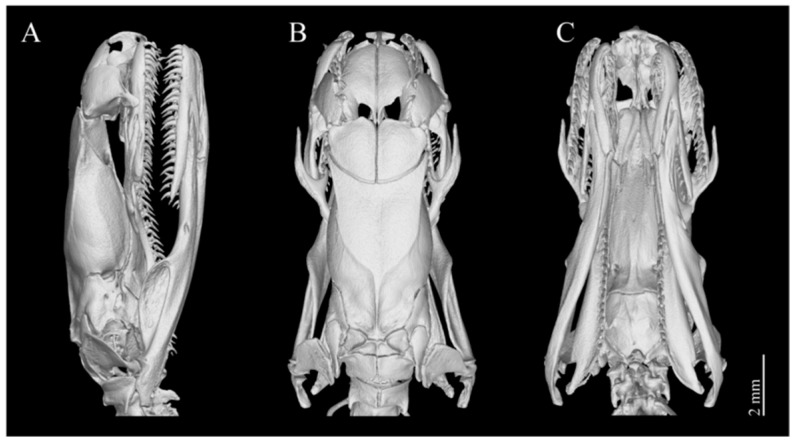
3D-reconstructed skull model of the holotype of Achalinus ningshanensis ningshanensis (ANU 20220001). (A) lateral view; (B) dorsal view; and (C) ventral view. Scale bars: 2 mm.
Chresonymy.
Achalinus ningshanensis: Li et al. [15]; Ma et al. [4]; Ma et al. [13]; Nguyen [48]; Xu et al. [18]; Yang et al. [5]; Yang et al. [12]; Zhang et al. [14].
Achalinus sp.: Xu et al. [18].
Examined specimens. A total of 14 specimens of A. n. ningshanensis were examined in this study. Females (n = 12): ANU 20220001–ANU 20220007, type series, collected by the team of Ke Jiang in summer of 2008 from Xunyangba Town, Ningshan County, Shaanxi Province, China (33°32′36.24″ N, 108°32′38.04″ E, 1372 m a. s. l.); QHU 2023006, QHU 2023007, QHU 2023009, and QHU 2023010, collected by the team of Lifang Peng in July of 2023 from Ningshan County, Shaanxi Province, China (33°26′40.2″ N, 108°29′04.56″ E, 1895 m a. s. l.); QHU 2024032, collected by Fengcheng Zhu, Zheming Zhang, and Fangyi Lai in September of 2024 from Chengguan Town, Ningshan County, Shaanxi Province, China (33°22′28.92″ N, 108°20′47.40″ E, 922 m a. s. l.). Males (n = 2): QHU 2023008, collected by Tianxuan Gu in July of 2023 from Mt. Yuquan, Wanyuan City, Sichuan Province, China (31°57′36.72″ N, 108°15′29.16″ E, 1453 m a. s. l.); QHU 2024017, collected by the team of Lifang Peng in April of 2024 from Ningshan County, Shaanxi Province, China (33°26′40.2″ N, 108°29′04.56″ E, 1895 m a. s. l.).
Revised diagnosis. Achalinus ningshanensis ningshanensis can be distinguished from its congeners by the following features: (1) the dorsum is uniformly brown to dark brown and lacks a longitudinal vertebral line; (2) the venter surface is brown; (3) the dotted black streak in the middle of the subcaudals is absent; (4) tail length is relatively short, with a TAL/TL ratio of 0.198–0.211 in males, and 0.121–0.161 in females; (5) fewer subcaudals, 51–56 in males, and 41–47 in females; (6) 23 rows of dorsal scales throughout, strongly keeled, and the outer-most rows on both sides of the body also keeled and slightly enlarged; (7) one loreal; (8) internasal not fused to prefrontal; (9) the length of the suture between internasals almost equal to that between prefrontals; (10) six supralabials; (11) four to five infralabials, with the first two or three touching the first pair of chin-shields; (12) three to four pairs of chin-shields; and (13) 20–22 maxillary teeth.
Description. Measurements and scalation data of A. n. ningshanensis specimens used in this study (n = 14) are presented in Table 3. The body is slender and cylindrical, TL 179–577 mm (363–412 mm in males, 179–577 mm in females); the tail is relatively short, with a TAL/TL ratio of 0.198–0.211 in males, and 0.121–0.161 in females; the head is slightly distinct from the neck, with an HW/HL ratio of 0.48–0.52 in males, and 0.37–0.57 in females; the eye is small, with an ED/HL ratio of 0.06–0.08 in males, and 0.07–0.10 in females; the rostrum is small, triangular, and slightly visible from above; the internasals are paired, and the length of the suture between the internasals is almost equal to that between the prefrontals, with an LSBI/LSBP ratio of 0.904–1.106; the nasal is divided into two sections by nasal cleft, with the nostrils in the anterior part of the nasal; the prefrontals are paired; the frontal area is pentagonal, pointed to the rear, slightly wider than high, and much shorter than the parietals; one loreal, subrectangular, LorH/LorL ratio 0.452–0.651; supraocular one, pentagonal; TEMP 7–9, arranged in three rows: (1–2) + (1–3) + (3–4), the anterior row contacts the eye; six supralabials, the fourth to fifth contact the eye, the last one is very elongated; three to four pairs of chin-shields; one mental; four to five infralabials, the first one in contact with each other after the mental area and before the first chin-shields. The first to third (rarely, second) touch the first pair of chin-shields.
Table 3.
Main morphological characteristics of Achalinus ningshanensis ningshanensis obtained from specimens examined in this study. K + K + K = keeled on anterior, mid, and posterior body.
| Voucher Number | QHU 2023006 | QHU 2023007 | QHU 2023008 | QHU 2023009 | QHU 2023010 | QHU 2024017 | QHU 2024032 |
|---|---|---|---|---|---|---|---|
| Location | Ningshan, Shaanxi | Ningshan, Shaanxi | Wanyuan, Sichuan | Ningshan, Shaanxi | Ningshan, Shaanxi | Ningshan, Shaanxi | Ningshan, Shaanxi |
| Sex | Female | Juvenil female | Male | Female | Female | Male | Female |
| TL | 577 | 179 | 363 | 571 | 428 | 412 | 472 |
| TAL | 83 | 28 | 72 | 89 | 67 | 87 | 70 |
| TAL/TL | 0.144 | 0.156 | 0.198 | 0.156 | 0.157 | 0.211 | 0.148 |
| HW | 7.8 | 4.7 | 6.1 | 8.3 | 6.9 | 6.5 | 7.2 |
| HL | 16.3 | 8.7 | 12.7 | 14.5 | 13.7 | 12.4 | 13 |
| ED | 1.33 | 0.84 | 0.7 | 1.29 | 1.01 | 1.0 | 1.3 |
| MT | 20 | 21 | 20 | 21 | 20 | 20 | 22 |
| SL | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| IL | 4/4 | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| Chin | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 4/3 | 4/4 |
| IL-1st Chin | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd |
| Lor | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| LorH | 0.98 | 0.68 | 0.80 | 1.27 | 1.11 | 0.95 | 0.91 |
| LorL | 2.07 | 1.12 | 1.47 | 1.95 | 1.63 | 1.54 | 1.53 |
| LorH/LorL | 0.473 | 0.607 | 0.544 | 0.651 | 0.681 | 0.617 | 0.595 |
| LSBI/LSBP | = | = | = | = | = | = | = |
| TEMP | 2 + 2 + 4/2 + 2 + 3 | 2 + 2 + 3/2 + 2 + 3 | 2 + 3 + 3/2 + 3 + 3 | 2 + 3 + 4/2 + 2 + 4 | 1 + 2 + 4/1 + 2 + 3 | 2 + 2 + 4/2 + 2 + 4 | 2 + 1 + 3/2 + 1 + 3 |
| ATEMP-Eye | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| DSR | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 |
| KOD | K + K + K | K + K + K | K + K + K | K + K + K | K + K + K | K + K + K | K + K + K |
| VS | 175 | 175 | 165 | 176 | 168 | 161 | 177 |
| SC | 47 | 47 | 51 | 47 | 45 | 56 | 44 |
| CP | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Voucher Number | ANU 20220001 | ANU 20220002 | ANU 20220003 | ANU 20220004 | ANU 20220005 | ANU 20220006 | ANU 20220007 |
| Location | Ningshan, Shaanxi | Ningshan, Shaanxi | Ningshan, Shaanxi | Ningshan, Shaanxi | Ningshan, Shaanxi | Ningshan, Shaanxi | Ningshan, Shaanxi |
| Sex | Female | Female | Female | Female | Female | Female | Female |
| TL | 436 | 491 | 527 | 416 | 398 | 450 | 430 |
| TAL | 62 | 72 | 64 | 63 | 64 | 69 | 65 |
| TAL/TL | 0.142 | 0.147 | 0.121 | 0.151 | 0.161 | 0.153 | 0.151 |
| HW | 7.16 | 7.48 | 4.75 | 6.27 | 5.5 | 5.94 | 4.79 |
| HL | 13.72 | 13.32 | 12.69 | 11.74 | 12.35 | 11.17 | 11.47 |
| ED | 1.12 | 1.19 | / | 1.1 | 1.12 | 0.82 | 0.96 |
| MT | 22 | 21 | 21 | 22 | / | / | / |
| SL | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| IL | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| Chin | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
| IL-1st Chin | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-2nd/1st-2nd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | / |
| Lor | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| LorH | 0.97 | 0.91 | 0.93 | 1.04 | 0.88 | 0.83 | / |
| LorL | 1.90 | 2.01 | 1.71 | 1.8 | 1.92 | 1.59 | / |
| LorH/LorL | 0.511 | 0.452 | 0.544 | 0.578 | 0.458 | 0.522 | / |
| LSBI/LSBP | = | = | = | = | = | = | = |
| TEMP | 2 + 3 + 4/2 + 3 + 4 | 2 + 3 + 4/2 + 3 + 4 | 2 + 2 + 4/2 + 2 + 4 | 2 + 2 + 3/2 + 2 + 3 | 2 + 2 + 4/2 + 2 + 4 | 2 + 2 + 3/2 + 2 + 3 | 2 + 2 + 4/2 + 2 + 4 |
| ATEMP-Eye | 2 | 2 | 2 | / | / | / | / |
| DSR | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-21 |
| KOD | K + K + K | K + K + K | K + K + K | K + K + K | K + K + K | K + K + K | K + K + K |
| VS | 167 | 174 | 171 | 170 | 159 | 174 | 164 |
| SC | 43 | 46 | 43 | 43 | 45 | 45 | 41 |
| CP | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Dorsal scale rows 23-23-23(21) are strongly keeled and lanceolate, and the outer-most row is also keeled and slightly enlarged. There are 161–165 in males vs. 159–177 in females; anal entire; SC 51–56 in males, and 41–47 in females, not paired.
Dentition. There are 20–22 maxillary teeth, without diastema, and they are nearly equal in length except for the first two, which are slightly smaller (Figure 9).
Coloration in life. In life, the dorsum is uniformly brown to dark brown with a metallic luster under light, and the interstitial skin of the dorsal area is dark brown. The ventral ground color is slightly lighter than that of the dorsum, and the posterior margins of ventral scales are light gray (Figure 6).
Coloration in preservation. In preservation, the coloration still resembles the specimen in life, except that the coloration of the dorsum deepens further, the background color of the venter becomes uniform light brownish, and posterior margins of ventral scales become grayish white (Figure 7 and Figure 8).
Distribution and natural history. Achalinus ningshanensis ningshanensis is currently known from Ningshan County, Ankang City, Shaanxi Province, and Wanyuan City, Dazhou City, Sichuan Province, China. Its known activity period spans from April to September, with peak activity occurring in the summer months. This species is typically found in grasslands, farmlands, and among stone piles near water sources at night, at elevations ranging from 800 to 1500 m. The surrounding habitat consists of secondary mixed forests of coniferous and broad-leaved trees (cf. this study, [12]).
Remarks. In 1983, Fang and Wang [49] reported a new provincial record for Achalinus rufescens in Shaanxi Province, based on two female specimens (voucher number 60,013 and 80019) collected from Hu County (now Huyi District, Xi’an City, at an altitude of 1400 m) and Ningshan County (at an altitude of 1500 m), Shaanxi Province. The main morphological characteristics were as follows: TL 517 + 77 mm in specimen 60013, and 400 + 70 mm in specimen 80019; the rostrum is triangular, its width is greater than its height, and is slightly visible from above; the internasals are paired, the length of the suture between the internasals is significantly shorter than that between the prefrontals, with the LSBI/LSBP ratio of 0.5; SL 6, the first one is the smallest, and the last one is very elongated; IL 5, the first one contact with each other after the mental; chin-shields 3/2; DSR 23-23-23, lanceolate and strongly keeled, the outer-most row smooth; VS 171–175; SC 43–46, not paired; CP complete. Based on the described morphology and collection locations, we suspect these specimens may actually be misidentified as A. ningshanensis. However, certain characteristics, such as the suture between internasals being much shorter than that between the prefrontals, the smooth outer-most dorsal scale row, and chin-shields 3/2, differ from A. ningshanensis ningshanensis. Since we have not been able to directly examine these specimens, we retained this record as it currently stands.
Achalinus ningshanensis occidentalis ssp. nov. Xu, Ma, Yang, Cai, Huang and Peng, 2024
http://zoobank.org/urn:lsid:zoobank.org:act:75A7E98C-EAB7-4C42-B02D-D0BAE362E3D9 (accessed on 2 November 2024)
Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15D–F and Figure 16
Figure 10.
Dorsal (A) and ventral (B) views of Achalinus ningshanensis occidentalis ssp. nov. in life. (A1,B1): QHU 2023013, holotype, adult female, from Longquanyi District, Sichuan Province; (A2,B2): QHU 2023014, paratype, adult male, from Longquanyi District, Sichuan Province; (A3,B3): QHU 2024016, paratype, adult male, from Hongya County, Sichuan Province. Photos by Yuhao Xu.
Figure 11.
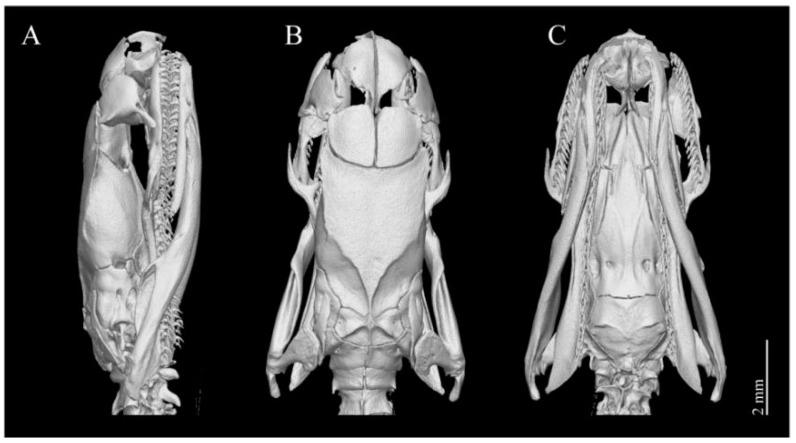
3D-reconstructed skull model of the paratype of Achalinus ningshanensis occidentalis ssp. nov. (QHU 2023014). (A) lateral view; (B) dorsal view; and (C) ventral view. Scale bars: 2 mm.
Figure 12.
Preserved specimen of the holotype of Achalinus ningshanensis occidentalis ssp. nov. (QHU 2023013, adult female). Photos by Yuhao Xu. Scale bars: 10 mm.
Figure 13.
Preserved specimen of the paratypes of Achalinus ningshanensis occidentalis ssp. nov. (A) QHU 2024016, adult male, from Hongya County, Sichuan Province; (B) QHU 2024093, subadult female, from Dayi County, Sichuan Province. Photos by Yuhao Xu. Scale bars: 10 mm.
Figure 14.
Habitats of Achalinus ningshanensis occidentalis ssp. nov. (A) Mt. Tiantai, Qionglai City, Sichuan Province, photo by Tianxuan Gu; (B) Lushan County, Yaan City, Sichuan Province, photo by Bo Cai; and (C) Wenchuan County, Aba Tibetan and Qiang Autonomous Prefecture, Sichuan Province, photo by Maozhou Xu.
Chresonymy.
Achalinus spinalis: Zhao et al. [50]; Peng et al. [44]; Ziegler et al. [2]; Li et al. [6]; Luu et al. [7]; Miller et al. [8]; Li et al. [10]; Li et al. [43].
Achalinus sp.: Yang et al. [5]; Xu et al. [18].
Holotype. QHU 2023013, an adult female, collected by the team of Lifang Peng on 25 September, 2023 from Longquanyi district, Chengdu City, Sichuan Province, China (30°32′19.67″ N, 104°19′18.12″ E; 845 m a.s.l.)
Paratypes. Females (n = 9): QHU 2024020, adult, collected on 6 May 2024 from Chongzhou City, Chengdu City, Sichuan Province, China (30°47′08.16″ N, 103°19′32.88″ E; 1024 m a.s.l.); QHU 2024021, QHU 2024022, adult, collected in May 2024 from Lushan County, Yaan City, Sichuan Province, China (30°29′10.68″ N, 103°6′15.48″ E; 1107 m a.s.l.); QHU 2024025, QHU 2024026, adult, collected by Maozhou Xu and Tianxuan Gu on 31 May 2024 from Mt. Tiantai, Qionglai City, Sichuan Province, China (30°16′12.36″ N, 103°07′07.68″ E; 1300 m a.s.l.); QHU 2024028, adult, collected on 18 June 2024 from Chongzhou City, Chengdu City, Sichuan Province, China (30°50′00.06″ N, 103°23′30.73″ E; 1232 m a.s.l.); and QHU 2024029, subadult, collected on 18 June 2024 from Dayi County, Chengdu City, Sichuan Province, China (30°38′45.96″ N, 103°19′01.92″ E; 1030 m a.s.l.) by the team of Lifang Peng. CIB MS761, adult, collected by Bo Cai on 5 August 2024 from Dujiangyan City, Chengdu City, Sichuan Province, China (30°57′43.92″ N, 103°35′24.00″ E; 750 m a.s.l.). ANU 20220008 (also CHS 007 in Li et al. [43], and HUM201200001 and HS 12093 in Peng et al. [44]), adult, collected by the team of Song Huang on 05 August, 2012 from Taibai County, Baoji City, Shaanxi Province, China (34°01′51.59″ N, 107°13′41.16″ E; 1677 m a.s.l.). Males (n = 4): QHU 2023014, adult, with the same collected information as the holotype; QHU 2024016, adult, ollected on 21 April 2024 from Hongya County, Meishan City, Sichuan Province, China (29°37′17.75″ N, 103°15′2.88″ E; 737 m a.s.l.); QHU 2024019, subadult, collected on 29 April 2024 from Wenchuan County, Aba Tibetan and Qiang Autonomous Prefecture, Sichuan Province, China (31°29′20.40″ N, 103°37′53.76″ E; 1400 m a.s.l.); and QHU 2024024, juvenile, collected on 11 June 2024 from Dayi County, Chengdu City, Sichuan Province, China (30°37′42.96″ N, 03°10′19.20″ E; 1300 m a.s.l.) by the team of Lifang Peng.
Diagnosis. (1) the tail is relatively short, with a TAL/TL ratio of 0.202–0.226 in males, and 0.155–0.178 in females; (2) there are two pairs of chin-shields; (3) there are 21–22 maxillary teeth; (4) the length of the suture between internasals is significantly shorter than that between prefrontals, with the LSBI/LSBP ratio of 0.502–0.773; (5) there are six supralabials, and the fourth and fifth are in contact with the eye; (6) there are five to six infralabials, and the first to third or fourth touch the first pair of chin-shields; (7) there is one loreal, hexagonal, with an LorH/LorL ratio of 0.612–1.040; (8) the two anterior temporals are in contact with eye; (9) there are 155–160 ventrals in males, and 165–174 in females; (10) there are 60–65 subcaudals in males, and 49–53 in females, not paired; (11) the dorsum is iridescent and uniformly charcoal black, lacks a longitudinal vertebral line, and the ventral area is dark brown or dark gray.
Description of the holotype. Measurements and scalation. The adult female had a TL of 367 mm (SVL 302 mm and TAL 65 mm), complete tail, and TAL/TL ratio of 0.177; the body was slender and cylindrical; the head was slightly distinct from the neck, with an HW of 5.95 mm, HL of 13.12 mm, and HW/HL ratio of 0. 47; the eye is small; ED of 0.8 mm; the rostrum is small, triangular, and slightly visible from above; the length of the suture between the internasals is significantly shorter than that between the prefrontals, LSBI of 1.12 mm, LSBP of 1.71 mm, and LSBI/LSBP ratio of 0.654; one supraocular, hexagonal, width significantly greater than height, in contact with loreal, prefrontal, frontal, parietal, and the upper anterior temporal; prefrontals paired; frontal single, pentagonal, pointing to the rear, the width slightly larger than the length; one loreal, hexagonal, LorH 1.07 mm, LorL 1.22 mm, LorH/LorL ratio 0.877; TEMP 6, arranged in three rows (2 + 1 + 3); the anterior pair is elongated, in contact with the eye, the upper anterior temporal is small, the lower anterior temporal is large, in contact with fifth and sixth supralabials; SL 6/6, the first one is the smallest, the fourth and fifth are in contact with the eye, and the sixth is the longest; two pairs of chin-shields; one mental area; IL 5/5, the first three touching the first pair of chin-shields.
Dorsal scale rows 21-22-21, strongly keeled and lanceolate, the outer-most row slightly enlarged, smooth in the anterior part and the middle of the body and keeled toward the posterior section. VS 167; SC 50, not paired; CP entire.
Dentition. A total of 21 maxillary teeth, without diastema, nearly equal in length except for the first two, which are slightly smaller (Figure 13).
Coloration of holotype in life. The dorsum, including the head, body, and tail, is charcoal black with a slight iridescent sheen and lacks a longitudinal vertebral line. The dorsal head scales match the dorsum in color, while the interstitial skin and scale sutures are light gray. The mental and chin-shields are dark brown, and the gular region is a lighter brown. The anterior part of the ventral surface of the body is dark brown, gradually deepening toward the posterior, and the ventral surface of the tail is almost black. The posterior margins of the ventral scales are grayish-white (Figure 10(A1,B1)).
Coloration of holotype in preservation. In preservation, the coloration still resembles the specimen in life, except that the coloration of the ventral area is lightened (Figure 12).
Variation. The main morphological characters of Achalinus ningshanensis occidentalis ssp. nov. are listed in Table 4. The paratypes exhibit a similar morphological pattern to the holotype, but the majority of paratypes have dorsal scale rows in 23-23-23. Moreover, there is pronounced sexual dimorphism: compared to females, the examined males have a significantly long tail, TAL/TL ratio of 0.202–0.226 (vs. 0.155–0.178 in females), fewer ventrals (155–160 vs. 165–174 in females), and more subcaudals (60–65 vs. 49–53 in males).
Table 4.
Main morphological characteristics of Achalinus ningshanensis occidentalis ssp. nov. obtained from specimens examined in this study. S + S + K = smooth on anterior and mid, keeled on posterior body; S + K + K = smooth on anterior, keeled on mid and posterior body.
| Voucher Number |
QHU 2023013 | QHU 2023014 | QHU 2024016 | QHU 2024019 | QHU 2024020 | QHU 2024021 | QHU 2024022 |
|---|---|---|---|---|---|---|---|
| Holotype | Paratype | Paratype | Paratype | Paratype | Paratype | Paratype | |
| Location | Longquanyi, Sichuan | Longquanyi, Sichuan | Hongya, Sichuan | Wenchuan, Sichuan | Chongzhou, Sichuan | Lushan, Sichuan | Lushan, Sichuan |
| Sex | Female | Male | Male | Subadultmale | Female | Female | Female |
| TL | 367 | 364 | 421 | 280 | 408 | 440+ | 403 |
| TAL | 65 | 81 | 95 | 60 | 71 | 64+ | 64 |
| TAL/TL | 0.177 | 0.223 | 0.226 | 0.214 | 0.174 | / | 0.159 |
| HW | 5.6 | 5.8 | 6.3 | 5.0 | 6.0 | 5.9 | 6.1 |
| HL | 12.0 | 12.4 | 12.5 | 9.3 | 12.4 | 13.4 | 10.8 |
| ED | 0.8 | 0.9 | 1.56 | 0.87 | 1.0 | 1.2 | 1.2 |
| MT | 21 | 21 | 21 | 21 | 21 | 22 | 21 |
| SL | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| IL | 5/5 | 5/5 | 5/6 | 5/5 | 5/5 | 5/5 | 5/5 |
| Chin | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 |
| IL-1st Chin | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-4th | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd |
| Lor | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| LorH | 1.07 | 1.17 | 1.41 | 1.22 | 1.33 | 1.62 | 1.23 |
| LorL | 1.22 | 1.54 | 1.60 | 1.29 | 1.57 | 1.85 | 1.46 |
| LorH/LorL | 0.877 | 0.760 | 0.881 | 0.946 | 0.847 | 0.876 | 0.842 |
| LSBI/LSBP | 0.654 | 0.599 | 0.722 | 0.773 | 0.640 | 0.657 | 0.771 |
| TEMP | 2 + 1 + 3/2 + 1 + 3 | 2 + 2 + 3/2 + 2 + 3 | 2 + 2 + 4/2 + 2 + 3 | 2 + 2 + 3/2 + 2 + 4 | 2 + 2 + 3/2 + 2 + 3 | 2 + 2 + 3/2 + 2 + 3 | 2 + 2 + 3/2 + 2 + 4 |
| ATEMP-Eye | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 |
| DSR | 21-22-21 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 |
| KOD | S + S + K | S + K + K | S + K + K | S + S + K | S + S + K | S + S + K | S + S + K |
| VS | 167 | 155 | 160 | 158 | 171 | 165 | 171 |
| SC | 50 | 60 | 61 | 65 | 50 | 43+ | 49 |
| CP | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
|
Voucher
Number |
QHU 2024024 | QHU 2024025 | QHU 2024026 | QHU 2024028 | QHU 2024029 | CIB MS761 | ANU 20220008 |
| Paratype | Paratype | Paratype | Paratype | Paratype | Paratype | Paratype | |
| Location | Dayi, Sichuan | Qionglai, Sichuan | Qionglai, Sichuan | Chongzhou, Sichuan | Dayi, Sichuan | Dujiangyan, Sichuan | Taibai, Shaanxi |
| Sex | Juvenil male | Female | Female | Female | Subadult female | Female | Female |
| TL | 178 | 409 | 455 | 366 | 309 | 387 | 493+ |
| TAL | 36 | 71 | 79 | 65 | 48 | 69 | 86+ |
| TAL/TL | 0.202 | 0.174 | 0.174 | 0.178 | 0.155 | 0.178 | / |
| HW | 4.1 | 5.4 | 6.4 | 5.6 | 5.4 | 6.2 | 8.55 |
| HL | 8.2 | 12.3 | 12.4 | 11.4 | 10.3 | 11.2 | 16.94 |
| ED | 0.70 | 1.11 | 1.18 | 1.07 | 0.9 | / | 1.47 |
| MT | / | 22 | 21 | 21 | 22 | 21 | / |
| SL | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| IL | 6/5 | 5/5 | 6/6 | 5/5 | 6/5 | 5/5 | 5/5 |
| Chin | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 |
| IL-1st Chin | 1st-4th/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd | 1st-3rd/1st-3rd |
| Lor | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| LorH | 0.97 | 1.07 | 1.29 | 1.51 | 1.05 | 1.12 | 1.62 |
| LorL | 1.09 | 1.43 | 1.63 | 1.45 | 1.64 | 1.83 | 2.38 |
| LorH/LorL | 0.890 | 0.748 | 0.791 | 1.040 | 0.640 | 0.612 | 0.681 |
| LSBI/LSBP | 0.599 | 0.648 | 0.659 | 0.703 | 0.657 | 0.502 | 0.616 |
| TEMP | 2 + 2 + 2/2 + 2 + 3 | 2 + 2 + 4/2 + 3 + 3 | 2 + 2 + 3/2 + 2 + 3 | 2 + 2 + 3/2 + 2 + 3 | 2 + 2 + 3/2 + 1 + 3 | 2 + 2 + 3/2 + 2 + 3 | 2 + 2 + 4/2 + 2 + 4 |
| ATEMP-Eye | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/1 |
| DSR | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 | 23-23-23 |
| KOD | S + S + K | S + S + K | S + S + K | S + S + K | S + S + K | / | S + K + K |
| VS | 158 | 172 | 174 | 173 | 169 | 172 | 166 |
| SC | 60 | 51 | 51 | 53 | 49 | 52 | 46+ |
| CP | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Etymology. The subspecific epithet “occidentalis”, meaning “western”, signifies that the new subspecies is found in western China (western Sichuan and southwestern Shaanxi), with a range lying west of A. n. ningshanensis. We suggest “Western China Odd-scaled Snake” as its English common name and “Níng Shǎn Jǐ Shé Huá Xī Yà Zhŏng (宁陕脊蛇华西亚种)” as its Chinese common name.
Distribution and natural history. Achalinus ningshanensis occidentalis ssp. nov. is currently known to be distributed in western China, including Sichuan Province (Longquanyi District, Chongzhou City, Dayi County, Qionglai City, Dujiangyan City, Mt. Qingcheng, Hongya County, Lushan County, and Wenchuan County) and Shaanxi Province (Taibai County). Its known activity period spans from April to September, with peak activity occurring in early summer. Its activity period spans from April to September, with peak activity in early summer. This species is nocturnal and lives in subtropical mountainous regions at elevations of 737–1677 m, typically found within the leaf litter of subtropical broadleaf forests, mixed conifer–broadleaf forests, or coniferous forests, as well as in grasslands, farmland, or rock piles (Figure 14). Notably, specimen QHU 2024019 was discovered in a dry-hot valley in Wenchuan County, indicating a possible adaptation to diverse microhabitats within its range.
Comparisons. Achalinus ningshanensis occidentalis ssp. nov. can be separated from A. n. ningshanensis as follows: (1) LSBI is significantly shorter than LSBP (vs. suture between internasals is similar size when compared to the suture between prefrontals); (2) two pairs of chin-shields (vs. three or four pairs); (3) VS 155–160 in males (vs. 161–165 in males); (4) SC 60–65 in males, 49–53 in females (vs. 51–56 in males, 41–47 in females; (5) loreal is relatively high, LorH/LorL ratio of 0.760–0.946 in males, and 0.612–1.040 in females (vs. LorH/LorL ratio of 0.544–0.617 in males, 0.458–0.681 in females), and (6) dorsum is uniformly charcoal black (vs. uniformly brown to dark brown dorsum) (Figure 15, Table 5). A detailed comparison between Achalinus ningshanensis occidentalis ssp. nov. and its congeners is summarized in Table 6.
Table 5.
Comparisons of main morphological characteristics of Achalinus ningshanensis occidentalis ssp. nov. and A. n. ningshanensis. S + S + K = smooth on anterior and mid, keeled on posterior body; S + K + K = smooth on anterior, keeled on mid, and posterior body; K + K + K = keeled on anterior, mid and posterior body.
| Species | A. n. occidentalis ssp. nov. | A. n. ningshanensis | ||
|---|---|---|---|---|
| Sex | ♂ | ♀ | ♂ | ♀ |
| N | 4 | 10 | 2 | 12 |
| TL | 178–421 | 309–493+ | 363–412 | 179–577 |
| TAL | 36–95 | 48–86+ | 72–87 | 28–89 |
| TAL/TL | 0.202–0.226 | 0.155–0.178 | 0.198–0.211 | 0.121–0.161 |
| HW/HL | 0.47–0.54 | 0.44–0.57 | 0.48–0.52 | 0.37–0.57 |
| ED | 0.70–1.56 | 0.81–1.47 | 0.7–1.0 | 0.82–1.33 |
| MT | 21 | 21–22 | 20 | 20–22 |
| SL | 6 | 6 | 6 | 6 |
| IL | 5 or 6 | 5 or 6 | 5 | 4 or 5 |
| Chin | 2 | 2 | 3–4 | 3–4 |
| Lor | 1 | 1 | 1 | 1 |
| LorH/LorL | 0.760–0.946 | 0.612–1.040 | 0.544–0.617 | 0.458–0.681 |
| LSBI/LSBP | < | < | = | = |
| TEMP | 2 + 2 + 2/3/4 | 2 + 1/2 + 3/4 | 2 + 2/3 + 3/4 | 1/2 + 1/2/3 + 3/4 |
| ATEMP-Eye | 2 | 1 or 2 | 2 | 1 or 2 |
| DSR | 23-23-23 | 23(21)-23(22)-23(21) | 23-23-23 | 23-23-23(21) |
| KOD | S + K + K or S + S + K | S + K + K or S + S + K | K + K + K | K + K + K |
| VS | 155–160 | 165–174 | 161–165 | 159–177 |
| SC | 60–65 | 49–53 | 51–56 | 41–47 |
| CP | 1 | 1 | 1 | 1 |
Table 6.
Morphological characteristics of Achalinus obtained from specimens examined in this study and the literature. Int. fus.: internasal fused to prefrontal; Pre. fus.: prefrontal fused to loreal; TAL/TL-M or F: TAL/TL in males or females; VEN-M or F: VEN in males or females; SC-M or F: SC in males or females; ?: further verification required.
| Species | TAL/TL-M | TAL/TL-F | MT | Int fus. | Pre fus. | LorH/LorL | LSBI/LSBP | DSR | SL |
|---|---|---|---|---|---|---|---|---|---|
| A. n. occidentalis ssp. nov. | 0.202–0.226 | 0.155–0.178 | 21–22 | 0 | 0 | 0.61–1.04 | <1 | 23(21)-23(22)-23(21) | 6 |
| A. ater | 0.190–0.220 | – | 0 | 0 | 0.40 | >1 | (21–23)-(21–25)-(21–25) | 6 | |
| A. dabieshanensis | 0.177–0.223 | 0.168 | – | 0 | 0 | 0.73–0.83 | <1 | 23-23-23 | 6 |
| A. damingensis | 0.246 | ? | – | 0 | 0 | 0.65 | >1 | 23-23-23 | 6 |
| A. dehuaensis | 0.263–0.286 | 0.206–0.217 | 31–33 | 0 | 0 | – | >1 | 23-23-23 | 6 |
| A. emilyae | ? | 0.183–0.203 | 27–28 | 0 | 0 | – | >1 | 23-23-23 | 6 |
| A. f. chigirai | 0.32 | 14 | 0 | 1 | – | =1 | (25–27)-(25–27)-25 | 6 | |
| A. f. formosanus | 0.16 | 17 | 0 | 1(usually) | – | =1 | 29-27-25 | 6 | |
| A. hainanus | ? | 0.258–0.266 | – | 0 | 0 | – | =1 | 23-23-23 | 6 |
| A. huangjietangi | 0.197–0.232 | 0.152–0.158 | – | 0 | 0 | 0.70–0.74 | <1 | 23-23-23 | 6 |
| A. hunanensis | 0.221–0.225 | ? | 23 | 0 | 0 | 0.62–0.70 | >1 | 23-23-23 | 6 |
| A. jinggangensis | 0.217 | 0.174 | – | 0 | 1 | – | >1 | 23-23-23 | 6 |
| A. juliani | 0.264–0.365 | 0.224 | 28 | 0 | 0 | – | >1 | 25-23-23 | 6 (7) |
| A. meiguensis | 0.142–0.238 | 17 | 1 | 0 | – | – | (21–23)-(19–21)-(19–21) | 6 | |
| A. nanshanensis | 0.215–0.246 | ? | 18 | 0 | 0 | 0.47–0.53 | > | (23–25)-(23–25)-(23–25) | 6 |
| A. niger | 0.151–0.179 | – | 0 | 0 | 0.67 | </=1 | 25-25-23 | 6 | |
| A. n. ningshanensis | 0.198–0.211 | 0.121–0.161 | 20–22 | 0 | 0 | 0.458–0.681 | =1 | 23-23-23(21) | 6 |
| A. panzhihuaensis | 0.246 | ? | 28 | 1 | 0 | 0.67 | – | 23-23-19 | 6 |
| A. pingbianensis | 0.243 | 0.172 | 20 | 0 | 1 | – | =1 | 23-23-23 | 7 |
| A. quangi | 0.283–0.304 | 0.218–0.262 | 27–29 | 0 | 0 | – | >1 | (23–25)-23-(21–23) | 6 |
| A. rufescens | 0.191–0.276 | 23 | 0 | 0 | 0.80–1.00 | >1 | 23-(23–25)-23 | 6 | |
| A. sheni | 0.183–0.224 | 0.149–0.164 | 24–25 | 0 | 0 | 0.53–0.93 | =1 | 23-23-23 | 6 |
| A. spinalis | 0.213 | 0.158–0.275 | 16–20 | 0 | 0 | – | </=1 | 23-(21–23)-(21–23) | 6 |
| A. timi | 0.213 | ? | 27 | 0 | 1 | – | >1 | 25-25-23 | 6 |
| A. tranganensis | ? | 0.254 (+) | 29 | 0 | 0 | – | >1 | 25-23-23 | 6 |
| A. werneri | 0.250–0.300 | – | 0 | 0 | – | =1 | ?-(21–23)-? | 6 | |
| A. yangdatongi | 0.261–0.262 | 0.180–0.200 | 24–26 | 0 | 0 | 0.57 | >1 | 23-23-23 | 6 |
| A. yunkaiensis | 0.185–0.200 | 0.156–0.204 | 20–24 | 0 | 0 | 0.49–0.64 | =1 | 23-23-23 | 6 |
| A. vanhoensis | 0.264 | ? | 32 | 0 | 1 | – | >1 | 25-23-23 | 6/7 |
| A. zugorum | 0.229 | ? | 28 | 0 | 1 | – | >1 | 25-23-23 | 6 |
| Species | SL-Eye | IL | Chin | TEMP | aTEMP-Eye | VEN-M | VEN-F | SC-M | SC-F |
| A. n. occidentalis ssp. nov. | 4–5 | 5 or 6 | 2 | 2 + 1/2/3 + 3/4 | 1–2 | 155–160 | 165–174 | 60–65 | 49–53 |
| A. ater | 4–5 | 5–6 | 2 or 3 | 2 + 2 + 3 | 2 | 156–170 | 47–70 | ||
| A. dabieshanensis | 4–5 | 5 | 2 | 2 + 2 + 3/4 | 2 | 141–151 | 155 | 46–55 | 45 |
| A. damingensis | 4–5 | 6 | 2 | 2 + 2 + 3 | 2 | 162 | ? | 74 | ? |
| A. dehuaensis | 4–5 | 5 | 2 | 2 + 2/3 + 3/4 | 1–2 | 142–147 | 152–154 | 74–81 | 63–65 |
| A. emilyae | 4–5 | 5 | 2 | 2 + 2 + 3 | 1 | ? | 157–161 | ? | 56–63 |
| A. f. chigirai | 4–5 | 5–6 | 2 or 3 | 2 + 2 | 2 | 161–167 | ? | 96–97 | ? |
| A. f. formosanus | 4–5 | 6–7 | 2 or 3 | 2 + 2 | 1 | 158–176 | 164–184 | 62–83 | 61–70 |
| A. hainanus | 4–5 | 5 | 2 | 1 + 2 + 3/4 | 1 | ? | 165–168 | ? | 67–69 |
| A. huangjietangi | 4–5 | 5–6 | 2 | 2 + 2 + 3/4 | 2 | 157–160 | 170 | 59–67 | 47 |
| A. hunanensis | 4–5 | 5–6 | 2 | 2 + 2 + 4 | 2 | 163–165 | ? | 69–72 | ? |
| A. jinggangensis | 4–5 | 6 | 2 | 1/2 + 2 + 3/4 | 2 | 156 | 164 | 64 | 51 |
| A. juliani | 4–5 (5–6) | 6 | 2 | 2 + 2 + 4 | 2 | 163–169 | 179 | 91 | 77 |
| A. meiguensis | 4–5 | 6 | 3 | 2/3 + 2/3 | 1 | 146–173 | 39–62 | ||
| A. nanshanensis | 4–5 | 6 | 2 | 2 + 2/3 + 4 | 2 | 147–158 | ? | 64–71 | ? |
| A. niger | 4–5 | 6 | 2 or 3 | 2 + 2/3 | 2 | 169–170 | 172–185 | 68–72 | 52–58 |
| A. n. ningshanensis | 4–5 | 5 | 3 or 4 | 1/2 + 1/2/3 + 2/3/4 | 1–2 | 161–165 | 159–177 | 51–56 | 41–47 |
| A. panzhihuaensis | 4–5 | 6 | 3 | 2 + 2 + 3 | 1 | 160 | ? | 73 | ? |
| A. pingbianensis | 5–6 | 6 | 2 | 2 + 2 + 3 | 1 | 164 | 167 | 56 | 48 |
| A. quangi | 4–5 | 5 | 2 | 2 + 2 + 4 | 1–2 | 139–141 | 141–154 | 75–84 | 69 |
| A. rufescens | 4–5 | 5 | 2 | 1/2 + 2 + 3/4 | 1–2 | 132–156 | 150–158 | 58–82 | 56–61 |
| A. sheni | 4–5 | 5–6 | 2 | 2 + 1/2 + 3/4 | 2 | 161–170 | 172–174 | 55–61 | 46–49 |
| A. spinalis | 4–5 | 5–6 | 3 | 2 + 2/3 | 1–2 | 146–168 | 45–62 | ||
| A. timi | 4–5 | 6 | 2 | 2 + 2 + 3 | 1 | 170 | ? | 72 | ? |
| A. tranganensis | 4–5 | 6 | 2 | 2 + 2 + 3 | 2 | ? | 171 | ? | 73(+) |
| A. werneri | 4–5 | 6 | 2 | 2 + 3/4 | – | 157–171 | 174–191 | 90–98 | 67–85 |
| A. yangdatongi | 4–5 | 5–6 | 2 | 2 + 2/3 + 2/3 | 2 | 155 | 170–171 | 76 | 59–64 |
| A. yunkaiensis | 4–5 | 6 | 2 | 2 + 2 + 3/4 | 2 | 151–162 | 144–156 | 49–56 | 51–55 |
| A. vanhoensis | 4–5/5–6 | 6 | 2 | 2 + 2 + 3 | 2 | 176 | ? | 84 | ? |
| A. zugorum | 4–5 | 7 | 2 | 2 + 2 + 3 | 2 | 173 | ? | 70 | ? |
Figure 15.
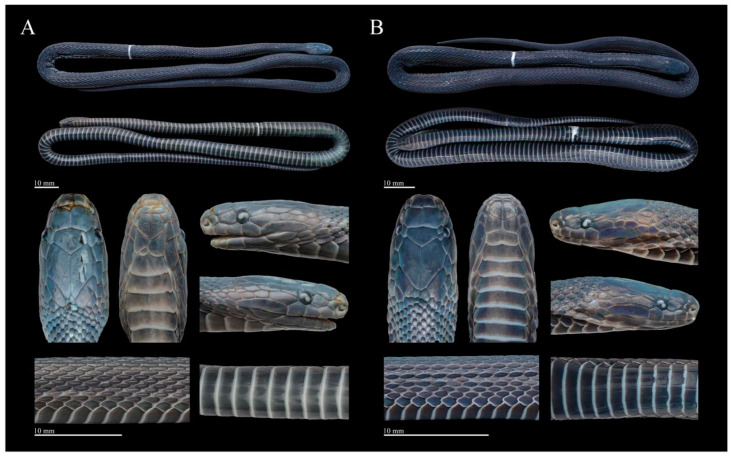
Dorsal (left), lateral (middle), and ventral (right) area of the head comparisons between Achalinus ningshanensis ningshanensis and Achalinus ningshanensis occidentalis ssp. nov. (A–C) A. n. ningshanensis: (A) QHU 2024017, adult male, from Ningshan County, Shaanxi Province; (B) QHU 2023006, adult female, from Ningshan County, Shaanxi Province; C. QHU 2023009, adult female, from Ningshan County, Shaanxi Province. (D–F) A. n. occidentalis ssp. nov.: (D) QHU 2023014, adult male, from Longquanyi District, Sichuan Province; (E) QHU 2023013, adult female, from Longquanyi District, Sichuan Province; and (F) QHU 2024022, adult female, from Lushan County, Sichuan Province. Photos by Yuhao Xu. Scale bars are not shown.
Achalinus ningshanensis occidentalis ssp. nov. can be separated from A. huangjietangi, A. dabieshanensis and A. spinalis due to the lack of a longitudinal vertebral line and fewer infralabials (usually 5 vs. 6). Moreover, it can be separated from A. spinalis due to having more maxillary teeth (21–22 vs. 16–20) and from A. dabieshanensis due to having more subcaudals in males (60–65 vs. 46–55) and more ventrals (155–174 vs. 141–155).
By the internasal separated from prefrontal, Achalinus ningshanensis occidentalis ssp. nov. can be separated from A. meiguensis Hu and Zhao, 1966 and A. panzhihuaensis Hou, Wang, Guo, Chen, Yuan & Che, 2021 (vs. internasal fused to prefrontal). Furthermore, it can be separated from A. meiguensis due to having 23 (rarely 22) DSR at the midbody (vs. 19–21), and from A. panzhihuaensis due to having fewer subcaudals in males (60–65 vs. 73), and 23 (rarely 21) DSR on the posterior body (vs. 19).
By LSBI/LSBP < 1, Achalinus ningshanensis occidentalis ssp. nov. can be easily separated from A. ater Bourret, 1937, A. damingensis Xu, Yang, Wu, Gong, Huang and Huang, 2023, A. dehuaensis Li, Wu, Xu, Zhu, Ren, Guo and Dong, 2021, A. emilyae Ziegler, Nguyen, Pham, Nguyen, Pham, van Schingen, Nguyen and Le, 2019, A. hunanensis Ma, Shi, Xiang, Shu and Jiang, 2023, A. jinggangensis (Zong and Ma, 1983), A. juliani Ziegler, Nguyen, Pham, Nguyen, Pham, van Schingen, Nguyen and Le, 2019, A. nanshanensis, A. quangi Pham, Pham, Le, Ngo, Ong, Ziegler and Nguyen, 2023, A. rufescens, A. timi Ziegler, Nguyen, Pham, Nguyen, Pham, Van Schingen, Nguyen and Le, 2019, A. tranganensis Luu, Ziegler, Ha, Lo, Hoang, Ngo, Le, Tran and Nguyen, 2020, A. yangdatongi Hou, Wang, Guo, Chen, Yuan and Che, 2021, A. vanhoensis Ha, Ziegler, Sy, Le, Nguyen and Luu, 2022, and A. zugorum Miller, Davis, Luong, Do, Pham, Ziegler, Lee, De Queiroz, Reynolds and Nguyen, 2020 (vs. >1), and from A. hainanus Huang, 1975, A. sheni Ma, Xu, Qi, Wang, Tang, Huang and Jiang, 2023, A. werneri Van Denburgh, 1912, and A. yunkaiensis Wang, Li and Wang, 2019 (vs. =1). Moreover, it can be distinguished from A. damingensis, A. dehuaensis, A. hunanensis, A. juliani, A. nanshanensis, A. quangi, A. timi, A. tranganensis, A. yangdatongi, A. vanhoensis, and A. zugorum due to it having fewer subcaudals (49–65 vs. 74 in A. damingensis, 74–81 in A. dehuaensis, 69–72 in A. hunanensis, 81–91 in A. juliani, 64–77 in A. nanshanensis, 69–84 in A. quangi, 72 in A. timi, 73+ in A. tranganensis, 76–82 in A. yangdatongi, 84 in A. vanhoensis, and 70 in A. zugorum). It can be separated from A. hainanus due to it having fewer subcaudals in females (49–53 vs. 67–69), and a comparatively shorter tail length in females (TAL/TL ratio 0.155–0.178 vs. 0.258–0.266). It can be separated from A. sheni due to it having fewer ventrals in males (155–160 vs. 161–170). It can be separated from A. werneri due to it having a comparatively shorter tail (TAL/TL ratio 0.155–0.226 vs. 0.250–0.300). It can be separated from A. yunkaiensis due to it having more subcaudals (60–65 vs. 49–56) and a comparatively longer tail length (0.202–0.226 vs. 0.185–0.200) in males, and more ventrals (166–174 vs. 144–156) in females.
By the loreal separated from prefrontal, Achalinus ningshanensis occidentalis ssp. nov. can be separated from A. formosanus chigirai Ota & Toyama, 1989, A. f. formosanus Boulenger, 1908, and A. pingbianensis Li, Yu, Wu, Liao, Tang, Liu & Guo, 2020 (vs. loreal fused to prefrontal).
Remarks. In 2017, Peng et al. [44] provided the mitochondrial genome of an Achalinus specimen (voucher number CHS 007 in Li et al. [43], HUM 201200001 and HS 12093 in Peng et al. [44], and ANU 20220008 in this study) from Taibai County, Shaanxi Province, China, which was identified as A. spinalis. The specimen was initially deposited at Huangshan University and was then transferred to Anhui Normal University Museum (ANU). This specimen has since been included in many studies of the genus Achalinus, where its original identification has been consistently used [1,2,3,6,7,8,43]. In 2023, Yang et al. [5], based on a phylogenetic analysis, reclassified it as Achalinus sp., but without providing any morphological description. In the present study, we examined this specimen (Figure 16, Table 4) and found the morphology of this specimen to be consistent with that of Achalinus ningshanensis occidentalis ssp. nov. Subsequent phylogenetic analyses further support the morphology results. Thus, we re-identify the specimen ANU 20220008 here as Achalinus ningshanensis occidentalis ssp. nov.
Figure 16.
Preserved specimen of the ANU 20220008 (adult female, paratype of Achalinus ningshanensis occidentalis ssp. nov., from Taibai County, Shaanxi Province). Photos by Diancheng Yang. Scale bars: 10 mm.
4. Discussion
Achalinus spinalis was once considered to be widely distributed across eastern and southeastern Asia, from Laos, Vietnam (Lao Cai, Vinh Phuc, and Thai Nguyen Province) and China (Jiangsu, Zhejiang, Anhui, Fujian, Jiangxi, Hubei, Hunan, Guangxi, Sichuan, Guizhou, Yunnan, Shaanxi, and Gansu Province) to Japan (Kyushu Island, Honshu Island, Koshiki Island, and Tokuno-shima Island) [17,51,52,53,54,55,56,57,58,59,60]. However, recent studies have shown that in various regions, the currently recognized A. spinalis has not formed a monophyletic group, with significant genetic divergence between lineages [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. This suggests the A. spinalis sensu stricto may be a range-restricted species that is only found in Japan, and the taxonomic status of the populations recognized in other regions need to be re-evaluated. Through taxonomic work, several populations previously identified as A. spinalis have been described as new species (e.g., A. huangjietangi, A. dabieshanensis and A. sheni), while some erroneous distribution records have been gradually corrected [4,9,13,48]. However, due to the lack of genetic information of A. spinalis from the type locality, there is a gap remaining about the classification and evolutionary history within this species.
Achalinus spinalis weigoldi Mell, 1909 was described by Mell [23] based on the specimen form “Wa Shan, eastern Szechwan”; then, it was synonymized with Achalinus spinalis by Pope [52]. The original description of Achalinus spinalis weigoldi is as follows: “V 160–167, a 162; Sc 63–67, a 65; 3 pair of chin-shields; Sq (mid-dorsal scale rows) 21; length 600 mm”. Since Mell [23] did not designate the type of specimens or mention where the specimens were deposited, we can only compare the morphology of Achalinus ningshanensis occidentalis ssp. nov. with the few provided characteristics of Achalinus spinalis weigoldi in the original description. Morphologically, Achalinus ningshanensis occidentalis ssp. nov. can be separated from Achalinus spinalis weigoldi due to it having two pairs of chin-shields (vs. three), twenty-three mid-dorsal scale rows, rarely twenty-two (vs. twenty-one), and a significantly smaller body size (178–493+ mm vs. 600 mm). Additionally, the real type locality of Achalinus spinalis weigoldi cannot be confirmed either. In 2024, Nguyen et al. [48] discussed the taxonomic issues within the genus Achalinus. For Achalinus spinalis weigoldi, the authors suggested that the type locality “Wa Shan”, likely corresponds to Mt. Wawu, as mentioned by Inger et al. [61]. They also identified the specimen of A. spinalis from Inger et al. [61] as A. cf. weigoldi. However, “Wa Shan” in Sichuan has historically referred to at least two different mountains, Mt. Wawu (in Hongya County, Meishan City) and Mt. Dawa (in Leshan City), which are at least 50 km apart. Since Mell [23] did not provide a detailed description of the location, it is impossible to accurately determine the type locality of Achalinus spinalis weigoldi. Consequently, we suppose that Achalinus spinalis weigoldi Mell, 1909 is a nomen nudum and exclude it from formal use.
Clear species boundaries are essential for effective biodiversity conservation planning and action [62,63,64,65,66,67,68]. In this study, we re-evaluated the systematic position of A. ningshanensis and described the populations, which were previously identified as A. spinalis, from southwestern Shaanxi and western Sichuan Province, as Achalinus ningshanensis occidentalis ssp. nov. As for morphology, Achalinus ningshanensis occidentalis ssp. nov. can be identified from A. n. ningshanensis according to the fact that the LSBI is significantly shorter than the LSBP (vs. suture between internasals is similar size when compared to the suture between prefrontals); there are two pairs of chin-shields (vs. three or four pairs); VS 155–160 in males (vs. 161–165 in males); SC 60–65 in males, 49–53 in females (vs. 51–56 in males, 41–47 in females; and the dorsum is uniformly charcoal black (vs. a uniformly brown to dark brown dorsum). In terms of molecular systematics, the population from western Sichuan and southwestern Shaanxi forms two distinct, moderately divergent lineages with the population from southern Shaanxi and northeastern Sichuan Province, with an uncorrected p-distance from 3.6% to 4.3% in CO1. Although the distances are comparable to some other recognized species, i.e., A. nanshanensis vs. A. yangdatongi (CO1 4.4%) [15], A. quangi vs. A. emilyae (CO1 4.0–4.7%) [33], we currently conservatively consider this distinct population as a subspecies of A. ningshanensis. Isolation factors, such as geographic barriers, are likely critical in restricting the gene flow between populations, potentially driving genetic divergence and speciation events. Future research requires broader surveys combined with ecological and genetic data to understand these isolation mechanisms and explore the role of isolation and connectivity in evolution and conservation [69,70,71].
5. Conclusions
We revised the taxonomic status of Achalinus ningshanensis, and described a new subspecies, Achalinus ningshanensis occidentalis ssp. nov., based on ten female and four male specimens collected from western Sichuan and south western Shaanxi Province, China. Due to their cryptic lifestyle, the discovery of the new subspecies is largely accidental, which makes it difficult for us to make accurate judgments on the distribution and population status of this species. Further research is needed to elucidate the true distribution range and ecological niche of the new subspecies. Broader surveys in this area are also essential to gather additional genetic information from various populations, along with phylogenomic analyses to understand evolutionary patterns and assess the potential gene flow between these populations, ultimately clarifying species boundaries.
Acknowledgments
We thank Hongwen Ma from Sichuan Dragonfly International Travel Service Co., Ltd. and Zheming Zhang, Fangyi Lai, and Maozhou Xu for their assistance in field surveys and providing information on animal distribution and habitats. We thank Jingsong Shi from the Institute of Vertebrate Paleontology and Paleoanthropology (IVPP), Chinese Academy of Sciences, for X-ray scanning of the specimens. We are also thankful to our workmates for their help and advice.
Author Contributions
Conceptualization, Y.X., S.M., S.H. and L.P.; data curation, S.M., Y.X., F.Z., T.Z., T.G. and L.P.; funding acquisition, L.P.; methodology, Y.X., S.M., D.Y., B.C., T.Z., T.G., F.Z. and L.P.; resources, Y.X. and L.P.; software, Y.X., B.C., S.M. and L.P.; supervision, L.P., S.H. and B.C.; visualization, D.Y. and L.P.; writing—original draft, Y.X.; writing—review and editing, Y.X., S.M., D.Y., B.C., S.H. and L.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All sampling and procedures involving live lizards were performed in accordance with the Wild Animals Protection Law of the People’s Republic of China.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. ZooBank Code: urn:lsid:zoobank.org:act:75A7E98C-EAB7-4C42-B02D-D0BAE362E3D9; urn:lsid:zoobank.org:pub:DAC31888-5B7F-4C8D-A875-59BD204A25EC.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China [32301325], the Project of Qinghai Science & Technology Department [2024-ZJ-965], and the Open Project of State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University [2023-ZZ-08].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wang J., Li Y., Zeng Z.C., Lyu Z.T., Sung Y.H., Li Y.Y., Lin C.Y., Wang Y.Y. A new species of the genus Achalinus from southwestern Guangdong Province, China (Squamata: Xenodermatidae) Zootaxa. 2019;4674:471–481. doi: 10.11646/zootaxa.4674.4.6. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler T., Nguyen T.Q., Pham C.T., Nguyen T.T., Pham A.V., Schingen M.V., Nguyen T.T., Le M.D. Three new species of the snake genus Achalinus from Vietnam (Squamata: Xenodermatidae) Zootaxa. 2019;4590:249–269. doi: 10.11646/zootaxa.4590.2.3. [DOI] [PubMed] [Google Scholar]
- 3.Hou S.B., Wang K., Guo P., Chen J.M., Yuan Z.Y., Che J. Two new species and a new country record of the genus Achalinus (Reptilia: Squamata: Xenodermidae) from China. Zootaxa. 2021;4950:528–546. doi: 10.11646/zootaxa.4950.3.6. [DOI] [PubMed] [Google Scholar]
- 4.Ma S., Shi S.C., Xiang S.J., Shu F., Jiang J.P. A new species of Achalinus Peters, 1869 (Squamata, Xenodermidae) from Hunan Province, China. ZooKeys. 2023;1166:315–331. doi: 10.3897/zookeys.1166.103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang D.C., Xu Y.H., Wu J.X., Gong Y.A., Huang R.Y., Xiang J., Feng Z.L., Huang T.Q., Huang S. A new species of the genus Achalinus (Squamata: Xenodermidae) from Nanning, Guangxi, China. Zootaxa. 2023;5319:389–402. doi: 10.11646/zootaxa.5319.3.5. [DOI] [PubMed] [Google Scholar]
- 6.Li K., Yu M., Wu Y.Y., Liao L.H., Tang K., Liu Q., Guo P. A new species of the genus Achalinus (Squamata: Xenodermatidae) from southeastern Yunnan Province, China. Zootaxa. 2020;4860:116–128. doi: 10.11646/zootaxa.4860.1.6. [DOI] [PubMed] [Google Scholar]
- 7.Luu V.Q., Ziegler T., Van H.N., Van L.O., Hoang T.T., Ngo H.T., Le M.D., Tran D.H., Nguyen T.Q. A new species of Achalinus (Squamata: Xenodermidae) from Trang An Landscape Complex, Ninh Binh Province, Vietnam. Zootaxa. 2020;4877:174–184. doi: 10.11646/zootaxa.4877.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Miller A.H., Davis H.R., Luong A.M., Do Q.H., Pham C.T., Ziegler T., Lee J.L., Queiroz K.D., Reynolds R.G., Nguyen T.Q. Discovery of a new species of enigmatic odd-scaled snake (Serpentes: Xenodermidae: Achalinus) from Ha Giang Province, Vietnam. Copeia. 2020;108:796–808. doi: 10.1643/CH2020060. [DOI] [Google Scholar]
- 9.Huang R.Y., Peng L.F., Yu L., Huang T.Q., Jiang K., Ding L., Chang J.K., Yang D.C., Xu Y.H., Huang S. A new species of the genus Achalinus from Huangshan, Anhui, China (Squamata: Xenodermidae) Asian Herpetol. Res. 2021;12:178–187. doi: 10.16373/j.cnki.ahr.200075. [DOI] [Google Scholar]
- 10.Li K., Wu Y.Y., Xu R.Y., Zhu F., Ren J.L., Guo P., Dong B.J. A new species of the Achalinus rufescens complex (Xenodermidae: Achalinus) from Fujian Province, China. Zootaxa. 2021;5026:239–254. doi: 10.11646/zootaxa.5026.2.5. [DOI] [PubMed] [Google Scholar]
- 11.Ha N.V., Ziegler T., Sy T.D., Le M.D., Nguyen T.Q., Luu V.Q. A new species of the genus Achalinus (Squamata: Xenodermidae) from Son La Province, Vietnam. Zootaxa. 2022;5168:375–387. doi: 10.11646/zootaxa.5168.3.8. [DOI] [PubMed] [Google Scholar]
- 12.Yang D.C., Huang R.Y., Jiang K., Burbrink F.T., Gong Y.A., Yu J., Zhang Y., Huang T.Q., Huang S. A new species of the genus Achalinus (Squamata: Xenodermidae) from Ningshan County, Shaanxi Province, China. Zootaxa. 2022;5190:127–140. doi: 10.11646/zootaxa.5190.1.5. [DOI] [PubMed] [Google Scholar]
- 13.Ma S., Xu Y.H., Qi S., Wang Y.Y., Tang S.S., Huang S., Jiang J.P. Discovery of a new cryptic Achalinus Peters, 1869 (Serpentes, Xenodermidae) species from Hunan Province, China. ZooKeys. 2023;1181:9–27. doi: 10.3897/zookeys.1181.109462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C.W., Liu K., Huang R.Y., Hu T.L., Yu L., Sun R.L., Zhang Y.C., Wen J., Zhang B.W. A new species of the genus Achalinus (Squamata: Xenodermidae) from the Dabie Mountains, Anhui, China. Animals. 2023;13:708. doi: 10.3390/ani13040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H., Zhu L.Q., Xiao B., Huang J., Wu S.W., Yang L.X., Zhang Z.Q., Mo X.Y. A new species of the genus Achalinus (Squamata, Xenodermatidae) from southwest Hunan Province, China. ZooKeys. 2024;1189:257–273. doi: 10.3897/zookeys.1189.112784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uetz P., editor. The Reptile Database. [(accessed on 26 October 2024)]. Available online: https://www.reptile-database.org.
- 17.Zhao E.M. Snakes of China. Anhui Science and Technology Publishing House; Hefei, China: 2006. [Google Scholar]
- 18.Xu Y.H., Wang S., Ma S., Burbrink F.T., Peng L.F., Huang S. First report of albinism for Achalinus sheni (Serpentes, Xenodermidae), with extended diagnosis of the species. ZooKeys. 2024;1209:1–17. doi: 10.3897/zookeys.1209.128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darko Y.A., Voss O., Uetz P. A dictionary of abbreviations used in reptile descriptions. Zootaxa. 2022;5219:421–432. doi: 10.11646/zootaxa.5219.5.2. [DOI] [PubMed] [Google Scholar]
- 20.Boulenger G.A. Description of two new snakes from Hongkong, and note on the dentition of Hydrophis viperina. Ann. Mag. Nat. Hist. 1888;2:43–44. doi: 10.1080/00222938809460874. [DOI] [Google Scholar]
- 21.Boulenger G.A. Catalogue of the Snakes in the British Museum (Nature History). I. British Museum (Natural History); London, UK: 1893. [Google Scholar]
- 22.Boulenger G.A. Catalogue of the Snakes in the British Museum (Natural History). III. British Museum (Natural History); London, UK: 1896. [Google Scholar]
- 23.Boulenger G.A. Description of a new frog and a new snake from Formosa. Ann. Mag. Nat. Hist. 1908;8:221–222. doi: 10.1080/00222930808692472. [DOI] [Google Scholar]
- 24.Mell R. List of Chinese snakes. Lingnan Sci. J. 1931;8:199–219. (Published in 1929) [Google Scholar]
- 25.Bourret R. Notes herpétologiques sur l‘Indochine française. VIII. Sur les Achalinus d’Indochine. Bulletin général de l’Instruction Publique. Jan 5, 1935. pp. 101–104. Hanoi, 14e Année (1934–1935); [Separate: 1–4]
- 26.Bourret R. Notes herpétologiques sur l’Indochine française. XV. Lézards et serpents reçus au Laboratoire des Sciences Naturelles de l’Université au cours de l’année 1937. Descriptions de deux espèces et de deux variétés nouvelles. Bulletin général de l’Instruction Publique. Dec 4, 1937. pp. 57–80. Hanoi, 17e Année (1937–1938); Annexe: 57–80.
- 27.Hu S.Q., Zhao E.M. Three new species of reptiles from Szechwan. Acta Zootaxon Sin. 1966;3:158–164. (In Chinese with English abstract) [Google Scholar]
- 28.Hu S.Q., Zhao E.M., Liu C.Z. A survey of amphibians and reptiles in Kweichow Province, including a herpetological analysis. Acta Zool. Sin. 1973;19:149–181. (In Chinese with English abstract) [Google Scholar]
- 29.Koshikawa A. Three new species of reptiles from Hainan Island, Guangdong Province. Smithson. Herpetol. Inf. Serv. 1982;53:1–10. doi: 10.5479/si.23317515.53.1. [DOI] [Google Scholar]
- 30.Ota H., Toyama M. Taxonomic re-definition of Achalinus formosanus Boulenger, 1908 (Xenoderminae: Colubridae: Ophidia), with description of a new subspecies. Copeia. 1989;1989:597–602. doi: 10.2307/1445485. [DOI] [Google Scholar]
- 31.Yu M., Li K., Liu Q., Yang K., Wu Y.Y., Guo P. First record of the Achalinus yunkaiensis from Maoershan National Nature Reserve, Guangxi, China. Chin. J. Zool. 2020;55:793–796. (In Chinese with English abstract) [Google Scholar]
- 32.Chen C.W., Zhang C.W., Ding G.H., Zhang B.W., Wang Y.P. A new snake record of Achalinus huangjietangi in Zhejiang Province, China. Chin. J. Wildl. 2022;43:1149–1150. (In Chinese with English abstract) [Google Scholar]
- 33.Pham A.V., Pham C.T., Le M.D., Ngo H.T., Ong A.V., Ziegler T., Nguyen T.Q. Achalinus quangi, a new odd-scaled snake species from Vietnam. Zootaxa. 2023;5270:48–66. doi: 10.11646/zootaxa.5270.1.2. [DOI] [PubMed] [Google Scholar]
- 34.Green M.D., Orlov N.L., Murphy R.W. Toward a phylogeny of the kukri snakes, genus Oligodon. Asian Herpetol. Res. 2010;1:1–21. [Google Scholar]
- 35.Parkinson C.L., Zamudio K.R., Greene H.W. Phylogeography of the pitviper clade Agkistrodon: Historical ecology, species status, and conservation of cantils. Mol. Ecol. 2000;9:411–420. doi: 10.1046/j.1365-294x.2000.00854.x. [DOI] [PubMed] [Google Scholar]
- 36.Che J., Chen H.M., Yang J.X., Jin J.Q., Jiang K., Yuan Z.Y., Murphy R.W., Zhang Y.P. Universal COI primers for DNA barcoding amphibians. Mol. Ecol. Resour. 2012;12:247–258. doi: 10.1111/j.1755-0998.2011.03090.x. [DOI] [PubMed] [Google Scholar]
- 37.Burbrink F.T., Lawson R., Slowinski J.B. Mitochondrial DNA phylogeography of the polytypic North American rat snake (Elaphe obsolete): A critique of the subspecies concept. Evolution. 2000;54:2107–2118. doi: 10.1554/0014-3820(2000)054[2107:MDPOTP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Burland T.G. DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 2000;132:71–91. doi: 10.1385/1-59259-192-2:71. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen L.T., Schmidt H.A., Haeseler A.V., Minh B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoang D.T., Chernomor O., Haeseler A.V., Minh B.Q., Vinh L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephane G., Jean-franc O.D., Vincent L., Maria A., Wim H., And O.G. New algorithms and methods to estimate Maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 43.Li J.N., Liang D., Wang Y.Y., Guo P., Huang S., Zhang P. A large-scale systematic framework of Chinese snakes based on a unified multilocus marker system. Mol. Phylogenet. Evol. 2020;148:106807. doi: 10.1016/j.ympev.2020.106807. [DOI] [PubMed] [Google Scholar]
- 44.Peng L.F., Yang D.C., Duan S.Q., Huang S. Mitochondrial genome of the Common burrowing snake Achalinus spinalis (Reptilia: Xenodermatidae) Mitochondrial DNA Part B. 2017;2:571–572. doi: 10.1080/23802359.2017.1365643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deepak V., Lalronunga S., Lalhmingliani E., Das A., Narayanan S., Das I., Gower D.J. Phylogenetic relationships of xenodermid snakes (Squamata: Serpentes: Xenodermidae), with the description of a new genus. Vertebr. Zool. 2021;71:747–762. doi: 10.3897/vz.71.e75967. [DOI] [Google Scholar]
- 46.Vidal N., Hedges S.B. Higher-level relationships of snakes inferred from four nuclear and mitochondrial genes. C. R. Biol. 2002;325:977–985. doi: 10.1016/S1631-0691(02)01510-X. [DOI] [PubMed] [Google Scholar]
- 47.Teynié A., David P., Lottier A., Le M.D., Vidal N., Nguyen T.Q. A new genus and species of xenodermatid snake (Squamata: Caenophidia: Xenodermatidae) from northern Lao People’s Democratic Republic. Zootaxa. 2015;3926:523–540. doi: 10.11646/zootaxa.3926.4.4. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen T.V., David P. First record of Achalinus pingbianensis Li et al., 2020 (Serpentes: Xenodermidae) from Vietnam, with the first description of a female specimen and an expanded diagnosis. Russ. J. Herpetol. 2024;31:157–167. doi: 10.30906/1026-2296-2024-31-3-157-167. [DOI] [Google Scholar]
- 49.Fang R., Wang T. Three new records of snakes from Shaanxi. Acta Herpetol. Sin. 1983;2:75–76. (In Chinese with English abstract) [Google Scholar]
- 50.Zhao E.M., Huang M.H., Zong Y. Fauna Sinica, Reptilia, Vol. 3: Squamata, Serpentes. Science Press; Beijing, China: 1999. [Google Scholar]
- 51.Deuve J. Serpents du Laos. Mémoires de l’Orstom; Paris, France: 1970. [Google Scholar]
- 52.Pope C.H. The Reptiles of China. Turtles, Crocodilians, Snakes, Lizards. Natural History of Central Asia, Vol. X. American Museum of Natural History; New York, NY, USA: 1935. [Google Scholar]
- 53.Shen Y.H., Ye Y.Y., Deng X.J. Fauna Hunan: Reptilia. Hunan Science and Technology Press; Changsha, China: 2014. (In Chinese) [Google Scholar]
- 54.Stejneger L.H. Herpetology of Japan and Adjacent Territory. Volume 58 Smithsonian Institution; Washington, DC, USA: 1907. Bulletin of the United States National Museum. [Google Scholar]
- 55.Nguyen S.V., Ho C.T. A Checklist of the Reptiles and Amphibians of Vietnam. Faculty of Science and Technology Publishing House; Hanoi, Vietnam: 1996. (In Vietnamese) [Google Scholar]
- 56.Nguyen S.V., Ho C.T., Nguyen T.Q. Agriculture Publishing House; Hanoi, Vietnam: 2005. A checklist of Amphibians and Reptiles of Vietnam. (In Vietnamese) [Google Scholar]
- 57.Nguyen S.V., Ho C.T., Nguyen T.Q. Herpetofauna of Vietnam. Edition Chimaira; Frankfurt am Main, Germany: 2009. [Google Scholar]
- 58.Nishio K., Kawase M. Records of Achalinus spinalis (Squamata, Xenodermatidae) from Aichi, Gifu, Mie Prefecture, central Japan and thier environments. Bull. Nagoya Biodivers. Cent. 2017;4:31–41. (In Japanese with English abstract) [Google Scholar]
- 59.Teynié A., David P. Voyages Naturalistes au Laos. Les Reptiles. Editions Revoir; Nohanent, France: 2010. [Google Scholar]
- 60.Wallach V., Williams K.L., Boundy J. Snakes of the World: A Catalogue of Living and Extinct Species. CRC Press; Boca Raton, FL, USA: 2014. [Google Scholar]
- 61.Inger R.F., Zhao E., Shaffer B.H., Wu G. Report on a Collection of Amphibians and Reptiles from Sichuan, China. Fieldiana Zool. 1990;58:i–iii, 1–24. [Google Scholar]
- 62.Bickford D., Lohman D.J., Sodhi N.S., Ng P.K.L., Meier R., Winker K., Ingram K.K., Das I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007;22:148–155. doi: 10.1016/j.tree.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 63.de Queiroz K. Species concepts and species delimitation. Syst. Biol. 2007;56:879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- 64.Funk W.C., Caminer M., Ron S.R. High Levels of Cryptic Species Diversity Uncovered in Amazonian Frogs. Proc. R. Soc. B Biol. Sci. 2012;279:1806–1814. doi: 10.1098/rspb.2011.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujita M.K., Leaché A.D., Burbrink F.T., McGuire J.A., Moritz C. Coalescent-Based Species Delimitation in an Integrative Taxonomy. Trends Ecol. Evol. 2012;27:480–488. doi: 10.1016/j.tree.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Lin H.Y., Yang Y., Li W.H., Luo Y.X., Bai X.H., OhiToma T., Kim C., Kim J.H., Zhao Y.P. Species boundaries and conservation implications of Cinnamomum japonicum, an endangered plant in China. J. Syst. Evol. 2024;62:73–83. doi: 10.1111/jse.12950. [DOI] [Google Scholar]
- 67.Mahony S., Kamei R.G., Brown R.M., Chan K.O. Unnecessary splitting of genus-level clades reduces taxonomic stability in amphibians. Vertebr. Zool. 2024;74:249–277. doi: 10.3897/vz.74.e114285. [DOI] [Google Scholar]
- 68.Sites J.W., Marshall J.C. Delimiting Species: A Renaissance Issue in Systematic Biology. Trends Ecol. Evol. 2003;18:462–470. doi: 10.1016/S0169-5347(03)00184-8. [DOI] [Google Scholar]
- 69.Gao W., Yu C.X., Zhou W.W., Zhang B.L., Chambers E.A., Dahn H.A., Jin J.Q., Murphy R.W., Zhang Y.P., Che J. Species Persistence with Hybridization in Toad-Headed Lizards Driven by Divergent Selection and Low Recombination. Mol. Biol. Evol. 2022;39:msac064. doi: 10.1093/molbev/msac064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang G.D., Zhang B.L., Zhou W.W., Li Y.X., Jin J.Q., Shao Y., Yang H.C., Liu Y.H., Yan F., Chen H.M., et al. Selection and Environmental Adaptation Along a Path to Speciation in the Tibetan Frog Nanorana parkeri. Proc. Natl. Acad. Sci. USA. 2018;115:E5056–E5065. doi: 10.1073/pnas.1716257115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang K., Yu Z.-B., Wu Y.-H., Hou S.-B., Gao W., Duan S.-H., Xiong Y., Jiang Y.-F., Liu K.C., Li J.-H., et al. Herpetofauna Diversity of the Gaoligong Mountain in China: Checklist, Zoogeography, and Conservation. Zool. Res. Divers. Conserv. 2024;1:169–179. doi: 10.24272/j.issn.2097-3772.2024.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. ZooBank Code: urn:lsid:zoobank.org:act:75A7E98C-EAB7-4C42-B02D-D0BAE362E3D9; urn:lsid:zoobank.org:pub:DAC31888-5B7F-4C8D-A875-59BD204A25EC.