Simple Summary
Cryptosporidium and Giardia are single-cell parasites that can infect both animals and humans, causing diarrhea and risks to public health. Both parasites can be found in nearly all mammals, including domestic dogs, red foxes, and raccoon dogs, which can act as natural carriers and spread them through direct contact, food, and the environment. This study aimed to understand how common these protozoans are in Latvia and what factors influence their occurrence in domestic and wild canids. We collected fecal samples from 373 domestic dogs, 219 red foxes, and 78 raccoon dogs and tested for the presence of Cryptosporidium and Giardia duodenalis. Red foxes and raccoon dogs had higher prevalence of both parasites than domestic dogs. Puppies showed higher infection rates with both parasites. All infected canids were more likely to have both parasites simultaneously. This research highlights the need for responsible pet care and a potential zoonotic risk for humans.
Keywords: domestic dog, red fox, raccoon dog, protozoans, Latvia, zoonosis, prevalence
Abstract
Cryptosporidium spp. and Giardia duodenalis are zoonotic food- and water-borne protozoa of veterinary importance. They are one of the main causes of diarrhea in domestic dogs (Canis familiaris). Wild canids, such as red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides), can act as natural reservoirs for these parasites. The aim of this study was to estimate the prevalence of these pathogens and analyze possible factors that may affect the infection in wild and domestic canids. A total of 373 domestic dog, 219 red fox, and 78 raccoon dog fecal samples were collected and tested for Cryptosporidium spp. and G. duodenalis presence with direct immunofluorescent staining. The dog owners filled a questionnaire on the dog’s habits and lifestyle to identify and analyze potential factors that may affect the occurrence of these parasites in dogs. Cryptosporidium spp. were found in 16.3%, 28.3%, and 28.2% and G. duodenalis in 0.7%, 27.4%, and 30.8% of the examined dogs, red foxes, and raccoon dogs, respectively. The highest prevalence was observed in puppies—15.4% and 18.5% for Cryptosporidium spp. and G. duodenalis, respectively. In all three canid species, infection with Cryptosporidium increases the risk of co-infection with Giardia and vice versa.
1. Introduction
Cryptosporidium spp. (Tyzzer, 1907) and Giardia duodenalis (Stiles, 1902) are important zoonotic protozoans that can cause diarrhea of varying severity in the vast majority of vertebrates, including canids—domestic dogs (Canis familiaris), red foxes (Vulpes vulpes), and raccoon dogs (Nyctereutes procyonoides)—as well as humans [1]. Currently, at least 44 Cryptosporidium species with 120 genotypes have been identified, with C. canis often reported in dogs and other canids [1,2]. However, numerous reports of the zoonotic C. parvum have also been recorded in dogs [3,4,5]. Of the eight Giardia species currently recognized, G. duodenalis is of particular interest in public health. There are eight assemblages (A–H), out of which assemblages A and B are zoonotic and can infect a vast range of mammals [2,6]. Canids are mainly affected by assemblages C and D, but there have also been sporadic reports of infections with assemblages A and B [6].
Cryptosporidiosis in domestic dogs is usually asymptomatic, but clinical signs, such as diarrhea, can be observed in younger dogs, especially if the animal is under chronic stress [7]. High infection rates are observed in three-, four-, and six-month-old dogs, with the infection declining in dogs above one year old [8,9]. The global estimated prevalence of Cryptosporidium in dogs reaches 8.0%, and in Europe, it can vary between 1.1% and 20.5% [10,11,12].
Giardia is one of the most commonly detected parasites in dogs; its worldwide prevalence reaches 15.2%, with a higher prevalence observed in shelter dogs and dogs from kennels [13,14,15]. In Europe, however, Giardia prevalence can vary between 15% and 30.5% [12,16,17]. Likewise, the risk of giardiasis increases if dogs are placed in a shelter for a prolonged period of time [18]. In contrast to cryptosporidiosis, the clinical manifestation of giardiasis in dogs is more severe: abdominal pain, cramping, watery diarrhea, steatorrhea, and malabsorption [19,20]. Often, the course of the disease can be asymptomatic, chronic, or intermittent, complicating the diagnosis [19,20].
Scarce information is available on the clinical signs of both parasite infections in red foxes and raccoon dogs. In red foxes, Cryptosporidium prevalence varies from 2.2% to 3.2%, but Giardia prevalence ranges from 2.8% to 44.0% in various European countries, such as Norway, Bosnia and Herzegovina, and Sweden [21,22,23]. In raccoon dogs, the prevalence of Cryptosporidium is estimated to be 17.6% in Poland [24]. Additionally, wild canids can act as possible reservoirs for both pathogens for domestic dogs and humans due to the zoonotic C. parvum and G. duodenalis assemblages A and B [21,25,26].
Identifying the prevalence of these pathogens in domestic dog, red fox, and raccoon dog populations is important to establish whether wild and domestic canids also pose such infection risks to humans. The aim of this study is to estimate the prevalence of Cryptosporidium and G. duodenalis in canids of Latvia—domestic dogs, red foxes, and raccoon dogs—and analyze factors affecting both infections in canids.
2. Materials and Methods
2.1. Study Area of Domestic Dogs
The necessary sample size was calculated using the OpenEpi v.2.3.1. open access program, assuming a 95% confidence interval (95%CI) and a 40% infection frequency in the domestic dog population [27]. The officially registered Latvian domestic dog population of 132,750 was retrieved from the Agricultural Data Centre Republic of Latvia (accessed on 17 January 2020) [28].
To reach a larger target audience, owners, veterinarians, and shelter owners were invited to participate in the study either through private contact or by responding to social media advertisements. Questionnaires, together with sterile collection containers, were provided to the participating veterinary clinics, shelters, and client centers of the Institute of Food Safety, Animal Health and Environment “BIOR” (Institute “BIOR”). It was recommended that fecal samples be collected for three days in a row. After the collected samples and the filled-out questionnaires arrived at the laboratory, they were labeled, fecal consistency was noted (liquid, soft, or formed), and the samples were stored at 4 °C until further testing.
2.2. Questionnaire for Domestic Dogs
A questionnaire was designed to gather general information about the dogs (breed, sex, age, living conditions), their daily activities (walks in the city, parks, meadow, and forest), health (general information about diarrhea), deworming (frequency of medication and deworming medicine used), feeding (raw, commercial, home-cooked, game meat), contact with other animals (including through home slaughter of livestock) (Supplementary File S1). The dog population was stratified by age: puppies (up to 2 years), adults (2 to 7 years old), seniors (8 to 11 years old), and geriatric (above 12 years old) [29]. The owner filled a questionnaire in Latvian.
2.3. Study Area of Wild Canids
Fecal samples from red foxes and raccoon dogs were collected during the Latvian State program for the Control and Eradication of Rabies organized by the Food and Veterinary Service [30]. Carcasses of hunted foxes and raccoon dogs were delivered to the scientific Institute “BIOR” by hunters, mainly from the eastern part of Latvia, together with additional information about the age, based on dental wear, of the animal (if recorded) and the location (forestry) of where the animal was obtained. Feces from the gastrointestinal tract were collected and stored in a clean container, labeled, and stored at 4 °C until further testing.
2.4. Sample Preparation
One gram of fecal sample was prepared in a clean 15 mL centrifuge tube (SARSTEDT, Nümbrecht, Germany), using equal parts of a saturated sodium chloride (NaCl; density 1.18) solution and distilled water and submitted to a one-minute centrifugation step at 1560× g. In the next step, the supernatant was poured into a clean 50 mL centrifuge tube (SARSTEDT, Nümbrecht, Germany), and distilled water was added to the 45 mL mark. Afterward, the 50 mL tube was centrifuged thrice for 10 min at 1560× g. Between these steps, the top layer was poured out till the 5 mL mark; the sediment was vortexed, and distilled water was added again, resulting in 2 mL of purified and concentrated sediment [31,32].
For immunofluorescence staining, according to the manufacturer’s instructions, 10 μL of thoroughly mixed sample was stained with fluorescein isothiocyanate (FITC)-labelled anti-Cryptosporidium/Giardia monoclonal antibodies (AquaGloTM, Waterborne Inc., New Orleans, LA, USA). For each batch, the positive control provided in the AquaGloTM stain kit was added once to assess the quality of the stain. All bright green (oo)cysts with typical morphology and size were counted, and the total count was multiplied by 200 to calculate the total (oo)cysts per gram of feces [32].
2.5. Data Analysis
Descriptive statistics (medians and means) for oocysts (OPG) and cysts per gram (CPG) were calculated for age groups, breed, and sex. The 95% CI was calculated using the Mid-p exact test using the OpenEpi v.2.3.1. open access program [27].
Standard deviation (SD) was calculated for the animal ages. A two-tailed Mid-p exact test was used to calculate the p-value for differences between purebred and mixed dog species, as well as sexes; p < 0.05 was considered statistically significant. The chi-square test was used to calculate the p-value between dog age groups, and p < 0.05 was considered statistically significant. An animal was considered positive if its fecal sample had at least one Cryptosporidium or/and Giardia (oo)cyst present.
Using QGIS (v.3.36.0-Maidenhead), a map displaying the canid sampling locations and the infection status of Cryptosporidium spp. and G. duodenalis was created [33].
Forward and backward logistic regression model selection was performed using R (v.4.3.2), based on the lowest Akaike information criterion (AIC and AICc) values (from performance package, function performance) for generalized linear models (GLMs) with the family set to binomial [34,35]. The final models had to align with the assumptions (multicollinearity was checked for with function vif from the car package) and included factors that appeared to be significant in single-factor logistic regression analyses to assess if they remained significant [36]. The response (dependent) variable was testing either positive or negative for Cryptosporidium spp. or G. duodenalis. The significance of each independent variable was assessed with the function summary or Anova function with type III sum of squares from the car package [36]. A result was considered statistically significant at p < 0.05, while p < 0.1 was considered to indicate a trend and was further investigated. Tjur’s coefficient of discrimination (Tjur’s R2) was calculated from the performance package function performance to assess how much a particular model explained the probability of having or not a particular infection.
3. Results
3.1. Overall Prevalence of Cryptosporidium spp. and G. duodenalis in Dogs
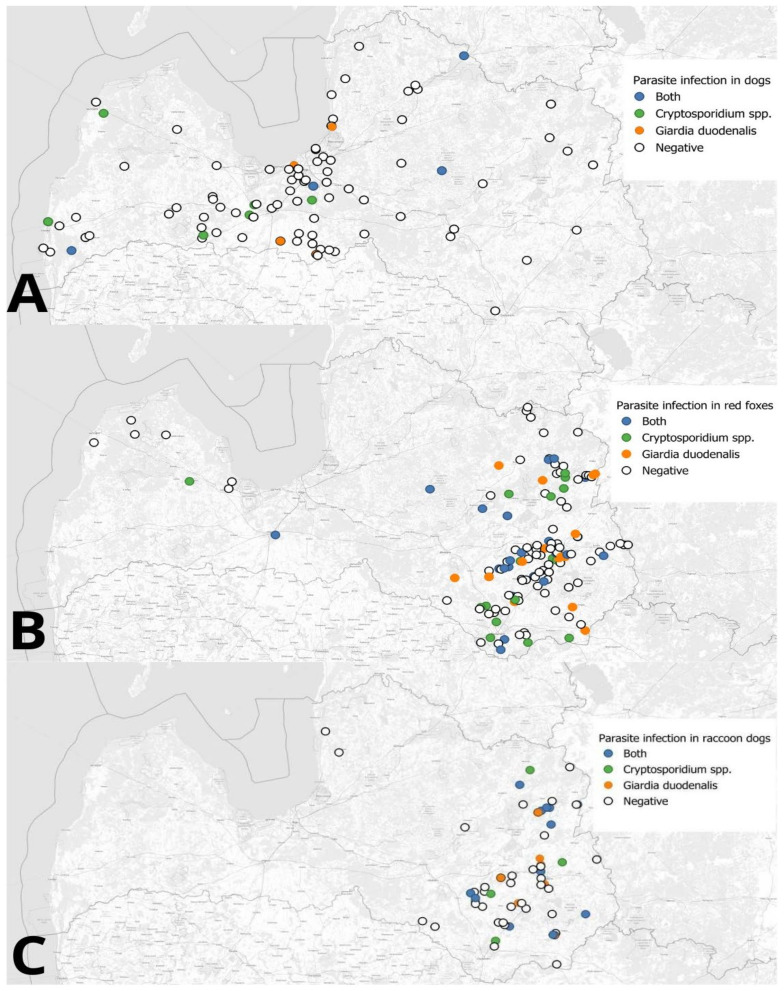
From February 2020 to June 2023, a total of 373 fecal samples from domestic dogs with a mean age of 5.4 years (SD ± 4.1; min 0.2; max 17; median 4) were collected (Figure 1).
Figure 1.
Fecal sample collection sites in Latvia for canids and positive results of Cryptosporidium spp. and G. duodenalis. (A) Domestic dogs; (B) red foxes; (C) raccoon dogs.
In total, 61 dogs (16.3%; 95%CI: 12.9–20.5) were positive for one or both parasites. Cryptosporidium spp. were detected in 9.9% (n = 37; 95%CI: 7.2–13.4) of the animals, with the highest prevalence observed in puppies (Table 1). The mean OPG was 5400 (min 200; max 30′800; median 800), with the highest OPG observed in geriatric dogs (Table 1). No significant statistical significance was observed for Cryptosporidium spp. between age groups, purebred and mixed breeds, and sexes (p > 0.05) (Table 1).
Table 1.
The overall prevalence of Cryptosporidium spp. and G. duodenalis in different domestic dogs by age, breed (purebred and mixed breed), and sex.
| Parasite Species/ Factor |
Sub-Factor | Total No. of Analyzed/ Infected Animals |
Prevalence (95% CI) | Mean OPG/CPG |
Median OPG/CPG |
Min–Max OPG/CPG | p-Value |
|---|---|---|---|---|---|---|---|
| Cryptosporidium spp. | |||||||
| Age group | Puppy | 65/10 | 15.4 (8.8–25.2) | 3680 | 2000 | 200–25,200 | 0.426 |
| Adults | 193/16 | 8.3 (5.1–26.2) | 5688 | 1000 | 200–25,000 | ||
| Seniors | 96/9 | 9.4 (4.4–15.7) | 7533 | 2200 | 200–30,600 | ||
| Geriatric | 19/2 | 10.5 (1.7–32.6) | 21,100 | 21,100 | 11,400–30,800 | ||
| Breed | Purebred | 218/23 | 10.5 (7.1–15.4) | 8100 | 2400 | 200–30,800 | 0.792 |
| Mixed | 155/14 | 9.0 (5.3–14.7) | 8160 | 3200 | 200–30,600 | ||
| Sex | Female | 183/16 | 8.4 (5.4–13.8) | 6109 | 2000 | 200–25,000 | 0.463 |
| Male | 190/21 | 11.0 (7.2–16.4) | 9679 | 2800 | 200–30,800 | ||
| G. duodenalis | |||||||
| Age group | Puppy | 65/12 | 18.5 (10.7–29.7) | 39,650 | 24,800 | 400–157,800 | 0.162 |
| Adults | 193/18 | 9.3 (5.9–14.3) | 35,225 | 3900 | 200–33,000 | ||
| Seniors | 96/9 | 9.4 (4.8–17.1) | 46,488 | 1600 | 200–393,600 | ||
| Geriatric | 19/1 | 5.3 (0.1–26.5) | NA | NA | NA | ||
| Breed | Purebred | 218/22 | 10.1 (6.7–14.9) | 30,522 | 22,700 | 200–157,800 | 0.641 |
| Mixed | 155/18 | 11.6 (7.4–17.7) | 30,600 | 3200 | 200–36,600 | ||
| Sex | Female | 183/12 | 6.6 (3.7–11.2) | 27,250 | 8800 | 200–89,600 | 0.011 * |
| Male | 190/28 | 14.7 (10.3–20.5) | 56,440 | 5300 | 200–393,600 | ||
Abbreviations: CI—confidence interval; NA—not applicable; * p < 0.05.
G. duodenalis was found in 10.7% (n = 40; 95%CI: 8.0–14.3) of the examined dogs, with a mean CPG of 35,368 (min 200; max 393,600; median 4200). Similarly to Cryptosporidium, puppies had the highest prevalence of giardiasis (Table 1). Male dogs had a significantly higher prevalence of Giardia than female dogs (Table 1 and Table 2), with 14.7% and 6.6% prevalence, respectively.
Table 2.
Analysis of deviance (type III tests) for binomial generalized linear models to test if the respective fixed effects (factors) affected the Cryptosporidium spp. or G. duodenalis infection status in domestic dogs, red foxes, and raccoon dogs.
| Canid Species | Parasite | Factor | LR Chisq | Df | p-Value |
|---|---|---|---|---|---|
| Domestic dog | Cryptosporidium spp. | G. duodenalis | 12.2 | 1 | <0.001 *** |
| Home slaughter of livestock | 3.3 | 1 | 0.07 | ||
| Fecal consistency | 1.6 | 1 | 0.2 | ||
| Activity outside the city—on leash | 2.7 | 1 | 0.1 | ||
| G. duodenalis | Cryptosporidium spp. | 28.3 | 1 | <0.001 *** | |
| Sex | 5.4 | 1 | 0.02 * | ||
| Activity outside the city—on leash | 3.5 | 1 | 0.06 | ||
| Red fox | Cryptosporidium spp. | G. duodenalis | 32.1 | 1 | <0.001 *** |
| Forestry | 11.2 | 3 | 0.01 * | ||
| Age (years) | 0.5 | 1 | 0.5 | ||
| G. duodenalis | Cryptosporidium spp. | 17.2 | 1 | <0.001 *** | |
| Age (years) | 7.6 | 1 | 0.006 ** | ||
| Cryptosporidium spp./Age (years) | 5.6 | 1 | 0.02 * | ||
| Raccoon dog | Cryptosporidium spp. | G. duodenalis | 10.9 | 1 | <0.001 *** |
| Age (years) | 0.1 | 1 | 0.7 | ||
| Forestry | 0.8 | 2 | 0.7 | ||
| G. duodenalis | Cryptosporidium spp. | 10.8 | 1 | 0.001 *** | |
| Age (years) | 2.7 | 1 | 0.09 |
Abbreviations: LR Chisq—likelihood ratio chi-square test; Df—degrees of freedom; * p < 0.05; ** p < 0.01; *** p < 0.001.
Co-infections were identified in 4.3% (n = 16; 95%CI: 2.6–6.9) of all the examined dogs. On average, the dogs that were positive with both parasites excreted 3263 (median 800) OPG and 25,012 (median 4800) CPG. The mean age of the dogs infected with both parasites was 4.4 years (SD ± 3.9, median 3.5).
3.2. Overall Prevalence of Cryptosporidium spp. and G. duodenalis in Wild Canids
A total of 219 red fox fecal samples were collected from 2020 to 2023 (Figure 1). Age in years was recorded for 169 foxes, with an average age of 2.1 years (SD ± 0.81, min 1; max 5).
In total, Cryptosporidium spp. prevalence was 28.3% (n = 62; 95%CI: 22.7–34.6), and G. duodenalis prevalence was 27.4% (n = 60; 95%CI: 21.9–33.7). Both parasites were detected in 16.4% of all examined red foxes (n = 36; 95%CI: 13.8–24.8). The average OPG was 5364 (min 200; max 62,800; median 1200), and CPG was 3133 (min 200; max 47,600; median 700).
A total of 78 fecal samples from raccoon dogs were collected during 2020–2023 (Figure 1). Age in years was recorded for 55 raccoon dogs, with an average age of 2 years (SD ± 0.64, min 1; max 4.5).
In total, Cryptosporidium spp. were detected in 28.2% (n = 22; 95%CI: 19.4–39.1), and G. duodenalis in 30.8% (n = 24; 95%CI: 21.6–41.7) of the raccoon dogs. Both parasites were detected in 20.5% (n = 16; 95%CI: 12.9–30.9) of the raccoon dogs. The average OPG was 6072 (min 200; max 51,400; median 1400), and CPG was 15,082 (min 200; max 224,000; median 1200).
3.3. Models
3.3.1. Cryptosporidium spp. and G. duodenalis in Domestic Dogs
The final model for Cryptosporidium spp. in domestic dogs with the lowest AIC values (AIC: 129.804, AICc: 130.094) was established by forward selection based on a single dependent variable model with p < 0.1. The risk factors included in the model were co-infections with G. duodenalis, home-based slaughter of livestock (chickens, ruminants, pigs), fecal consistency, and activity on a leash outside the city. Dogs with cryptosporidiosis were more likely to have G. duodenalis co-infection (Table 2), while the final model explained 19% (Tjur’s R2) of the probability of having Cryptosporidium spp. infection. Dogs with giardiasis were also more likely to have Cryptosporidium spp. co-infection (Table 2). Sex was a risk factor for domestic dogs with G. duodenalis infection (Table 2). The dog age did not affect either cryptosporidiosis or giardiasis (p = 0.15; p = 0.6, respectively).
3.3.2. Cryptosporidium spp. and G. duodenalis in Red Foxes
For Cryptosporidium spp., the final model with the lowest AIC values (AIC: 162.443, AICc: 162.961) was retrieved by backward selection with the following factors affecting Cryptosporidium spp. in red foxes: co-infection with G. duodenalis, forestry, and old age (age in years; z = 0.7, Table 2). However, only co-infection with G. duodenalis and forestry were statistically significant (p < 0.05, Table 2). The final model explained 26% (Tjur’s R2) of the probability of having Cryptosporidium spp. infection.
For G. duodenalis, the final model with the lowest AIC values (AIC: 165.681, AICc: 165.925) was retrieved by forward selection based on single dependent variable models with p < 0.05, which included co-infection with Cryptosporidium spp., old age (age in years; z = 2.7), and their interaction, with all of them being statistically significant (p < 0.05, Table 2). In red foxes, the probability of G. duodenalis infection increased if the animal was already infected with Cryptosporidium spp. and was older (Table 2). The final model explained 25% (Tjur’s R2) of the probability of having G. duodenalis infection.
3.3.3. Cryptosporidium spp. and G. duodenalis in Raccoon Dogs
In raccoon dogs, both models showed that if an animal was already infected with either Cryptosporidium or Giardia, then there was a higher probability of also being infected with the other pathogen (p < 0.01, Table 2), which was also observed in red foxes.
For Cryptosporidium spp., the final model with the lowest AIC values (AIC: 62.490, AICc: 64.240) was retrieved by backward selection, which included co-infection with G. duodenalis, younger age (age in years; z = −1.5), and forestry; only G. duodenalis co-infection appeared to be statistically significant (p < 0.001, Table 2). It was concluded that in raccoon dogs, Cryptosporidium spp. infection was positively affected only by G. duodenalis co-infection (Table 2), while the final model explained 27% (Tjur’s R2) of the probability of having Cryptosporidium spp. infection.
For G. duodenalis, the final model with the lowest AIC values (AIC: 53.576, AICc: 54.047) was retrieved by forward selection based on single dependent variable models with p < 0.08, which included Cryptosporidium spp. and younger age (age in years; z = −0.2), with only Cryptosporidium spp. being statistically significant (p < 0.01, Table 2). It was concluded that in raccoon dogs, G. duodenalis infection was positively affected by Cryptosporidium spp. co-infection (Table 2). The final model explained 27% (Tjur’s R2) of the probability of having G. duodenalis infection.
4. Discussion
Both parasites were prevalent in the domestic dog population, with prevalence reaching 9.9% for Cryptosporidium spp. and 10.7% for G. duodenalis.
A high overall Cryptosporidium spp. prevalence was observed in dogs under two years old (puppies), which is consistent with other studies [11,37,38]. Even though there was no statistical significance for the difference between age groups, Cryptosporidium showed a tendency to affect younger animals due to their immature immune system—puppies acquire passive immunity via the colostrum that is provided by the bitch [39]. Geriatric dogs excreted high levels of oocysts in their feces, possibly due to advanced age-related health decline, decreased number of T-cells, and changes in the intestinal microbiota [40,41]. These changes reduce the body’s ability to fight pathogens, including parasites, increasing the probability of shedding oocysts for a prolonged period [40]. It is also worth mentioning that the small sample size of the geriatric dog group positive for Cryptosporidium should be considered, and the results should be interpreted cautiously.
Similarly to Cryptosporidium, the highest prevalence of Giardia and the highest cyst output were observed in puppies (Table 1). Giardia is one of the most commonly detected parasites in dogs [17,42,43,44]. Studies show that dogs under one year old are more likely to shed Giardia cysts, but no association between the presence of pathogens and changes in fecal consistency was observed [9,45,46,47]. Several studies have been focusing on Giardia-induced clinical signs in dogs; however, the results are inconclusive: in some, a correlation between Giardia and diarrhea was observed, whereas, in others, no such association between infection and diarrhea was found [44,48]. These differences could be explained by the diverse clinical manifestations of giardiasis—ranging from asymptomatic to severe diarrhea—and sampling strategies (sampling from seemingly healthy dogs versus that from dogs with gastrointestinal signs). Nevertheless, Giardia should be considered a possible cause of gastrointestinal problems, especially if intermittent diarrhea is present [17,44]. Before the new 2024 World Small Animal Veterinary Association (WSAVA) vaccination guidelines, it was recommended to socialize puppies only after the final core vaccines were administered, which was at around 16 weeks of age; hence, puppies may get infected with Cryptosporidium and Giardia right after they start to go outside and socialize with other dogs [49,50].
Co-infections with both parasites were mostly observed in adult dogs, who shed high amounts of (oo)cysts in the environment. Additionally, in the final models (Table 2), a significant association was observed between Cryptosporidium spp. and G. duodenalis co-infections, indicating that the presence of one infection increased the likelihood of the other. Even though there have been observations of Giardia co-infections with other parasites, such as Toxocara canis and Isospora spp., especially in young dogs, no direct statistical correlation has been observed [17,51,52].
Even though there are still uncertainties about whether dogs pose a risk for human infections, several studies have shown that dogs are positive for the zoonotic C. parvum or G. duodenalis assemblages A and B [3,4,5,6,47]. Additionally, there have been several reports of humans being infected with C. canis [37,53,54]. Therefore, personal hygiene measures should be upheld when working or living with dogs, even if the animal does not show clinical signs. Regular routine parasitological examinations to detect and, if needed, treat these animals to minimize environmental contamination and risk for humans should also be considered. It is not clear whether cryptosporidiosis in dogs increases the probability of giardiasis and vice versa; however, prioritizing the diagnostics of both parasites should be strongly considered. Additionally, field experts should educate dog and dog shelter owners and handlers about the importance of feces collection from the environment after defecation to minimize the parasite load in the environment [55,56].
For both parasites, higher prevalence was observed in male dogs; however, in the final model, sex appeared to be a significant factor only for Giardia. Other studies show that either female dogs have higher Cryptosporidium and Giardia prevalence, or sex has no correlation at all with these infections [9,57,58]. Some studies show that male dogs are at a higher risk of developing diarrhea, which could be explained by their increased roaming and sniffing behavior, compared to female dogs, which increases the risk of contact with pathogens, including parasites [59,60].
In the initial model, home slaughter of livestock and poultry appeared to be a factor that increased the likelihood of cryptosporidiosis in dogs, but the final model showed only a trend toward significance (Table 2). Ruminants, especially cattle, shed high amounts of Cryptosporidium spp. oocysts in the environment [61]. Therefore, it is possible that these dogs were coincidentally infected through contact with ruminants, and this is not a direct representation of the home slaughter of livestock and poultry. However, feeding by-products of animal origin to dogs can be a route of infection for various other pathogens [62]. Even though the questionnaire used to gather information from the dog owners was comprehensive, it was not validated, which could have introduced some variability in the responses.
A higher prevalence of both parasites was observed in red foxes and raccoon dogs compared to domestic dogs. A high prevalence of G. duodenalis has also been observed in red foxes from Sweden; however, prevalences as low as 4.5% and 2.2% have been observed in Romania and Croatia [21,26,63]. Red foxes are widespread in Latvia, and because they tend to roam in urban areas, they can also pose a risk for humans due to their potential carrying of the zoonotic G. duodenalis assemblages A and B [21,26,63]. Red foxes also carry several Cryptosporidium species that pose a zoonotic risk—C. parvum, C. hominis, and C. canis [25,64]. Limited information is available about both parasites in raccoon dogs in Europe; however, G. duodenalis assemblage D has been reported in Poland and Romania, and C. canis in Poland [65,66,67]. Similar to dogs, also in wild canids, both parasites seem to impact each other (Table 2).
An interesting pattern was observed regarding G. duodenalis infection, which appeared to significantly increase with the age of red foxes (Table 2). This was an unexpected finding due to the fact that G. duodenalis infection was previously reported from juvenile Swedish foxes [22]. The same pattern was observed for Cryptosporidium spp. infection; however, all the results were insignificant. This is an unusual finding, especially since previous studies have shown that both parasites are more commonly associated with higher infection rates in dogs under one year of age [11,12]. This pattern was also observed in our study for both domestic dogs and raccoon dogs (Table 1 and Table 2). In this study, all wild canids were above one year old, and although both parasites tend to affect younger animals, if the animal is subjected to chronic stress or has an accompanying disease, such as sarcoptic mange infection, both parasites can develop chronic manifestations that can last for several months, or the animals can acquire the infection later in life due to a compromised immune system [8,68]. However, it is worth noting that age determination was performed by the hunters who sent in the samples; therefore, the exact age of these animals may be different.
This study did not use molecular methods to confirm the zoonotic Cryptosporidium species and G. duodenalis assemblages; hence, the true zoonotic risk from domestic dogs, red foxes, and raccoon dogs in Latvia still needs to be determined.
5. Conclusions
There was a highly significant association between Cryptosporidium spp. and G. duodenalis co-infections in all the canid species studied, suggesting a possible interaction or shared risk factors between the two pathogens. Both infections are prevalent in Latvian domestic dogs, with the highest prevalence observed in puppies. Because Cryptosporidium and Giardia are likely to cause co-infection, routine testing for both parasites should be prioritized if diarrhea is present, especially if it is intermittent. The prevalence of both parasites in red foxes and raccoon dogs was higher than in domestic dogs. Therefore, they could be a potential infectious source for humans and dogs.
Acknowledgments
We would like to give our sincerest gratitude to the veterinarians, dog owners, and dog shelters that participated in this study as well as to our colleagues from the Institute of Food Safety, Animal Health and Environment “BIOR”, i.e., Maija Rozenfelde, Lelde Šuksta, Anastasija Ahadova for helping with the sample preparation, Andris Avotiņš for the advice on statistical analysis, Janīna Zute and Aleksandrs Uļjanovs for the sample gathering.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14233484/s1, File S1: Table with raw dataset.
Author Contributions
Conceptualization, M.M., A.C., M.S., D.P.Š., M.T. and G.D.; methodology, M.M., A.C., M.S. and G.D; data curation, M.M. and A.C.; writing—original draft preparation, M.M. and A.C.; writing—review and editing, A.C., M.S., D.P.Š., M.T. and G.D.; visualization, M.M., A.C., M.S., D.P.Š., M.T. and G.D.; supervision, M.T. and G.D.; project administration, G.D.; funding acquisition, G.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study did not require ethical approval, as all domestic dog fecal samples were collected with the explicit, written consent of the dog owners, and all data were anonymous.
Informed Consent Statement
The dog owners provided informed consent by signing the questionnaire, which included a detailed explanation of the research conducted, and all data were made anonymous.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Ministry of Education and Science, Latvian Council of Science, Republic of Latvia, within the fundamental and applied research project “Transmission of Foodborne Parasitic pathogen from animals to humans: TRANSPAR” (lzp-2021/1-0055).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ryan U.M., Feng Y., Fayer R., Xiao L. Taxonomy and Molecular Epidemiology of Cryptosporidium and Giardia–A 50-Year Perspective (1971–2021) Int. J. Parasitol. 2021;51:1099–1119. doi: 10.1016/j.ijpara.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Adam R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosanowski S.M., Banica M., Ellis E., Farrow E., Harwood C., Jordan B., James C., McKenna D., Fox M., Blake D.P. The Molecular Characterisation of Cryptosporidium Species in Relinquished Dogs in Great Britain: A Novel Zoonotic Risk? Parasitol. Res. 2018;117:1663–1667. doi: 10.1007/s00436-018-5857-z. [DOI] [PubMed] [Google Scholar]
- 4.Simonato G., Frangipane di Regalbono A., Cassini R., Traversa D., Tessarin C., Di Cesare A., Pietrobelli M. Molecular Detection of Giardia duodenalis and Cryptosporidium spp. in Canine Fecal Samples Contaminating Public Areas in Northern Italy. Parasitol. Res. 2017;116:3411–3418. doi: 10.1007/s00436-017-5671-z. [DOI] [PubMed] [Google Scholar]
- 5.Sotiriadou I., Pantchev N., Gassmann D., Karanis P. Molecular Identification of Giardia and Cryptosporidium from Dogs and Cats. Parasite. 2013;20:8. doi: 10.1051/parasite/2013008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacciò S.M., Lalle M., Svärd S.G. Host Specificity in the Giardia duodenalis Species Complex. Infect. Genet. Evol. 2018;66:335–345. doi: 10.1016/j.meegid.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Thompson R.A., Olson M.E., Zhu G., Enomoto S., Abrahamsen M.S., Hijjawi N.S. Cryptosporidium and Cryptosporidiosis. Adv. Parasitol. 2005;59:77–158. doi: 10.1016/S0065-308X(05)59002-X. [DOI] [PubMed] [Google Scholar]
- 8.Thompson R.A., Palmer C.S., O’Handley R. The Public Health and Clinical Significance of Giardia and Cryptosporidium in Domestic Animals. Vet. J. 2008;177:18–25. doi: 10.1016/j.tvjl.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamnes I.S., Gjerde B.K., Robertson L.J. A Longitudinal Study on the Occurrence of Cryptosporidium and Giardia in Dogs During Their First Year of Life. Acta Vet. Scand. 2007;49:22. doi: 10.1186/1751-0147-49-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taghipour A., Olfatifar M., Bahadory S., Godfrey S.S., Abdoli A., Khatami A., Javanmard E., Shahrivar F. The Global Prevalence of Cryptosporidium Infection in Dogs: A Systematic Review and Meta-Analysis. Vet. Parasitol. 2020;281:109093. doi: 10.1016/j.vetpar.2020.109093. [DOI] [PubMed] [Google Scholar]
- 11.Murnik L.C., Daugschies A., Delling C. Cryptosporidium Infection in Young Dogs from Germany. Parasitol. Res. 2022;121:2985–2993. doi: 10.1007/s00436-022-07632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szydłowicz M., Zajączkowska Ż., Lewicka A., Łukianowski B., Kamiński M., Holubová N., Sak B., Kváč M., Kicia M. Host-Specific Cryptosporidium, Giardia, and Enterocytozoon bieneusi in Shelter Dogs from Central Europe. Parasitology. 2024;151:351–362. doi: 10.1017/S003118202400009X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouzid M., Halai K., Jeffreys D., Hunter P.R. The Prevalence of Giardia Infection in Dogs and Cats, a Systematic Review and Meta-Analysis of Prevalence Studies from Stool Samples. Vet. Parasitol. 2015;207:181–202. doi: 10.1016/j.vetpar.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Claerebout E., Casaert S., Dalemans A.C., De Wilde N., Levecke B., Vercruysse J., Geurden T. Giardia and Other Intestinal Parasites in Different Dog Populations in Northern Belgium. Vet. Parasitol. 2009;161:41–46. doi: 10.1016/j.vetpar.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira F.S., Pereira-Baltasar P., Parreira R., Padre L., Vilhena M., Tavira L.T., Atouguia J., Centeno-Lima S. Intestinal Parasites in Dogs and Cats from the District of Évora, Portugal. Vet. Parasitol. 2011;179:242–245. doi: 10.1016/j.vetpar.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Drake J., Sweet S., Baxendale K., Hegarty E., Horr S., Friis H., Goddu T., Ryan W.G., von Samson-Himmelstjerna G. Detection of Giardia and Helminths in Western Europe at Local K9 (Canine) Sites (DOGWALKS Study) Parasites Vectors. 2022;15:311. doi: 10.1186/s13071-022-05440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Šmit I., Potočnjak D., Matijatko V., Torti M., Jović I., Grden D., Crnogaj M., Beck R. The Influence of Giardia duodenalis on the Occurrence of Clinical Signs in Dogs. Vet. Sci. 2023;10:694. doi: 10.3390/vetsci10120694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubná S., Langrová I., Nápravník J., Jankovská I., Vadlejch J., Pekár S., Fechtner J. The Prevalence of Intestinal Parasites in Dogs from Prague, Rural Areas, and Shelters of the Czech Republic. Vet. Parasitol. 2007;145:120–128. doi: 10.1016/j.vetpar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Epe C., Rehkter G., Schnieder T., Lorentzen L., Kreienbrock L. Giardia in Symptomatic Dogs and Cats in Europe—Results of a European Study. Vet. Parasitol. 2010;173:32–38. doi: 10.1016/j.vetpar.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Tysnes K.R., Skancke E., Robertson L.J. Subclinical Giardia in Dogs: A Veterinary Conundrum Relevant to Human Infection. Trends Parasitol. 2014;30:520–527. doi: 10.1016/j.pt.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Debenham J.J., Landuyt H., Troell K., Tysnes K., Robertson L.J. Occurrence of Giardia in Swedish Red Foxes (Vulpes vulpes) J. Wildl. Dis. 2017;53:649–652. doi: 10.7589/2017-01-002. [DOI] [PubMed] [Google Scholar]
- 22.Hamnes I.S., Gjerde B.K., Forberg T., Robertson L.J. Occurrence of Giardia and Cryptosporidium in Norwegian Red Foxes (Vulpes vulpes) Vet. Parasitol. 2007;143:347–353. doi: 10.1016/j.vetpar.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Hodžić A., Alić A., Omeragić J. Occurrence of Cryptosporidium spp. and Giardia duodenalis in Red Foxes (Vulpes vulpes) in Bosnia and Herzegovina. Maced. Vet. Rev. 2014;37:189–192. doi: 10.14432/j.macvetrev.2014.04.012. [DOI] [Google Scholar]
- 24.Osten-Sacken N., Słodkowicz-Kowalska A., Pacoń J., Skrzypczak Ł., Werner A. Intestinal and External Parasites of Raccoon Dogs (Nyctereutes procyonoides) in Western Poland. Ann. Parasitol. 2017;63:37–44. doi: 10.17420/ap6301.83. [DOI] [PubMed] [Google Scholar]
- 25.Mateo M., de Mingo M.H., de Lucio A., Morales L., Balseiro A., Espí A., Barral M., Barbero J.F.L., Habela M.Á., Fernández-García J.L., et al. Occurrence and Molecular Genotyping of Giardia duodenalis and Cryptosporidium spp. in Wild Mesocarnivores in Spain. Vet. Parasitol. 2017;235:86–93. doi: 10.1016/j.vetpar.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Onac D., Oltean M., Mircean V., Jarca A., Cozma V. Occurrence of Giardia duodenalis Zoonotic Assemblages in Red Foxes from Romania. Sci. Parasitol. 2015;16:177–180. [Google Scholar]
- 27.Sullivan K.M., Dean A., Soe M.M. OpenEpi: A Web-Based Epidemiologic and Statistical Calculator for Public Health. Public Health Rep. 2009;124:471–474. doi: 10.1177/003335490912400320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agricultural Data Centre Public Statistics of Pet Animals. [(accessed on 1 March 2024)]; Available online: https://registri.ldc.gov.lv/pub_istabas_dz/pub_istabas_dz.php.
- 29.Harvey N.D. How Old Is My Dog? Identification of Rational Age Groupings in Pet Dogs Based Upon Normative Age-Linked Processes. Front. Vet. Sci. 2021;8:643085. doi: 10.3389/fvets.2021.643085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ministry of Agriculture, Food and Veterinary Service Latvian State Program for the Control and Eradication of Rabies. [(accessed on 1 January 2020)];2021 Available online: https://www.pvd.gov.lv/lv/media/1109/download.
- 31.Kuczynska E., Shelton D.R. Method for Detection and Enumeration of Cryptosporidium parvum Oocysts in Feces, Manures, and Soils. Appl. Environ. Microbiol. 1999;65:2820–2826. doi: 10.1128/AEM.65.7.2820-2826.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddox-Hyttel C., Langkjær R.B., Enemark H.L., Vigre H. Cryptosporidium and Giardia in Different Age Groups of Danish Cattle and Pigs—Occurrence and Management-Associated Risk Factors. Vet. Parasitol. 2006;141:48–59. doi: 10.1016/j.vetpar.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 33.QGIS.org. QGIS Geographic Information System; QGIS Association. [(accessed on 1 March 2024)]. Available online: http://www.qgis.org.
- 34.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2023. [(accessed on 1 March 2024)]. Version 4.3.2. Available online: https://www.R-project.org/ [Google Scholar]
- 35.Sakamoto Y., Ishiguro M., Kitagawa G. Akaike Information Criterion Statistics; Mathematics and Its Applications. 1st ed. D. Reidel Publishing Company; Dordrecht, The Netherlands: 1986. [Google Scholar]
- 36.Fox J., Weisberg S. An R Companion to Applied Regression. 3rd ed. SAGE Publications, Inc.; Thousand Oaks, CA, USA: 2018. [Google Scholar]
- 37.Li J., Ryan U., Guo Y., Feng Y., Xiao L. Advances in Molecular Epidemiology of Cryptosporidiosis in Dogs and Cats. Int. J. Parasitol. 2021;51:787–795. doi: 10.1016/j.ijpara.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Neves D., Lobo L., Simões P.B., Cardoso L. Frequency of Intestinal Parasites in Pet Dogs from an Urban Area (Greater Oporto, Northern Portugal) Vet. Parasitol. 2014;200:295–298. doi: 10.1016/j.vetpar.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Chastant S., Mila H. Passive Immune Transfer in Puppies. Anim. Reprod. Sci. 2019;207:162–170. doi: 10.1016/j.anireprosci.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Day M.J. Ageing, Immunosenescence, and Inflammageing in the Dog and Cat. J. Comp. Pathol. 2010;142:S60–S69. doi: 10.1016/j.jcpa.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Willems A., Paepe D., Marynissen S., Smets P., Van de Maele I., Picavet P., Duchateau L., Daminet S. Results of Screening of Apparently Healthy Senior and Geriatric Dogs. J. Vet. Intern. Med. 2017;31:81–92. doi: 10.1111/jvim.14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pipia A.P., Varcasia A., Tamponi C., Sanna G., Soda M., Paoletti B., Traversa D., Scala A. Canine Giardiosis in Sardinia Island, Italy: Prevalence, Molecular Characterization, and Risk Factors. J. Infect. Dev. Ctries. 2014;8:655–660. doi: 10.3855/jidc.4255. [DOI] [PubMed] [Google Scholar]
- 43.Remesar S., García-Dios D., Calabuig N., Prieto A., Díaz-Cao J.M., López-Lorenzo G., López C., Fernández G., Morrondo P., Panadero R., et al. Cardiorespiratory Nematodes and Co-Infections with Gastrointestinal Parasites in New Arrivals at Dog and Cat Shelters in North-Western Spain. Transbound. Emerg. Dis. 2022;69:e3141–e3153. doi: 10.1111/tbed.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.López-Arias Á., Villar D., López-Osorio S., Calle-Vélez D., Chaparro-Gutiérrez J.J. Giardia Is the Most Prevalent Parasitic Infection in Dogs and Cats with Diarrhea in the City of Medellín, Colombia. Vet. Parasitol. Reg. Stud. Rep. 2019;18:100335. doi: 10.1016/j.vprsr.2019.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adell-Aledón M., Köster P.C., de Lucio A., Puente P., Hernández-de-Mingo M., Sánchez-Thevenet P., Dea-Ayuela M.A., Carmena D. Occurrence and Molecular Epidemiology of Giardia duodenalis Infection in Dog Populations in Eastern Spain. BMC Vet. Res. 2018;14:26. doi: 10.1186/s12917-018-1353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mateo M., Montoya A., Bailo B., Köster P.C., Dashti A., Hernández-Castro C., Saugar J.M., Matas P., Xiao L., Carmena D. Prevalence and Public Health Relevance of Enteric Parasites in Domestic Dogs and Cats in the Region of Madrid (Spain) with an Emphasis on Giardia duodenalis and Cryptosporidium sp. Vet. Med. Sci. 2023;9:2542–2558. doi: 10.1002/vms3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barutzki D., Thompson R.C.A., Wielinga C., Parka U., Schaper R. Observations on Giardia Infection in Dogs from Veterinary Clinics in Germany. Parasitol. Res. 2007;101:153–156. doi: 10.1007/s00436-007-0623-7. [DOI] [Google Scholar]
- 48.Scorza A.V., Buch J., Franco P., McDonald C., Chandrashekar R., Lappin M.R. Evaluation for Associations Amongst Giardia duodenalis Assemblages and Diarrhea in Dogs. Vet. Parasitol. 2021;300:109581. doi: 10.1016/j.vetpar.2021.109581. [DOI] [PubMed] [Google Scholar]
- 49.Day M.J., Horzinek M.C., Schultz R.D., Squires R.A. WSAVA Guidelines for the Vaccination of Dogs and Cats. J. Small Anim. Pract. 2016;57:E1. doi: 10.1111/jsap.2_12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Squires R.A., Crawford C., Marcondes M., Whitley N. 2024 Guidelines for the Vaccination of Dogs and Cats–Compiled by the Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA) J. Small Anim. Pract. 2024;63:277–316. doi: 10.1111/jsap.13718. [DOI] [PubMed] [Google Scholar]
- 51.Barutzki D., Schaper R. Age-Dependent Prevalence of Endoparasites in Young Dogs and Cats up to One Year of Age. Parasitol. Res. 2013;112:S119. doi: 10.1007/s00436-013-3286-6. [DOI] [PubMed] [Google Scholar]
- 52.Sommer M.F., Rupp P., Pietsch M., Kaspar A., Beelitz P. Giardia in a Selected Population of Dogs and Cats in Germany–Diagnostics, Coinfections and Assemblages. Vet. Parasitol. 2018;249:49–56. doi: 10.1016/j.vetpar.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Leoni F., Amar C., Nichols G., Pedraza-Diaz S., McLauchlin J. Genetic Analysis of Cryptosporidium from 2414 Humans with Diarrhoea in England Between 1985 and 2000. J. Med. Microbiol. 2006;55:703–707. doi: 10.1099/jmm.0.46251-0. [DOI] [PubMed] [Google Scholar]
- 54.Lucio-Forster A., Griffiths J.K., Cama V.A., Xiao L., Bowman D.D. Minimal Zoonotic Risk of Cryptosporidiosis from Pet Dogs and Cats. Trends Parasitol. 2010;26:174–179. doi: 10.1016/j.pt.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Matos M., Alho A.M., Owen S.P., Nunes T., Madeira de Carvalho L. Parasite Control Practices and Public Perception of Parasitic Diseases: A Survey of Dog and Cat Owners. Prev. Vet. Med. 2015;122:174–180. doi: 10.1016/j.prevetmed.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Overgaauw P.A., Van Zutphen L., Hoek D., Yaya F.O., Roelfsema J., Pinelli E., Van Knapen F., Kortbeek L.M. Zoonotic Parasites in Fecal Samples and Fur from Dogs and Cats in The Netherlands. Vet. Parasitol. 2009;163:115–122. doi: 10.1016/j.vetpar.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 57.Bajer A., Bednarska M., Rodo A. Risk Factors and Control of Intestinal Parasite Infections in Sled Dogs in Poland. Vet. Parasitol. 2011;175:343–350. doi: 10.1016/j.vetpar.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 58.Mircean V., Györke A., Cozma V. Prevalence and Risk Factors of Giardia duodenalis in Dogs from Romania. Vet. Parasitol. 2012;184:325–329. doi: 10.1016/j.vetpar.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 59.Hubbard K., Skelly B.J., McKelvie J., Wood J.L.N. Risk of Vomiting and Diarrhoea in Dogs. Vet. Rec. 2007;161:755. doi: 10.1136/vr.161.22.755. [DOI] [PubMed] [Google Scholar]
- 60.Stavisky J., Radford A.D., Gaskell R., Dawson S., German A., Parsons B., Clegg S., Newman J., Pinchbeck G. A Case–Control Study of Pathogen and Lifestyle Risk Factors for Diarrhoea in Dogs. Prev. Vet. Med. 2011;99:185–192. doi: 10.1016/j.prevetmed.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deksne G., Mateusa M., Cvetkova S., Derbakova A., Keidāne D., Troell K., Schares G. Prevalence, Risk Factor and Diversity of Cryptosporidium in Cattle in Latvia. Vet. Parasitol. Reg. Stud. Rep. 2022;28:100677. doi: 10.1016/j.vprsr.2021.100677. [DOI] [PubMed] [Google Scholar]
- 62.Cardoso A.S., Costa I.M.H., Figueiredo C., Castro A., Conceição M.A.P. The Occurrence of Zoonotic Parasites in Rural Dog Populations from Northern Portugal. J. Helminthol. 2014;88:203–209. doi: 10.1017/S0022149X13000047. [DOI] [PubMed] [Google Scholar]
- 63.Beck R., Sprong H., Lucinger S., Pozio E., Caccio S.M. A Large Survey of Croatian Wild Mammals for Giardia duodenalis Reveals a Low Prevalence and Limited Zoonotic Potential. Vector-Borne Zoonotic Dis. 2011;11:1049–1055. doi: 10.1089/vbz.2010.0113. [DOI] [PubMed] [Google Scholar]
- 64.Barrera J.P., Carmena D., Rodríguez E., Checa R., López A.M., Fidalgo L.E., Gálvez R., Marino V., Fuentes I., Miró G., et al. The Red Fox (Vulpes vulpes) as a Potential Natural Reservoir of Human Cryptosporidiosis by Cryptosporidium hominis in Northwest Spain. Transbound. Emerg. Dis. 2020;67:2172–2182. doi: 10.1111/tbed.13569. [DOI] [PubMed] [Google Scholar]
- 65.Adriana G., Zsuzsa K., Oana D.M., Mircea G.C., Viorica M. Giardia duodenalis Genotypes in Domestic and Wild Animals from Romania Identified by PCR-RFLP Targeting the gdh Gene. Vet. Parasitol. 2016;217:71–75. doi: 10.1016/j.vetpar.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Perec-Matysiak A., Hildebrand J., Popiołek M., Buńkowska-Gawlik K. The Occurrence of Cryptosporidium spp. in Wild-Living Carnivores in Poland—A Question Concerning Its Host Specificity. Pathogens. 2023;12:198. doi: 10.3390/pathogens12020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solarczyk P., Majewska A.C., Jędrzejewski S., Górecki M.T., Nowicki S., Przysiecki P. First Record of Giardia Assemblage D Infection in Farmed Raccoon Dogs (Nyctereutes procyonoides) Ann. Agric. Environ. Med. 2016;23:696–698. doi: 10.5604/12321966.1226869. [DOI] [PubMed] [Google Scholar]
- 68.Soulsbury C.D., Iossa G., Baker P.J., Cole N.C., Funk S.M., Harris S. The Impact of Sarcoptic Mange Sarcoptes scabiei on the British Fox Vulpes vulpes Population. Mammal Rev. 2007;37:278–296. doi: 10.1111/j.1365-2907.2007.00100.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.