Abstract
Background: There are no clinical or laboratory markers that can diagnose acute mesenteric ischemia (AMI) accurately. This study aimed to find differences in clinical and laboratory markers between arterial occlusive AMI and other acute abdominal diseases where AMI was initially suspected. Methods: This was a post hoc study of an international prospective multicenter study where data on patients with suspected AMI were collected. Independent factors associated with arterial occlusive AMI were evaluated in a multivariable logistic regression analysis. Results: The number of patients with arterial occlusive AMI was 231, consisting of thrombotic (n = 104), embolic (n = 61), and indeterminate (n = 66) occlusions. The non-AMI group included 287 patients, of whom 128 had strangulated bowel obstruction. Current smoking (odds ratio [OR] 2.56, 95% confidence interval [CI] 1.31–5.03), hypertension (OR 2.08, 95% CI 1.09–3.97), bowel emptying (OR 3.25, 95% CI 1.59–6.63), and leukocytosis (OR 1.54, 95% CI 1.14–2.08) at admission were independently associated with arterial occlusive AMI compared to the non-AMI group. Conclusions: This study found clinical and laboratory data to be associated with arterial occlusive AMI in patients with suspicion of AMI, which can possibly be of value in screening for arterial occlusive AMI at the emergency department. Further studies are needed to find more accurate diagnostic markers.
Keywords: acute mesenteric ischemia, arterial occlusive AMI, acute superior mesenteric artery, clinical diagnosis, laboratory markers
1. Introduction
Acute mesenteric ischemia (AMI) is a life-threatening condition with a high mortality rate. AMI, if not treated adequately, leads to intestinal gangrene and death [1]. One of the main causes of AMI is acute occlusion of the superior mesenteric artery (SMA): the artery which primarily supplies the small intestines and the proximal parts of the colon [1,2]. Arterial occlusive AMI has been shown to be the most common reason for AMI [1]. Occlusion due to embolism or thrombosis may have various onsets and affect different patient categories. Thrombosis—which often is secondary to extensive atherosclerosis—often has a more gradual onset with diffuse symptoms, whereas embolic occlusion—which oftentimes affects patients with atrial fibrillation—may have a more acute onset [1]. The classic clinical triad for acute SMA embolus includes abdominal pain with minimal clinical findings, bowel emptying with vomiting and/or diarrhea, and source of emboli. However, studies have shown inconsistencies in the frequency of these findings [1].
I-FABP, D-lactate, and citrulline were believed to possibly have diagnostic value in patients with AMI; however, a recent systematic review [3] found that those markers failed to differentiate patients with AMI from patients with acute abdomen from other causes. Importantly, one of the most common differential diagnoses of AMI is strangulating bowel obstruction (SBO), and a plasma biomarker differentiating these conditions would be of great value in the clinical workup [4]. Currently, computed tomography (CT) angiography is the gold standard of diagnosis in patients with suspected arterial occlusive AMI [1,5], but it is not without flaws [6].
The AMESI (Acute MESenteric Ischemia) study was a prospective international multicenter study which included patients with AMI along with suspected but confirmed non-AMI patients [7]. The study included patients from 32 hospitals globally.
The aim of this post hoc AMESI study was to find a possible combination of early clinical data and laboratory markers in patients with a suspicion of arterial occlusive AMI to facilitate patient selection for immediate CT angiography.
2. Materials and Methods
2.1. Study Design and Population
The prospectively collected study data were retrieved from the AMESI study [7]. The data included all patients with arterial occlusive AMI and patients with suspected but confirmed to be non-AMI. The study included patients over 18 years of age with suspected or confirmed AMI who were either admitted or transferred to 32 hospitals between 6 June 2022 and 5 April 2023. Patients with non-occlusive mesenteric ischemia (NOMI) and mesenteric vein thrombosis were excluded from the present study.
2.2. Definitions
Suspicion of AMI was raised by the local investigators in a patient with acute abdominal pain in the absence of another obvious diagnosis or critically ill patients in the intensive care unit with increasing plasma lactate levels and suspicion of non-occlusive mesenteric ischemia. AMI was confirmed by CT, endoscopy, surgery, histology, or autopsy in all cases. Patients with confirmed non-AMI (including SBO) comprised a collection of baseline data and hospital survival. Disability was categorized as the need for assistance in daily living activities (no/yes). Atherosclerotic disease was defined as previous ischemic heart disease, stroke, and/or peripheral arterial disease (carotid artery disease, lower extremity arterial disease). The Charlson comorbidity index [8] was calculated (mdcalc.com/calc/3917/charlson-comorbidity-index-cci). Bowel emptying was defined as diarrhea and/or vomiting.
2.3. Statistics
Categorical data are presented as numbers and proportions (%) and continuous data as medians with interquartile ranges (IQR). Variables associated with thrombotic or embolic arterial occlusive AMI, compared to non-AMI patients or SBO, in univariable analysis (p < 0.1), were candidates for inclusion in the multivariable logistic regression analysis, expressed in odds ratios (OR) with 95% confidence intervals (CI). The normality of data was assessed by the Kolmogorov–Smirnov test, and all tested continuous variables were log10 transformed due to skewed distribution and converted to Z scores before entering as covariates into a multivariable logistic regression model. Continuous data after multivariable testing were expressed per one standard deviation (SD) increment. A maximum of one covariate per ten arterial occlusive AMI events was allowed in the multivariable model [9]. No imputation of missing data was conducted, except when developing the prediction model (Supplementary Materials). The level of statistical significance was p < 0.05. IBM SPSS Statistics, version 28 (SPSS, Chicago, IL, USA) was used for statistical analysis. A clinical prediction model was developed using logistic regression modeling (Supplementary Materials).
3. Results
3.1. Comparison of Clinical Background Between Arterial Occlusive AMI and Non-AMI Groups
The total number of patients with confirmed acute occlusive arterial AMI was 231, of which all but 9 had SMA occlusions; 5 had occlusions of the inferior mesenteric artery and 4 of the celiac trunk. The total number of patients with suspected and confirmed non-AMI was 287, of which 128 had SBO. The etiologies of arterial occlusive AMI were thrombotic (n = 104), embolism (n = 61), and indeterminate (n = 66).
Compared to the non-AMI group, patients with arterial occlusive AMI were more often current smokers (p < 0.001), had more atrial fibrillation (p < 0.001), atherosclerotic disease (p = 0.020), hypertension (p < 0.001), myocardial infarction (p = 0.004), previous thromboembolic arterial events (p = 0.003), higher Charlson comorbidity index (p = 0.029), and more frequently used anticoagulant drugs (p = 0.004) (Table 1).
Table 1.
Comparison of clinical background between patients with arterial occlusive AMI and non-AMI groups.
| Arterial Occlusive AMI (n = 231) | Non-AMI (n = 287) | p-Value | |
|---|---|---|---|
| Age, years (median, IQR) | 71 (60–80) | 69 (56–59) | 0.14 |
| Female gender (%) | 100 (43.3) | 138/284 (48.6) | 0.23 |
| Body Mass Index, kg/m2 (median, IQR) | 24.4 (21.3–27.7) (n = 180) | 24.7 (22.0–27.7) (n = 216) | 0.56 |
| Disability (%) | 49/219 (22.4) | 75/269 (27.9) | 0.11 |
| Current smoking (%) | 70/180 (38.9) | 51/225 (22.7) | <0.001 |
| Atrial fibrillation (%) | 84 (36.4) | 65 (22.6) | <0.001 |
| Atherosclerotic disease (%) | 100/217 (46.1) | 96/269 (35.7) | 0.020 |
| Hypertension (%) | 166/224 (74.1) | 169/280 (60.4) | 0.001 |
| Myocardial infarction (%) | 48/221 (21.7) | 33/274 (12.0) | 0.004 |
| Previous thromboembolic arterial event (%) | 25/202 (12.4) | 12/272 (4.4) | 0.003 |
| Charlson comorbidity index (median, IQR) | 4 (3–6) (n = 212) | 4 (2–5) (n = 268) | 0.029 |
| Anticoagulant drugs (%) | 70/217 (32.3) | 56/270 (20.7) | 0.004 |
| Antiplatelet drugs (%) | 77/212 (36.3) | 76/271 (28.0) | 0.052 |
| Statins (%) | 76/213 (35.7) | 85/270 (31.5) | 0.33 |
AMI; acute mesenteric ischemia, IQR; interquartile range.
3.2. Comparison of Clinical Presentation Between Arterial Occlusive AMI and Non-AMI Groups
Patients with arterial occlusive AMI presented more often with diarrhea (p = 0.001), vomiting (p = 0.009), bowel emptying (p < 0.001), and shock (p = 0.031) than the non-AMI group (Table 2).
Table 2.
Comparison of clinical presentation at admission between patients with arterial occlusive AMI and non-AMI groups.
| Arterial Occlusive AMI (n = 231) | Non-AMI (n = 287) | p-Value | |
|---|---|---|---|
| Pre-hospital symptom duration, hours (median; IQR) | 24 (9–48) (n = 172) | 24 (6–48) (n = 190) | 0.94 |
| Acute abdominal pain (%) | 206 (89.2) | 246 (85.7) | 0.24 |
| Diarrhea (%) | 48 (20.8) | 30 (10.5) | 0.001 |
| Bloody stool (%) (macroscopic) | 25 (10.8) | 18 (6.3) | 0.062 |
| Vomiting (%) | 30 (13.0) | 18 (6.3) | 0.009 |
| Bowel emptying * (%) | 68 (29.4) | 47 (16.4) | <0.001 |
| Shock (%) | 43 (18.6) | 34 (11.8) | 0.031 |
AMI; acute mesenteric ischemia, IQR; interquartile range. * diarrhea and/or vomiting.
3.3. Comparison of Laboratory Data Between Arterial Occlusive AMI and Non-AMI Groups
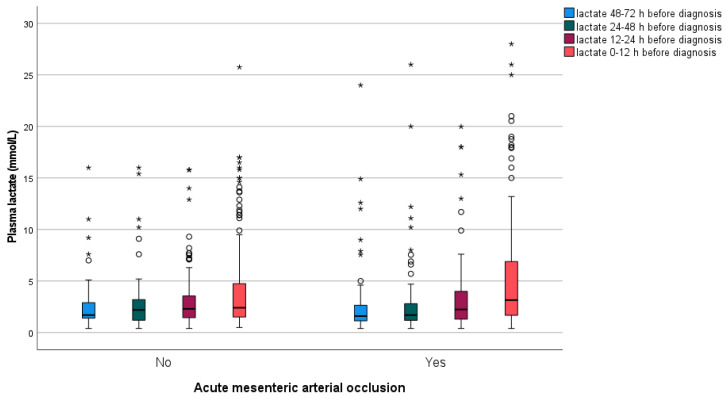
White blood cell count (WBC) (p < 0.001), C-reactive protein (CRP) (p < 0.001), and aspartate aminotransferase (ASAT) (p = 0.001) were more elevated in the arterial occlusive AMI group (Table 3). Base excess (p = 0.017) was lower in patients with arterial occlusive AMI. Plasma lactate levels at different time points prior to diagnosis were similar between the two groups when measured >12 h before verification of diagnosis but differed when measured 0–12 h before diagnosis, where plasma lactate levels were more elevated (p = 0.034) in the arterial occlusive AMI group (Appendix A, Figure A1).
Table 3.
Comparison of laboratory data at admission between patients with arterial occlusive AMI and non-AMI groups.
| Arterial Occlusive AMI (n = 231) | Non-AMI (n = 287) | p-Value | |
|---|---|---|---|
| White blood cell count, ×109/L (median, IQR) | 16.2 (11.4–20.5) (n = 225) | 12.5 (8.0–16.8) (n = 277) | <0.001 |
| CRP, mg/L (median, IQR) | 95 (21–216) (n = 188) | 44 (7.5–118.5) (n = 201) | <0.001 |
| eGFR, ml/min/1.73 m2 (median, IQR) | 50 (26–80) (n = 146) | 60 (36–85) (n = 206) | 0.083 |
| ASAT, U/L (median, IQR) | 39 (23–73) (n = 188) | 28 (19–55) (n = 187) | 0.001 |
| Amylase, U/L (median, IQR) | 66 (41–152) (n = 107) | 57 (34–122) (n = 140) | 0.16 |
| Troponin T, ng/L (median, IQR) | 31 (13–125) (n = 106) | 38 (10–183) (n = 64) | 0.90 |
| pH (median, IQR) | 7.33 (7.23–7.41) (n = 187) | 7.37 (7.27–7.42) (n = 209) | 0.12 |
| Base excess, (median, IQR) | −5.0 (−11.2–0.0) (n = 138) | −1.8 (−8.0–1.2) (n = 203) | 0.014 |
| D-dimer, mg/L (median, IQR) | 4.0 (1.2–9.0) (n = 71) | 4.5 (1.3–8.2) (n = 34) | 0.73 |
| Time point 1 *. Lactate, mmol/L (median, IQR) | 1.6 (1.1–2.7) (n = 71) | 1.7 (1.3–3.0) (n = 37) | 0.40 |
| Time point 2 **. Lactate, mmol/L (median, IQR) | 1.7 (1.2–2.8) (n = 80) | 2.2 (1.2–3.2) (n = 42) | 0.54 |
| Time point 3 ***. Lactate, mmol/L (median, IQR) | 2.2 (1.3–4.0) (n = 98) | 2.3 (1.4–3.7) (n = 75) | 0.52 |
| Time point 4 ****. Lactate, mmol/L (median, IQR) | 3.2 (1.7–6.9) (n = 196) | 2.4 (1.5–4.8) (n = 212) | 0.034 |
| Change in Lactate, mmol/L (median, IQR) between time points 4 and 1 | 0.0 (0.0–0.87) (n = 70) | 0.50 (−0.22–2.15) (n = 34) | 0.27 |
| Change in Lactate, mmol (median, IQR) between time points 4 and 3 | 0.0 (0.0–1.20) (n = 96) | 0.30 (−0.05–1.60) (n = 73) | 0.43 |
AMI; acute mesenteric ischemia, IQR; interquartile range, CRP; c-reactive protein, eGFR; estimated glomerular filtration rate, ASAT; aspartate aminotransferase. * (48–72 h before diagnosis). ** (24–48 h before diagnosis). *** (12–24 h before diagnosis). **** (0–12 h before diagnosis).
3.4. Factors Associated with Arterial Occlusive AMI
Current smoking (OR 2.56, 95% CI 1.31–5.03), hypertension (OR 2.08, 95% CI 1.09–3.97), elevated WBC (OR 1.54, 95% CI 1.14–2.08), and bowel emptying (OR 3.25, 95% CI 1.59–6.63) were independently more associated with arterial occlusive AMI compared to the non-AMI group (Table 4).
Table 4.
Variables associated with arterial occlusive AMI compared to non-AMI group in multivariable analysis.
| Variable | Multivariable Logistic Regression | |
|---|---|---|
| OR (95% CI) | p-Value | |
| Current smoking | 2.56 (1.31–5.03) | 0.006 |
| Hypertension | 2.08 (1.09–3.97) | 0.027 |
| Atherosclerotic disease | 0.70 (0.39–1.27) | 0.24 |
| Atrial fibrillation | 1.58 (0.84–2.99) | 0.16 |
| White blood cell count, ×109/L | 1.54 * (1.14–2.08) | 0.005 |
| CRP, mg/L | 1.19 * (0.91–1.56) | 0.21 |
| ASAT, U/L | 1.34 * (0.94–1.91) | 0.10 |
| Bowel emptying ** | 3.25 (1.59–6.63) | 0.001 |
AMI; acute mesenteric ischemia, OR; odds ratio, CI; confidence interval, CRP; c-reactive protein, ASAT; aspartate aminotransferase. * OR was expressed per one standard deviation increment. ** diarrhea and/or vomiting.
3.5. Comparison of Clinical and Laboratory Data Between Arterial Occlusive AMI and SBO Groups
Univariable analyses were made comparing clinical and laboratory data in arterial occlusive AMI and patients with SBO (Appendix A: Table A1, Table A2 and Table A3). When entering hypertension, atherosclerotic disease, atrial fibrillation, current smoking, bowel emptying, WBC, CRP, ASAT, and D-dimer in a multivariable logistic regression model, only elevated D-dimer (OR 9.32 per one standard deviation increment, 95% CI 1.31–66.39; p = 0.026) remained as an independent factor associated with arterial occlusive AMI.
3.6. Comparison of Clinical and Laboratory Data Between Embolic Arterial Occlusive AMI and Non-AMI Groups
Univariable analyses were made comparing clinical and laboratory data in embolic arterial occlusive AMI (n = 61) and patients with the non-AMI group (Appendix A: Table A4, Table A5 and Table A6). When entering age, hypertension, atrial fibrillation, bowel emptying, WBC, and CRP in a multivariable logistic regression model, atrial fibrillation (OR 4.6, 95% CI 2.1–10.0; p < 0.001), bowel emptying (OR 3.1, 95% CI 1.4–6.9; p = 0.007), and elevated WBC (OR 2.1 per one standard deviation increment, 95% CI 1.3–3.4; p = 0.002) remained as independent factors associated with embolic arterial occlusive AMI.
3.7. Predictive Nomogram for Arterial Occlusive AMI Using a Combination of Clinical and Laboratory Data
A nomogram based on the variables hypertension, bowel emptying, current smoker, CRP, and WBC was constructed to estimate the probability of arterial occlusive AMI (Supplementary Materials). The model had moderate discriminatory performance on internal validation (C-statistic 0.718).
4. Discussion
In this prospective multicenter study, we found that smoking, hypertension, increased WBC, and bowel emptying (diarrhea and/or vomiting) were independently associated with arterial occlusive AMI when compared with the non-AMI group. Smoking was an unsurprising association since it is a known risk factor for atherosclerotic diseases [10], and the best medical treatment for arterial occlusive AMI includes smoking cessation [1]. Previous studies have shown a high prevalence of cardiovascular diseases, such as hypertension, in patients with arterial occlusive AMI [11]. Significant leukocytosis, a marker of severe abdominal pain [12], has been shown to be a predictive marker for transmural bowel necrosis [13,14]. Bowel emptying, which includes both vomiting and/or diarrhea, is a common symptom of AMI [15]. The pathophysiology of this is unclear but could possibly be explained by damage to the mucosal villi and bowel wall distention triggering bowel emptying [16]. The present study showed that bowel emptying was indeed more common than in the non-AMI group, even though a large share consisted of SBO. These associations could possibly be used in a prediction model, and an attempt was made to establish such a model to use when there is a clinical suspicion of arterial occlusive AMI. However, the model was not efficient enough and cannot be used in a clinical setting. Probable reasons for this are, among others, that the markers used in the model are not disease-specific enough and thus are present in several other acute conditions. A new attempt is warranted, preferably when more specific biomarkers are available.
The present study found no difference in the proportion of acute abdominal pain between the groups, whereas another recent report on patients with acute abdomen found that patients with arterial AMI had a higher proportion of sudden onset of pain and morphine-requiring abdominal pain [17].
New biomarkers could be helpful in diagnosing AMI earlier and hopefully increase survival rates. CT angiography is currently the favored method of diagnosis [1]; however, it is a diagnostic modality which has varied availability globally [18,19,20] and may pose a great cost for the patient and the healthcare system [21]. In some cases, it may also be overused [22]. A recent systematic review found that radiological predictors of transmural bowel necrosis may differ according to the various causes of AMI [23]. There are two prospective multicenter studies on biomarkers in the prediction of AMI in pipeline [24,25], where sequential blood samples will be collected in patients with suspected AMI to possibly find biomarkers that can distinguish between different AMI subtypes and their severity. In addition, there is another prospective cohort study searching for biomarkers of acute intestinal ischemia (https://www.ichgcp.net/clinical-trials-registry/NCT03518099, accessed on 18 September 2024). These studies will hopefully find new diagnostic biomarkers to be used as a complement or alternative to CT.
D-dimer was independently associated with arterial occlusive AMI compared to SBO, but it was not significantly associated with the whole non-AMI group. This finding could suggest that fibrinolytic activity [26] is lower in the SBO group.
Atrial fibrillation was independently associated with embolic AMI, which can be expected since atrial fibrillation is a known independent risk factor for other conditions caused by arterial embolisms [27]. It can be argued that in a patient with acute abdomen and atrial fibrillation, arterial occlusive AMI should be one of the differential diagnoses. The present study could indicate that increasing levels of lactate are a finding associated with the severity of the condition and a late-stage marker [28]. Plasma lactate may therefore not be used as an early diagnostic marker in the emergency department since normal lactate in AMI is a diagnostic pitfall [29]. On the other hand, persistently high levels of plasma lactate or increasing plasma lactate levels >200% after cardiovascular surgery should raise suspicion of the development of occlusive or non-occlusive AMI [30]. The subtype of AMI in this study by Mothes et al. was not declared, but it is well-known that most patients with AMI after cardiovascular surgery have non-occlusive mesenteric ischemia [1,30,31].
One of the main limitations is the post hoc analyses of the prospective data since some clinical variables, such as inability to pass gas, constipation/obstipation, and bowel distention, were not pre-specified and therefore missing, making a comparative analysis between groups impossible. Moreover, all the different sites chose their own blood samples in this pragmatic observational study without additional study-specific blood samples, which resulted in missing data for some laboratory data. Another limitation is that the study was composed of almost 60% of patients with AMI [7], indicating recruitment bias. The high proportion of patients with AMI among all suspected cases is highly unlikely since it is known that AMI is a complex and hard diagnosis to make on clinical grounds [1]. It is not exactly known how the different sites defined suspicion of AMI since it was not pre-specified in detail (to avoid interference with local clinical practices), making the inclusions more arbitrary and thereby leading to information bias. Also, not knowing all the diagnoses in the non-AMI group makes the data less applicable since there is a risk of confounding. Furthermore, not all blood samples were analyzed with the same methods: D-dimer, for example, had different assays provided by different manufacturers, which may affect the results [32,33,34]. Another limitation is that the timing of the blood samples in relation to symptom onset was unknown.
5. Conclusions
This study found pre-existing conditions and clinical and laboratory markers independently associated with arterial occlusive AMI compared to non-AMI. It was not possible to attain a well-performing prediction model, possibly due to the high similarity in both groups and markers that are not specific enough for the pathophysiology of the disease. However, this post hoc study is the first to be performed on arterial occlusive AMI on this scale and a well-needed step in the right direction to understand the disease better. Further prospective studies with predefined tests of novel plasma biomarkers in a core lab and biobanking of blood samples are needed to find more accurate diagnostic markers.
Acknowledgments
Sites and Investigators (AMESI Collaborators): Intestinal Stroke Center, Department of Gastroenterology, IBD and Intestinal Failure, AP-HP. Nord, Beaujon Hospital, Paris Cité University, Paris, France: Olivier Corcos, Yves Castier and Maxime Ronot. Division of General Surgery, University Hospital of Trieste ASUGI, Trieste, Italy: Alan Biloslavo and Lucia Paiano. Universitätsklinikum Schleswig-Holstein, Campus Kiel, Kiel, Germany: Gunnar Elke, Denise Nagel and David Immanuel Radke. Hospital General San Martin de La Plata, Buenos Aires, Argentina: Jacqueline Vilca Becerra and María Elina Abeleyra. Lucerne Cantonal Hospital, Lucerne, Switzerland: Martin Cahenzli. University Hospital North Norway, Tromsø, Norway: Geir Ivar Nedredal and Øivind Irtun. Hadassah Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel: Oded Cohen-Arazi and Asaf Kedar. Nicolae Testemitanu, State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Moldova: Gheorghe Rojnoveanu and Tatiana Malcova. Virgen del Rocío University Hospital, Sevilla, Spain: Felipe Pareja Ciuró, Anabel García-Leon’ and Carlos Javier García-Sánchez. Sarawak General Hospital, Kuching, Malaysia: Lim Jia Hui and Loy Yuan Ling. Rabin Medical Center, University of Tel Aviv, Petah Tikva, Israel: Ilya Kagan and Pierre Singer. North Estonia Medical Centre, Tallinn, Estonia: Edgar Lipping. N. Kipshidze Central University Hospital, Tbilisi, Georgia: Ana Tvaladze. Royal Infirmary of Edinburgh, Edinburgh, United Kingdom: Dimitrios Damaskos and Darja Clinch. Hospital Melaka, Malacca, Malaysia: Too Xiao Qing and Mohammad Alif Yunus. Stavanger University Hospital, Stavanger, Norway: Morten Vetrhus. Azienda Ospedaliera Universitaria Careggi, Firenze, Italy: Jacopo Martellucci and Giulia Cerino. Fujian Provincial Hospital, Fuzhou, China: Donghuang Hong and Jinsheng Liu. Hospital Bintulu, Bintulu, Malaysia: Ernest Ong. Erciyes University Hospital, Kayseri, Turkey: Kursat Kundogan and Tutkun Talih. Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India: Lovenish Bains. AOU Cittá della Salute e della Scienza, Turin, Italy: Diego Visconti and Lorenzo Gibello. Hospital Ampang, Ampang, Malaysia: Ruhi Fadzlyana Jailani and Muhammad Amirul Ashra. School of Medical Sciences & Hospital Universiti Sains Malaysia, Kota Bharu, Malaysia: Andee Dzulkarnaen Zakaria and Ahmad Faiz Najmuddin Mohd Ghazi. Hospital Pengajar Universiti Putra, Serdang, Malaysia: Nur Suriyana Abd Ghani. Hospital Sultanah Nur Zahirah, Kuala Terengganu, Malaysia: Mohd Fadliyazid Ab Rahim. University Hospital Centre Zagreb, Zagreb, Croatia: Goran Augustin and Damir Halužan. Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India: Mohan Gurjar and Rahul Rahul. Queen Elisabeth Hospital, Kota Kinabalu, Malaysia: Firdaus Hayati and Jin-Jiun Mah.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diagnostics14232705/s1, Table S1. Univariate logistic regression towards the outcome, arterial occlusive AMI, for 21 pre-specified candidate predictors. Table S2. Trade-off from using the model based on a range of treatment thresholds for the prediction model-derived probability of arterial occlusive AMI. Figure S1. Calibration plot for the full arterial occlusive acute mesenteric ischemia (AMI) occlusion prediction model based on internal validation using 500 sample bootstrapping and the five model variables: Serum white cell blood count (WBC), bowel emptying history, serum C-reactive protein (CRP), arterial hypertension and current smoker. Figure S2. Nomogram for calculating the individual probability for arterial occlusive acute mesenteric ischemia (AMI) among adult patients presenting with abdominal pain in the emergency department. Summarize the scores for each of the five variables to a total score for a corresponding probability assessment [35,36].
Appendix A
Table A1.
Comparison of clinical background data between patients with arterial occlusive AMI and SBO.
| Arterial Occlusive AMI (n = 231) | SBO (n = 128) | p-Value | |
|---|---|---|---|
| Age, years (median, IQR) | 71 (60–80) | 70 (58–80) | 0.47 |
| Female gender (%) | 100 (43.3) | 67/125 (53.6) | 0.063 |
| Body Mass Index, kg/m2 (median, IQR) | 24.4 (21.3–27.7) (n = 180) | 24.2 (20.3–28.1) (n = 101) | 0.78 |
| Disability (%) | 49/219 (22.4) | 32/121 (26.4) | 0.040 |
| Current smoking (%) | 70/180 (38.9) | 20/99 (20.2) | 0.001 |
| Atherosclerotic disease (%) | 100/217 (46.1) | 35/122 (28.7) | 0.002 |
| Hypertension (%) | 166/224 (74.1) | 70/125 (56.0) | <0.001 |
| Myocardial infarction (%) | 48/221 (21.7) | 10/124 (8.1) | 0.001 |
| Previous thromboembolic arterial event (%) | 25/214 (11.7) | 3/124 (2.4) | 0.003 |
| Charlson comorbidity index (median, IQR) | 4 (3–6) (n = 212) | 4 (2–5) (n = 121) | 0.001 |
| Anticoagulant drugs (%) | 70/217 (32.3) | 25/127 (19.7) | 0.012 |
| Antiplatelet drugs (%) | 77/212 (36.3) | 30/126 (23.8) | 0.017 |
| Statins (%) | 76/213 (35.7) | 36/126 (28.6) | 0.18 |
AMI; acute mesenteric ischemia, SBO; strangulating bowel obstruction, IQR; interquartile range.
Table A2.
Comparison of clinical presentation between patients with arterial occlusive AMI and SBO.
| Arterial Occlusive AMI (n = 231) | SBO (n = 128) | p-Value | |
|---|---|---|---|
| Pre-hospital symptom duration, hours (median; IQR) | 24 (9–48) (n = 172) | 24 (8–48) (n = 87) | 0.93 |
| New atrial fibrillation (%) | 23 (10.0) | 4 (3.1) | 0.019 |
| Atrial fibrillation (%) | 84 (36.4) | 28 (21.9) | 0.005 |
| Acute abdominal pain (%) | 206 (89.2) | 120 (93.8) | 0.15 |
| Diarrhea (%) | 48 (20.8) | 7 (5.5) | <0.001 |
| Bloody stool (%) (macroscopic) | 25 (10.8) | 5 (3.9) | 0.023 |
| Vomiting (%) | 30 (13.0) | 13 (10.2) | 0.43 |
| Bowel emptying * (%) | 68 (29.4) | 20 (15.6) | 0.004 |
| Shock (%) | 43 (18.6) | 5 (3.9) | <0.001 |
AMI; acute mesenteric ischemia, SBO; strangulating bowel obstruction, IQR; interquartile range. * diarrhea and/or vomiting.
Table A3.
Comparison of laboratory data between patients with arterial occlusive AMI and SBO.
| Arterial Occlusive AMI (n = 231) | SBO (n = 128) | p-Value | |
|---|---|---|---|
| White blood cell count, ×109/L (median, IQR) | 16.2 (11.4–20.5) (n = 225) | 12.4 (8.2–16.0) (n = 123) | <0.001 |
| CRP, mg/L (median, IQR) | 95 (21–216) (n = 188) | 36 (6–112) (n = 83) | <0.001 |
| eGFR, ml/min/1.73 m2 (median, IQR) | 50 (26–80) (n = 146) | 60 (39–86) (n = 85) | 0.044 |
| ASAT, U/L (median, IQR) | 39 (23–73) (n = 188) | 24 (18–32) (n = 78) | <0.001 |
| Amylase, U/L (median, IQR) | 66 (41–152) (n = 107) | 67 (37–123) (n = 55) | 0.63 |
| Troponin T, ng/L (median, IQR) | 31 (13–125) (n = 106) | 30 (10–105) (n = 29) | 0.54 |
| pH (median, IQR) | 7.33 (7.23–7.41) (n = 187) | 7.38 (7.34–7.42) (n = 101) | <0.001 |
| Base excess, (median, IQR) | −5.0 (−11.0–0.0) (n = 137) | −0.80 (−5.50–2.0) (n = 99) | <0.001 |
| D-dimer, mg/L (median, IQR) | 4.0 (1.3–10.0) (n = 71) | 1.4 (0.3–5.0) (n = 15) | 0.009 |
| Time point 1 *. Lactate, mmol/L (median, IQR) | 1.6 (1.1–2.7) (n = 71) | 1.6 (0.6–1.8) (n = 11) | 0.40 |
| Time point 2 **. Lactate, mmol/L (median, IQR) | 1.7 (1.2–2.8) (n = 80) | 1.4 (0.6–2.5) (n = 11) | 0.33 |
| Time point 3 ***. Lactate, mmol/L (median, IQR) | 2.2 (1.3–4.0) (n = 98) | 2.0 (1.4–2.7) (n = 32) | 0.26 |
| Time point 4 ****. Lactate, mmol/L (median, IQR) | 3.2 (1.7–6.9) (n = 196) | 2.2 (1.5–3.5) (n = 98) | <0.001 |
AMI; acute mesenteric ischemia, SBO; strangulating bowel obstruction, CRP; c-reactive protein, eGFR; estimated glomerular filtration rate, ASAT; aspartate aminotransferase. * (48–72 h before diagnosis). ** (24–48 h before diagnosis). *** (12–24 h before diagnosis). **** (0–12 h before diagnosis).
Table A4.
Comparison of clinical background data between patients with embolic arterial occlusive AMI and non-AMI.
| Embolic Arterial Occlusive AMI (n = 61) | Non-AMI (n = 287) | p-Value | |
|---|---|---|---|
| Age, years (median, IQR) | 76 (66–83) | 69 (56–59) | 0.008 |
| Female gender (%) | 33 (43.1) | 138/284 (48.6) | 0.45 |
| Body Mass Index, kg/m2 (median, IQR) | 25.2 (23.3–28.4) (n = 49) | 24.7 (22.0–27.7) (n = 216) | 0.15 |
| Disability (%) | 15 (24.6) | 75/269 (27.9) | 0.62 |
| Current smoking (%) | 10/55 (16.4) | 51/225 (22.7) | 0.47 |
| Atherosclerotic disease (%) | 25 (41.0) | 96/269 (35.7) | 0.44 |
| Hypertension (%) | 49 (80.3) | 169/280 (60.4) | 0.003 |
| Myocardial infarction (%) | 12 (19.7) | 33/274 (12.0) | 0.11 |
| Previous thromboembolic arterial event (%) | 5/59 (8.5) | 12/272 (4.4) | 0.20 |
| Charlson comorbidity index (median, IQR) | 4 (3–6) (n = 58) | 4 (2–5) (n = 268) | 0.083 |
| Anticoagulant drugs (%) | 20/60 (33.3) | 56/270 (20.7) | 0.036 |
| Antiplatelet drugs (%) | 16/60 (26.7) | 76/271 (28.0) | 0.83 |
| Statins (%) | 22/60 (36.7) | 85/270 (31.5) | 0.44 |
AMI; acute mesenteric ischemia, IQR; interquartile range.
Table A5.
Comparison of clinical presentation at admission between patients with embolic arterial occlusive AMI and non-AMI.
| Embolic Arterial Occlusive AMI (n = 61) | Non-AMI (n = 287) | p-Value | |
|---|---|---|---|
| Pre-hospital symptom duration, hours (median; IQR) | 18 (4–48) (n = 47) | 24 (6–48) (n = 190) | 0.19 |
| New atrial fibrillation (%) | 11 (18.0) | 10 (3.5) | <0.001 |
| Atrial fibrillation (total) (%) | 41 (67.2) | 65 (22.6) | <0.001 |
| Acute abdominal pain (%) | 55 (90.2) | 246 (85.7) | 0.36 |
| Diarrhea (%) | 15 (24.6) | 30 (10.5) | 0.003 |
| Bloody stool (%) | 10 (16.4) | 18 (6.3) | 0.008 |
| Vomiting (%) | 10 (16.4) | 18 (6.3) | 0.008 |
| Bowel emptying * (%) | 19 (31.1) | 47 (16.4) | 0.008 |
| Shock (%) | 14 (23.0) | 34 (11.8) | 0.022 |
AMI; acute mesenteric ischemia, IQR; interquartile range. * diarrhea and/or vomiting.
Table A6.
Comparison of laboratory data between patients with embolic arterial occlusive AMI and non-AMI.
| Embolic Arterial Occlusive AMI (n = 61) | Non-AMI (n = 287) | p-Value | |
|---|---|---|---|
| White blood cell count, ×109/L (median, IQR) | 16.6 (11.4–19.0) (n = 59) | 12.5 (8.0–16.8) (n = 277) | <0.001 |
| CRP, mg/L (median, IQR) | 73 (19.2–174.8) (n = 52) | 44 (7.5–118.5) (n = 201) | 0.026 |
| eGFR, ml/min/1.73 m2 (median, IQR) | 56 (30–82) (n = 40) | 60 (36–85) (n = 206) | 0.41 |
| ASAT, U/L (median, IQR) | 34 (25–61) (n = 54) | 28 (19–55) (n = 187) | 0.12 |
| Amylase, U/L (median, IQR) | 69 (50–138) (n = 30) | 57 (34–122) (n = 140) | 0.15 |
| Troponin T, ng/L (median, IQR) | 39 (13–188) (n = 31) | 38 (10–183) (n = 64) | 0.82 |
| pH (median, IQR) | 7.34 (7.26–7.42) (n = 52) | 7.37 (7.27–7.42) (n = 209) | 0.92 |
| Base excess, (median, IQR) | −2.9 (−8.0–0.0) (n = 39) | −1.8 (−8.0–1.2) (n = 203) | 0.40 |
| D-dimer, mg/L (median, IQR) | 5.0 (2.0–9.5) (n = 21) | 4.5 (1.3–8.2) (n = 34) | 0.23 |
| Time point 1 *. Lactate, mmol/L (median, IQR) | 1.7 (1.3–2.3) (n = 15) | 1.7 (1.3–3.0) (n = 37) | 0.68 |
| Time point 2 **. Lactate, mmol/L (median, IQR) | 1.8 (1.3–2.9) (n = 19) | 2.2 (1.2–3.2) (n = 42) | 0.95 |
| Time point 3 ***. Lactate, mmol/L (median, IQR) | 2.4 (1.7–3.1) (n = 23) | 2.3 (1.4–3.7) (n = 75) | 0.98 |
| Time point 4 ****. Lactate, mmol/L (median, IQR) | 3.0 (1.8–5.4) (n = 54) | 2.4 (1.5–4.8) (n = 212) | 0.30 |
| Change in Lactate, mmol/L (median, IQR) between time points 4 and 1 | 0.2 (−0.1–0.8) (n = 15) | 0.50 (−0.22–2.15) (n = 34) | 0.28 |
AMI; acute mesenteric ischemia, IQR; interquartile range, CRP; c-reactive protein, ASAT; aspartate aminotransferase. * (48–72 h before diagnosis). ** (24–48 h before diagnosis). *** (12–24 h before diagnosis). **** (0–12 h before diagnosis).
Figure A1.
Box plot graph showing median and interquartile range (IQR) values of plasma lactate at different time ranges before diagnosis of arterial occlusive acute mesenteric ischemia (AMI) and non-AMI, respectively. The line across the box indicates the median, the box represents the interquartile range, and the whiskers are lines that extend from the box edges to the highest and lowest values, excluding outliers. Values more than 1.5 IQR’s but less than 3 IQR’s (o), and more than 3 IQR’s (*), from the box edges are labeled as outliers and extremes, respectively.
Author Contributions
All authors contributed to conceptualization, methodology, formal analysis, writing—review and editing, visualization, and project administration. All authors except Y.S.-N. and J.T. contributed to software, validation, investigation, resources, supervision, funding acquisition, and data curation. Y.S.-N., S.A. and J.T. contributed to writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and primary ethical approval was obtained from the Ethics Committee of the University of Tartu (357/T-8 and 364M-7, 01 February 2022). Thereafter, the 32 sites obtained ethical approval from their local ethics committees.
Informed Consent Statement
Informed consent was obtained from all participants with full data collection. Patients from whom only baseline data and information on hospital survival were collected were handled based on local ethics requirements at each site. Informed consent was not obtained for the aforementioned patients with limited data collection if general consent at the site was available and a waiver of informed consent was approved by the local ethics committee.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (S.A).
Conflicts of Interest
A.R.B has received speaker or consultancy fees from Nestlé, VIPUN Medical, Nutricia, and Fresenius Kabi, and holds a grant from the Estonian Research Council (PRG1255). AF has received speaker fees from B Braun and Fresenius Kabi. A.N. has received speaker or consultancy fees from Abbvie and Janssen, research funding from MSD-Avenir, and PhD grants from Fondation de l’Avenir and SNFGE. Y.S.-N., M.B. (Martin Björck), J.S., M.M., K.T., O.T., A.-L.V., M.K., M.B. (Miklosh Bala), Z.B., D.C., Z.D., M.D., V.D.M.-C., H.F., M.H.I., B.H., K.K., K.L., M.L., C.I.L., D.J.M., S.S., M.S., K.V., J.T., and S.A reports no conflicts of interest.
Funding Statement
The research was funded by the Estonian Research Council, grant number PRG1255, and The Swedish Gastroenterology and Hepatology Foundation.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bjorck M., Koelemay M., Acosta S., Bastos Goncalves F., Kolbel T., Kolkman J.J., Lees T., Lefevre J.H., Menyhei G., Oderich G., et al. Editor’s Choice—Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS) Eur. J. Vasc. Endovasc. Surg. 2017;53:460–510. doi: 10.1016/j.ejvs.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Geboes K., Geboes K.P., Maleux G. Vascular anatomy of the gastrointestinal tract. Best. Pract. Res. Clin. Gastroenterol. 2001;15:1–14. doi: 10.1053/bega.2000.0152. [DOI] [PubMed] [Google Scholar]
- 3.Reintam Blaser A., Starkopf J., Björck M., Forbes A., Kase K., Kiisk E., Laisaar K.-T., Mihnovits V., Murruste M., Mändul M., et al. Diagnostic accuracy of biomarkers to detect acute mesenteric ischaemia in adult patients: A systematic review and meta-analysis. World J. Emerg. Surg. 2023;18:44. doi: 10.1186/s13017-023-00512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rami Reddy S.R., Cappell M.S. A Systematic Review of the Clinical Presentation, Diagnosis, and Treatment of Small Bowel Obstruction. Curr. Gastroenterol. Rep. 2017;19:28. doi: 10.1007/s11894-017-0566-9. [DOI] [PubMed] [Google Scholar]
- 5.Anglaret S., Dallongeville A., Beaussier H., Touloupas C., Boulay I., Tardivel A.M., Béranger S., Silvera S., Chatellier G., Ronot M., et al. Influence of clinical suspicion on CT accuracy of acute mesenteric ischemia: Retrospective study of 362 patients. Eur. J. Radiol. 2021;138:109652. doi: 10.1016/j.ejrad.2021.109652. [DOI] [PubMed] [Google Scholar]
- 6.Mothes H., Mueller-Mau V., Lehmkuhl L., Lehmann T., Settmacher U., Teichgräber U., Ludewig S. The role of computed tomography in the diagnostic pathway of acute mesenteric ischemia: A nested case-control study. Acta Radiol. 2020;61:1444–1451. doi: 10.1177/0284185120905086. [DOI] [PubMed] [Google Scholar]
- 7.Reintam Blaser A., Mändul M., Björck M., Acosta S., Bala M., Bodnar Z., Casian D., Demetrashvili Z., D’Oria M., Durán Muñoz-Cruzado V., et al. Incidence, diagnosis, management and outcome of acute mesenteric ischaemia: A prospective, multicentre observational study (AMESI Study) Crit. Care. 2024;28:32. doi: 10.1186/s13054-024-04807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Hoore W., Sicotte C., Tilquin C. Risk adjustment in outcome assessment: The Charlson comorbidity index. Methods Inf. Med. 1993;32:382–387. [PubMed] [Google Scholar]
- 9.Harrell F.E., Jr., Lee K.L., Mark D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Chao T.H., Lin T.H., Cheng C.I., Wu Y.W., Ueng K.C., Wu Y.J., Lin W.W., Leu H.B., Cheng H.M., Huang C.C., et al. 2024 Guidelines of the Taiwan Society of Cardiology on the Primary Prevention of Atherosclerotic Cardiovascular Disease—Part I. Acta Cardiol. Sin. 2024;40:479–543. doi: 10.6515/acs.202409_40(5).20240724a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemma A., Tolonen M., Vikatmaa P., Mentula P., Kantonen I., But A., Leppaniemi A., Sallinen V. Epidemiology, diagnostics and outcomes of acute occlusive arterial mesenteric ischaemia—A population-based study. Eur. J. Vasc. Endovasc. Surg. 2022;64:646–653. doi: 10.1016/j.ejvs.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Deibener-Kaminsky J., Lesesve J.F., Kaminsky P. Leukocyte differential for acute abdominal pain in adults. Lab. Hematol. 2011;17:1–5. doi: 10.1532/LH96.10023. [DOI] [PubMed] [Google Scholar]
- 13.Emile S.H. Predictive Factors for Intestinal Transmural Necrosis in Patients with Acute Mesenteric Ischemia. World J. Surg. 2018;42:2364–2372. doi: 10.1007/s00268-018-4503-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H., Meng Y., Zhang P., Zhang Q., Wang F., Li Y. Predictors and risk factors for intestinal necrosis in patients with mesenteric ischemia. Ann. Transl. Med. 2021;9:337. doi: 10.21037/atm-20-8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cudnik M.T., Darbha S., Jones J., Macedo J., Stockton S.W., Hiestand B.C. The Diagnosis of Acute Mesenteric Ischemia: A Systematic Review and Meta-analysis. Acad. Emerg. Med. 2013;20:1087–1100. doi: 10.1111/acem.12254. [DOI] [PubMed] [Google Scholar]
- 16.Fruhwald S., Holzer P., Metzler H. Gastrointestinal motility in acute illness. Wien. Klin. Wochenschr. 2008;120:6–17. doi: 10.1007/s00508-007-0920-2. [DOI] [PubMed] [Google Scholar]
- 17.Nuzzo A., Peoc’H K., Vaittinada Ayar P., Tran-Dinh A., Weiss E., Panis Y., Ronot M., Garzelli L., Eloy P., Ben Abdallah I., et al. Improving clinical suspicion of acute mesenteric ischemia among patients with acute abdomen: A cross-sectional study from an intestinal stroke center. World J. Emerg. Surg. 2023;18:37. doi: 10.1186/s13017-023-00505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav H., Shah D., Sayed S., Horton S., Schroeder L.F. Availability of essential diagnostics in ten low-income and middle-income countries: Results from national health facility surveys. Lancet Glob. Health. 2021;9:e1553–e1560. doi: 10.1016/S2214-109X(21)00442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kithinji S.M., Lule H., Acan M., Kyomukama L., Muhumuza J., Kyamanywa P. Efficacy of extended focused assessment with sonography for trauma using a portable handheld device for detecting hemothorax in a low resource setting; a multicenter longitudinal study. BMC Med. Imaging. 2022;22:211. doi: 10.1186/s12880-022-00942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasbey J.C., Moore R., Ademuyiwa A., Adisa A., Biccard B., Chakrabortee S., Ghosh D., Harrison E., Jones C., Lapitan M.C., et al. Global guidelines for emergency general surgery: Systematic review and Delphi prioritization process. BJS Open. 2022;6:zrac005. doi: 10.1093/bjsopen/zrac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berge P., Darsonval A., Nedelcu C., Paisant A., Aubé C. Incidental findings on emergency CT scans: Predictive factors and medico-economic impact. Eur. J. Radiol. 2020;129:109072. doi: 10.1016/j.ejrad.2020.109072. [DOI] [PubMed] [Google Scholar]
- 22.Systermans B.J., Devitt P.G. Computed tomography in acute abdominal pain: An overused investigation? ANZ J. Surg. 2014;84:155–159. doi: 10.1111/ans.12360. [DOI] [PubMed] [Google Scholar]
- 23.Zeng Y., Yang F., Hu X., Zhu F., Chen W., Lin W. Radiological predictive factors of transmural intestinal necrosis in acute mesenteric ischemia: Systematic review and meta-analysis. Eur. Radiol. 2022;33:2792–2799. doi: 10.1007/s00330-022-09258-5. [DOI] [PubMed] [Google Scholar]
- 24.Duivenvoorden A.A.M., Clarysse M., Ceulemans L.J., Geelkerken R.H., Derikx J.P.M., De Vries J.-P.P.M., Buscher H.C.J.L., Olde Damink S.W.M., Van Schooten F.J., Lubbers T., et al. Diagnostic potential of plasma biomarkers and exhaled volatile organic compounds in predicting the different stages of acute mesenteric ischaemia: Protocol for a multicentre prospective observational study (TACTIC study) BMJ Open. 2023;13:e072875. doi: 10.1136/bmjopen-2023-072875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamme K., Acosta S., Biloslavo A., Björck M., Casian D., Damaskos D., Forbes A., Kase K., Kisand K., Lakbar I., et al. Biomarkers In Prediction of Acute Mesenteric Ischaemia: A prospective multicentre study (BIPAMI study): A study protocol. BMC Surg. 2024;24:201. doi: 10.1186/s12893-024-02491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson J.D. Chapter One—D-dimer: An Overview of Hemostasis and Fibrinolysis, Assays, and Clinical Applications. Adv. Clin. Chem. 2015;69:1–46. doi: 10.1016/bs.acc.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 28.Sumbal R., Ali Baig M.M., Sumbal A. Predictors of Mortality in Acute Mesenteric Ischemia: A Systematic Review and Meta-Analysis. J. Surg. Res. 2022;275:72–86. doi: 10.1016/j.jss.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Collange O., Lopez M., Lejay A., Pessaux P., Ouattara A., Dewitte A., Rimmele T., Girardot T., Arnaudovski D., Augustin P., et al. Serum lactate and acute mesenteric ischaemia: An observational, controlled multicentre study. Anaesth. Crit. Care Pain. Med. 2022;41:101141. doi: 10.1016/j.accpm.2022.101141. [DOI] [PubMed] [Google Scholar]
- 30.Mothes H., Wickel J., Sponholz C., Lehmann T., Kaluza M., Zanow J., Doenst T. Monitoring of the Progression of the Perioperative Serum Lactate Concentration Improves the Accuracy of the Prediction of Acute Mesenteric Ischemia Development After Cardiovascular Surgery. J. Cardiothorac. Vasc. Anesth. 2021;35:1792–1799. doi: 10.1053/j.jvca.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson J., Hansson E., Andersson B. Intestinal ischemia after cardiac surgery: Analysis of a large registry. J. Cardiothorac. Surg. 2013;8:156. doi: 10.1186/1749-8090-8-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannitsis E., Mair J., Christersson C., Siegbahn A., Huber K., Jaffe A.S., Peacock W.F., Plebani M., Thygesen K., Möckel M., et al. How to use D-dimer in acute cardiovascular care. Eur. Heart J. Acute Cardiovasc. Care. 2017;6:69–80. doi: 10.1177/2048872615610870. [DOI] [PubMed] [Google Scholar]
- 33.Linkins L.A., Takach Lapner S. Review of D-dimer testing: Good, Bad, and Ugly. Int. J. Lab. Hematol. 2017;39:98–103. doi: 10.1111/ijlh.12665. [DOI] [PubMed] [Google Scholar]
- 34.Favresse J., Lippi G., Roy P.-M., Chatelain B., Jacqmin H., Ten Cate H., Mullier F. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit. Rev. Clin. Lab. Sci. 2018;55:548–577. doi: 10.1080/10408363.2018.1529734. [DOI] [PubMed] [Google Scholar]
- 35.Steyerberg E.W. Statistics for Biology and Health. Springer Nature; Dordrecht, The Netherlands: 2009. Clinical Prediction Models. [DOI] [Google Scholar]
- 36.Steyerberg E.W., Vergouwe Y. Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur. Heart J. 2014;35:1925–1931. doi: 10.1093/eurheartj/ehu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (S.A).