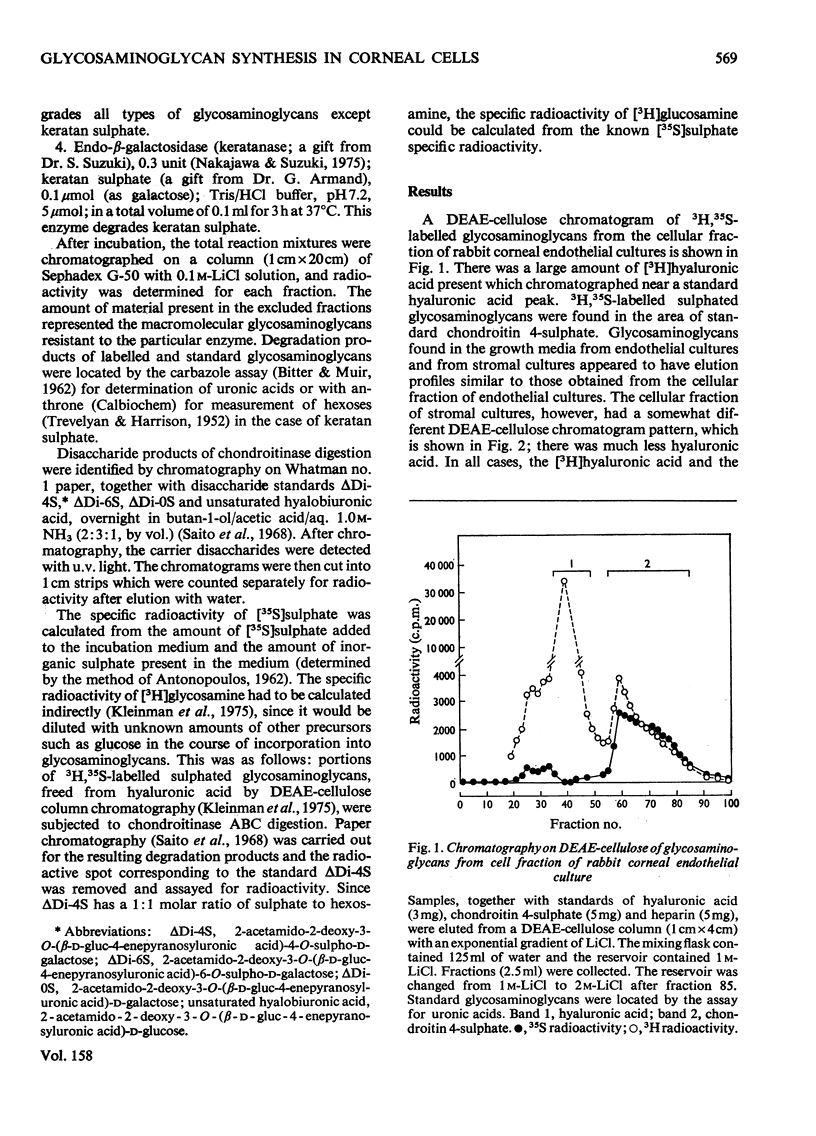
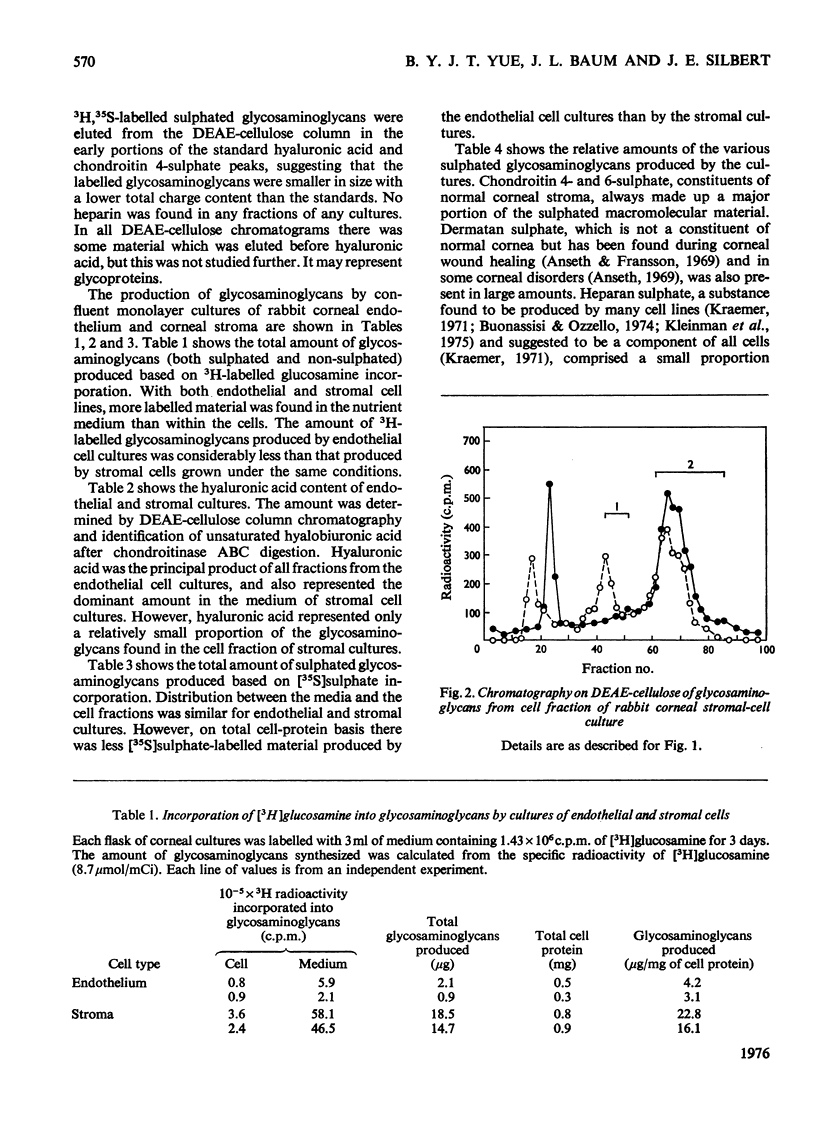
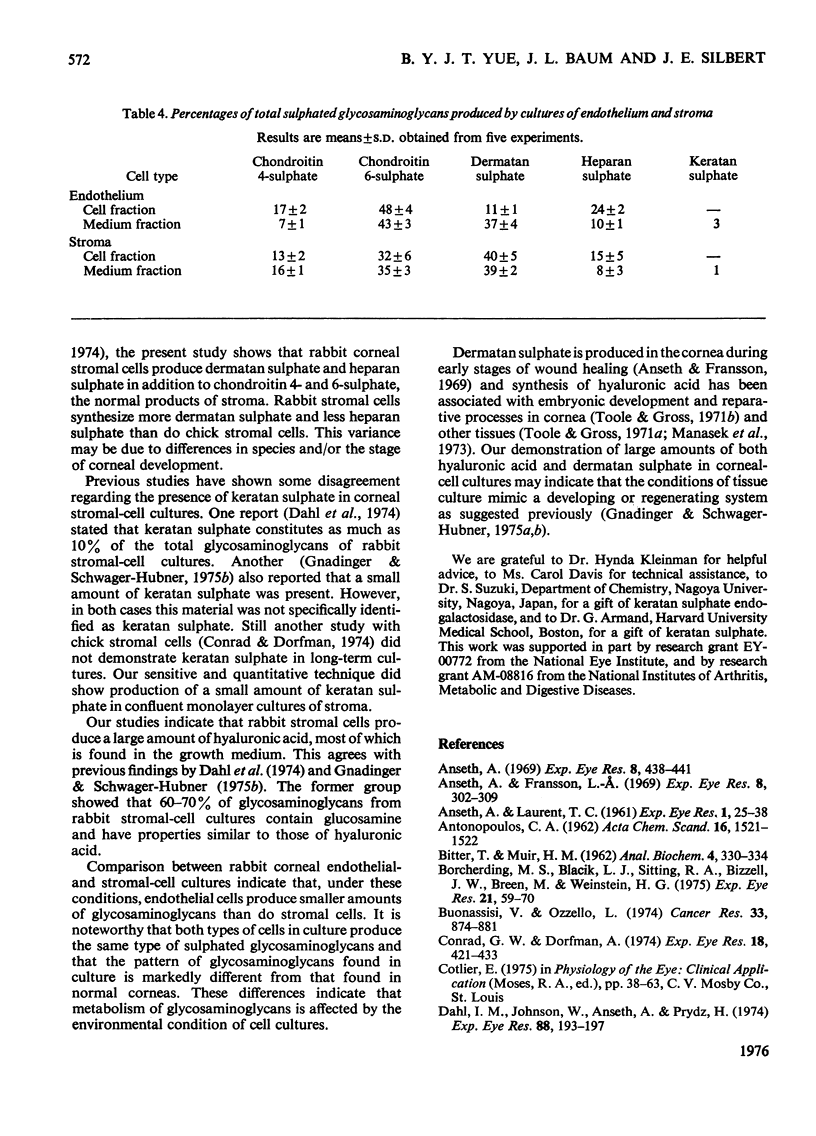
Abstract
Confluent monolayer cultures of rabbit corneal endothelial and stromal cells were incubated independently with [35S]sulphate and [3H]glucosamine for 3 days. AFter incubation, labelled glycosaminoglycans were isolated from the growth medium and from a cellular fraction. These glycosaminoglycans were further characterized by DEAE-cellulose column chromatography and by sequential treatment with various glycosamino-glycan-degrading enzymes. Both endothelial and stromal cultures synthesized hyaluronic acid as the principal product. The cell fraction from the stromal cultures, however, had significantly less hyaluronic acid than that from the endothelial cultures. In addition, both types of cells synthesized a variety of sulphated glycosaminoglycans. The relative amounts of each sulphated glycosaminoglycan in the two cell lines were similar, with chondroitin 4-sulphate, chondroitin 6-sulphate and dermatan sulphate as the major components. Heparan sulphate was present in smaller amounts. Keratan sulphate was also identified, but only in very small amounts (1-3%). The presence of dermatan sulphate and the high content of hyaluronic acid are similar to the pattern of glycosaminoglycans seen in regenerating or developing tissues, including cornea.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANSETH A., LAURENT T. C. Studies on corneal polysaccharides. I. Separation. Exp Eye Res. 1961 Sep;1:25–38. doi: 10.1016/s0014-4835(61)80005-5. [DOI] [PubMed] [Google Scholar]
- Anseth A., Fransson L. A. Studies on corneal polysaccharides. VI. Isolation of dermatan sulfate from corneal scar tissue. Exp Eye Res. 1969 Jul;8(3):302–309. doi: 10.1016/s0014-4835(69)80043-6. [DOI] [PubMed] [Google Scholar]
- Anseth A. Studies on corneal polysaccharides. 8. Changes in the glycosaminoglycans in some human corneal disorders. Exp Eye Res. 1969 Oct;8(4):438–441. doi: 10.1016/s0014-4835(69)80010-2. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Borcherding M. S., Blacik L. J., Sittig R. A., Bizzell J. W., Breen M., Weinstein H. G. Proteoglycans and collagen fibre organization in human corneoscleral tissue. Exp Eye Res. 1975 Jul;21(1):59–70. doi: 10.1016/0014-4835(75)90057-3. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Ozzello L. Sulfated mucopolysaccharide production by synovial sarcoma cells in vivo and in tissue culture. Cancer Res. 1973 Apr;33(4):874–881. [PubMed] [Google Scholar]
- Conrad G. W., Dorfman A. Synthesis of sulfated mucopolysaccharides by chick corneal fibroblasts in vitro. Exp Eye Res. 1974 May;18(5):421–433. doi: 10.1016/0014-4835(74)90079-7. [DOI] [PubMed] [Google Scholar]
- Dahl I. M., Johnsen W., Anseth A., Prydz H. The synthesis of glycosaminoglycans by corneal stroma cells in culture. Exp Cell Res. 1974 Sep;88(1):193–197. doi: 10.1016/0014-4827(74)90634-x. [DOI] [PubMed] [Google Scholar]
- Gnädinger M. C., Schwager-Hübner M. E. Biosynthesis of glycosaminoglycans by mammalian corneal epithelium and fibroblasts in vitro. I. Isolation and fractionation-differences of GAG from the two cell types. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975 Aug 4;196(1):9–19. doi: 10.1007/BF00410022. [DOI] [PubMed] [Google Scholar]
- Gnädinger M. C., Schwager-Hübner M. E. Biosynthesis of glycosaminoglycans by mammalian corneal epithelium and fibroblasts in vitro. II. Approach to specify the GAG from the two cell types. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975 Aug 4;196(1):21–30. doi: 10.1007/BF00410023. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Phelps C. F. The biosynthesis in vitro of keratan sulphate in bovine cornea. Biochem J. 1972 Jun;128(2):205–213. doi: 10.1042/bj1280205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Silbert J. E., Silbert C. K. Heparan sulfate of skin fibroblasts grown in culture. Connect Tissue Res. 1975;4(1):17–23. doi: 10.3109/03008207509152193. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. I. Membrane-associated and cell-sap species in Chinese hamster cells. Biochemistry. 1971 Apr 13;10(8):1437–1445. doi: 10.1021/bi00784a026. [DOI] [PubMed] [Google Scholar]
- Lewis R. G., Spencer A. F., Silbert J. E. Biosynthesis of glycosoaminoglycans by microsomal preparations from cultured mastocytoma cells. Biochem J. 1973 Jun;134(2):465–471. doi: 10.1042/bj1340465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER K., LINKER A., DAVIDSON E. A., WEISSMANN B. The mucopolysaccharides of bovine cornea. J Biol Chem. 1953 Dec;205(2):611–616. [PubMed] [Google Scholar]
- Manasek F. J., Reid M., Vinson W., Seyer J., Johnson R. Glycosaminoglycan synthesis by the early embryonic chick heart. Dev Biol. 1973 Dec;35(2):332–348. doi: 10.1016/0012-1606(73)90028-6. [DOI] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Synthesis of sulfated glycosaminoglycans by embryonic corneal epithelium. Dev Biol. 1973 Dec;35(2):318–331. doi: 10.1016/0012-1606(73)90027-4. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Suzuki S. Purification of Keratan Sulfate-endogalactosidase and its action on keratan sulfates of different origin. J Biol Chem. 1975 Feb 10;250(3):912–917. [PubMed] [Google Scholar]
- Perlman M., Baum J. L., Kaye G. I. Fine structure and collagen synthesis activity of monolayer cultures of rabbit corneal endothelium. J Cell Biol. 1974 Oct;63(1):306–311. doi: 10.1083/jcb.63.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman M., Baum J. L. Synthesis of a collagenous basal membrane by rabbit corneal endothelial cells in vitro. Arch Ophthalmol. 1974 Sep;92(3):238–239. doi: 10.1001/archopht.1974.01010010246015. [DOI] [PubMed] [Google Scholar]
- Perlman M., Baum J. L. The mass culture of rabbit corneal endothelial cells. Arch Ophthalmol. 1974 Sep;92(3):235–237. doi: 10.1001/archopht.1974.01010010243014. [DOI] [PubMed] [Google Scholar]
- STOCKER F. W., EIRING A., GEORGIADE R., GEORGIADE N. A tissue culture technique for growing corneal epithelial, stromal, and endothelial tissues separately. Am J Ophthalmol. 1958 Nov;46(5 Pt 2):294–298. doi: 10.1016/0002-9394(58)90811-0. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P., Gross J. The extracellular matrix of the regenerating newt limb: synthesis and removal of hyaluronate prior to differentiation. Dev Biol. 1971 May;25(1):57–77. doi: 10.1016/0012-1606(71)90019-4. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Trelstad R. L. Hyaluronate production and removal during corneal development in the chick. Dev Biol. 1971 Sep;26(1):28–35. doi: 10.1016/0012-1606(71)90104-7. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K., Toole B. P. Epithelial collagens and glycosaminoglycans in the embryonic cornea. Macromolecular order and morphogenesis in the basement membrane. J Cell Biol. 1974 Sep;62(3):815–830. doi: 10.1083/jcb.62.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]


