Abstract
Background/Objectives: The prognostic nutritional index (PNI) and Glasgow Prognostic Score (GPS) are associated with patients’ nutritional and immune statuses. One important factor in the pathophysiology of type 2 diabetes mellitus (T2DM) is inflammation. Being present in insulin-target tissues, chronic tissue inflammation has become recognized as a crucial aspect of obesity and type 2 diabetes. This study aimed to compare the PNI and GPS levels of the subjects with T2DM to those of prediabetes (preDM) individuals. Furthermore, the goal was to investigate how these inflammatory markers relate to different types of obesity and whether the combination of PNI, GPS, and obesity-related indices was associated with any particular prognostic variables. Methods: In this study, we enrolled one-hundred patients with newly diagnosed T2DM and one-hundred patients with preDM. Results: Four findings emerged from this observational study. As a first observation, 28% of patients with preDM and 15% of patients with T2DM had a normal weight, while up to 43% of patients with preDM and 60% of patients with T2DM were obese. The second important observation was that the PNI of the T2DM patients was significantly lower than the PNI of the patients with preDM (p < 0.0001). The PNI showed that patients with T2DM had a moderate-to-severe malnutrition status (median value of 38.00). Patients with preDM had a mild-to-moderate malnutrition status (median value of 61.00) at diagnosis. Third, observed in the current study, preDM patients with PNI < 61.00 and T2DM patients with a PNI < 38.00 were associated with significantly higher median values of the waist-to-height ratio (WHtR) (p = 0.041, and p = 0.034, respectively) and body mass index (BMI) (p = 0.016, and p = 0.041, respectively). Fourth, this study also revealed, in the T2DM group, a moderate and statistically significant negative correlation between PNI and weight (rho = −0.322, p = 0.035), waist circumference (WC) (rho = −0.308, p = 0.042), hip circumference (HC) (rho = −0.338, p = 0.039), WHtR (rho = −0.341, p = 0.022), body adiposity index (BAI) (rho = −0.312, p = 0.032), and fasting plasma glucose (FPG) (rho = −0.318, p = 0.029). Additionally, the PNI values expressed a weak negative correlation with BMI (rho = −0.279, p = 0.015), and glycated hemoglobin A1c (HbA1c) (rho = −0.245, p = 0.025). The PNI levels exhibited a single positive correlation, weak but statistically significant, with estimated glomerular filtration rate (eGFR-CKD-EPI) values (rho = 0.263, p = 0.018). Conclusions: The findings of this study regarding the correlations between PNI, GPS, and different obesity-related indices in people with diabetes or prediabetes suggest that these indices, which assess nutritional and inflammatory status, can be used as independent predictor factors associated with the four pillars of DM management (glucose, blood pressure, lipids, and weight control) recommended by the American Diabetes Association (ADA).
Keywords: prognostic nutritional index, Glasgow Prognostic Score, body mass index, waist to hip ratio, waist to height ratio, body adiposity index
1. Introduction
Diabetes is one of the twenty-first century’s worldwide health emergencies with the quickest growth rate, according to the findings of the 10th edition of the International Diabetes Federation (IDF) Diabetes Atlas [1]. Forecasts indicate that 537 million people worldwide had diabetes in 2021, which will rise to 643 million by 2030, and reach 783 million by 2045. Furthermore, it is projected that 541 million individuals will have impaired glucose tolerance by 2021 [2].
Prediabetes (preDM) is a condition preceding diabetes wherein blood glucose is higher than normal yet below the diabetes limit [3,4]. It is typically defined as an intermediate state of hyperglycemic concentration in the blood with a high potential to progress to type 2 diabetes mellitus (T2DM) [4,5].
According to the American Diabetes Association (ADA), prediabetes is diagnosed when the fasting plasma glucose (FPG) is between 100 and 125 mg/dL or glycated hemoglobin A1c (HbA1c) levels are between 5.7 and 6.4% [3,4,5]. The progression from prediabetes to diabetes can occur slowly over the years; once the disease is established, it will be irreversible [6,7]. Therefore, awareness of the condition and immediate intervention can be indispensable to prevent or at least delay the onset of T2DM [8].
Around 5–10% of all prediabetic subjects develop T2DM yearly in the United States [9,10]. Some studies showed a conversion to diabetes can occur within five years if prediabetes is left untreated [11].
Studies have confirmed a strong association between obesity and prediabetes [12,13]. Indeed, central (visceral) obesity is strongly linked to developing T2DM [13]. Other risk factors include physical inactivity, hypertension, dyslipidemia (high triglycerides or low high-density lipoprotein, cholesterol), family history of diabetes, gestational diabetes, and smoking [12,13,14].
Adults with body mass index (BMI) ≥ 25 kg/m2 and additional risk factors should be screened for prediabetes. If there are no risk factors, screening should not occur later than age 45. FPG, two-hour plasma glucose (2 h PG) after a 75 g oral glucose tolerance test, and HbA1c are all validated tests to diagnose prediabetes [12]. Previously, studies [15,16,17] have frequently employed BMI to measure weight, but it was unable to differentiate between patients who were abdominally or generally obese. Further research is needed to determine whether the waist-to-hip ratio (WHR) waist-to-height ratio (WHtR), and body adiposity index (BAI) are related to immunological and nutritional status.
Studies to explore the relationship between obesity and the nutritional and immunological status among Romanian people are also limited. Therefore, this retrospective study assessed the association of obesity-related indices with immunological and nutritional factors, such as the prognostic nutritional index (PNI) and Glasgow Prognostic Score (GPS), among T2DM and preDM patients using the data of two university clinical hospitals representative for Dolj County, Romania. We also wanted to identify the possible correlation between them.
PNI is evaluated by the lymphocyte (LYM) count of the peripheral blood and serum albumin (ALB), which is a biomarker that integrates nutritional status, immune state, and inflammation condition. It was originally founded by Japanese scholars Onodera et al. [18,19,20,21] in 1984 to evaluate the nutritional and immunological condition of cancer patients having gastrointestinal surgery. In recent years, PNI has increasingly emerged as a novel prognostic indicator for various disorders, including heart failure [22,23,24,25] and the coronavirus disease 2019 (COVID-19) [26]. Diabetes and obesity have recently been identified as risk factors for severe COVID-19 disease [27,28]. The PNI has been identified as a predictor of mortality in older patients with chronic kidney disease (CKD) [29]. The correlation between PNI and the prognosis of diabetic nephropathy (DN) in patients with T2DM remains ambiguous. Furthermore, a notable correlation existed between serum ALB levels and the severity of retinopathy in T2DM [30].
2. Materials and Methods
Over six months, we carried out an epidemiological, non-interventional, and cross-sectional study. In this study, one-hundred-eighty-five consecutive patients with newly diagnosed T2DM were enrolled, while one-hundred patients with preDM who matched the inclusion criteria in terms of age, gender ratio, and urban/rural location made up the control group. This study was conducted by the Declaration of Helsinki, and approved by the Ethics Committee of the Filantropia Municipal Clinical Hospital (no. 886/15 January 2024) and Emergency County Clinical Hospital of Craiova (no. 2371/14 January 2022), Dolj, Romania.
2.1. Patient Selection
The following conditions had to be met to be included in the study: individuals with type 2 diabetes who were older than eighteen years were chosen from the Outpatient Diabetes, Nutrition, and Metabolic Diseases Departments of the Filantropia Municipal Clinical Hospital and the Emergency County Clinical Hospital of Craiova. All the participants were voluntarily included in the study, after signing the informed consent.
Patients with chronic microvascular complications of T2DM at diagnosis, which include diabetic peripheral polyneuropathy, diabetic kidney disease, and diabetic retinopathy were excluded from the study. Diabetic retinopathy (DR) was diagnosed following a dilated fundus examination [31]. As advised by the American Diabetes Association (ADA), diabetic peripheral neuropathy was evaluated using a combination of temperature sensation (for small fiber function) and vibration sensation (for large fiber function) tests, as well as the presence of characteristic symptoms (pain, dysesthesias, numbness) [31]. Guidelines from Kidney Disease: Improving Global Outcomes (KDIGO) were used to assess the existence of CKD [32].
Patients under the age of 18, pregnant women, those who had experienced an acute infection or inflammatory disease in the last month, those with a chronic infection or inflammatory disease, and cancer patients were excluded from the study.
2.2. Assessment of Diabetes and Prediabetes
One of the following criteria can be used to define prediabetes: (1) a diagnosis made by a medical professional; (2) a hemoglobin A1c (HbA1c) level greater than 5.7% and less than 6.5%; (3) a fasting plasma glucose (FPG) level between 5.6 mmol/L and 7.0 mmol/L; or (4) a 2 h FPG value during an oral glucose tolerance test (OGTT) between 7.8 mmol/L and 11.0 mmol/L [33]. Patients who presented with obesity (especially abdominal or visceral obesity), dyslipidemia with elevated triglycerides and/or low HDL cholesterol, and hypertension, and met the criteria mentioned above, were included in the preDM group.
A diagnosis of diabetes is made if one or more of the following conditions are satisfied: a medical diagnosis that has been verified by the patient’s healthcare providers; an HbA1c level that is greater than 6.5%; an FPG level of 7.0 mmol/L or higher; a random blood glucose level of 11.1 mmol/L or higher; a two-hour blood glucose level that is greater than 11.1 mmol/L after an OGTT; or random glucose value accompanied by classic hyperglycemic symptoms (e.g., polyuria, polydipsia, and unexplained weight loss) or hyperglycemic crises [33].
One-hundred out of one-hundred-eighty-five patients T2DM patients completed the study and were included in the final analysis, while eighty-five were lost to follow-up for the following reasons: diabetic peripheral polyneuropathy (n = 30), diabetic kidney disease (n = 25), diabetic retinopathy (n = 20), unwillingness to continue (n = 5), and relocation (n = 5).
2.3. Medical History, Biometric Parameter Assessment, and Demographic Data
Data on anthropometric measures, medical variables, laboratory test results, and demographic and lifestyle details were all intended to be gathered through an interview questionnaire.
Age, sex, household income each month, and educational attainment were among the demographic factors. Factors related to lifestyle and health included the presence of a smoking history or drinking history; a family history of hypertension, diabetes mellitus, and cardiovascular diseases; and the amount of time spent engaging in intentional moderate physical activity each week.
2.4. Different Obesity-Related Indices (BMI, WHR, WHtR, and BAI) Assessment
We determined the body mass index (BMI) using the participant’s height and weight measurements. The calculation is BMI = weight (kilograms)/height2 (meters). The patient’s nutritional status was evaluated according to BMI, using the WHO criteria [28]. BMI was categorized into normal weight (18.5–22.9 kg/m2), overweight (23.0–25.0 kg/m2), and obese (>25.0 kg/m2), according to the WHO. We measured the weight with a weight scale. Using a measuring stick on the weight scale, the height was determined.
The hip circumference (HC) over the femoral trochanters and the waist circumference (WC) at the halfway between the upper iliac crest and the lower border of the rib cage were measured. The waist-to-hip ratio (WHR), which is computed using the formula WC (cm)/HC (cm), was another tool used to measure abdominal obesity. Visceral adiposity was also evaluated using the waist-to-height ratio (WHtR), which was computed using the formula WC (cm)/height (cm). The body adiposity index (BAI) was calculated: BAI = ((hip circumference)/((height)1.5) − 18) [34]. Due to the lack of standard categories, we classified WHR, WHtR, and BAI into quarters.
2.5. Laboratory Investigations
After collecting anthropometric data, we brought the subjects to the laboratory for further investigation.
Laboratory data, blood urea nitrogen (BUN), creatinine (CREA), uric acid (UA), fasting plasma glucose (FPG), two-hour plasma glucose after a 75 g oral glucose tolerance test (2hPG), glycosylated hemoglobin A1c (HbA1c), total cholesterol (TC), total triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), C-reactive protein (CRP), and albumin (ALB) were determined using the chemiluminescence immunological technique and an automatic immunoassay analyzer (Cobas e411, Roche Diagnostics GmbH, Mannheim, Germany).
Using flow cytometry and Coulter’s principle, we were able to obtain an extended leukocyte formula of 5 diff (Ruby Cell-Dyne, Abbott, Abbott Park, IL, USA) and determine the hemoleucogram markers: hemoglobin (Hb), white blood cells/leukocytes (WBC), neutrophils (NEU), lymphocytes (LYM), monocytes (MON), platelets (PLT), and hemoglobin (Hb).
Measurements of serum creatinine were made, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [35], and the Modification of Diet in Renal Disease Study (MDRD-Study) [36] were used to determine the estimated glomerular filtration rate (eGFR).
2.6. Prognostic Nutritional Index and Glasgow Prognostic Score Calculations
Based on the absolute lymphocyte count and serum ALB level, the prognosis nutritional index (PNI) is calculated. The PNI was calculated according to the acknowledged formula: 10 × serum albumin (g/dL) + 0.5% × total lymphocyte number (per mm3) [37]. Interpretation: PNI value ≥ 50—Normal, PNI value < 50—Mild malnutrition, PNI value < 45—Moderate-to-severe malnutrition, PNI value < 40—Serious malnutrition.
CRP and ALB levels were used to calculate the Glasgow Prognostic Score (GPS); patients with CRP ≤ 10 mg/L and ALB ≥ 35 g/L were allocated to the GPS0 group. Patients with only CRP > 10 mg/L were assigned to the GPS1 group. Patients who had both CRP > 10 mg/L and ALB < 35 g/L [38] were assigned to the GPS2 group.
Provided that 61.00 was the median value among the 100 preDM patients, and 38.00 for the 100 T2DM patients, respectively, we used the median of PNI scores as classified criteria and patients were divided into two groups: low PNI (<61.00, and <38.00, respectively) group and high PNI (≥61.00, and ≥38.00, respectively) group.
2.7. Statistical Analysis
Using Microsoft Excel, we processed and handled patient data from medical records. To analyze the data, we utilized GraphPad Prism 10.3.1 Version (GraphPad Software, LLC, San Diego, CA, USA). The D’Agostino and Pearson normality tests were used to determine whether the data were normal.
The following variables’ means are shown alongside their standard deviations (SD): Hb, WBC, NEU, LYM, MON, PLT, ALB, CRP, ESR, BUN, Crea, UA, FPG, 2hPG, HbA1c, TC, TG, LDL-C, HDL-C, and BUN all had normal distributions. It was demonstrated that the distributions of height, weight, WC, HC, WHR, WHtR, and BAI were non-normal, and the data are displayed as the median with interquartile range. The category values are stated as percentages.
Continuous variables were evaluated using the one-way ANOVA or the Kruskal–Wallis test (used for non-Gaussian distributions) to find the difference between groups and the χ2 test was used for categorical variables.
Spearman’s coefficients (−1 < rho < 1) were used to see if there were any significant correlations between the levels of BMI, height, weight, WC, HC, WHR, WHtR, BAI, ALB, CRP, WBC, NEU, LYM, MON, PLT, PNI, and ESR.
3. Results
3.1. Clinical and Demographic Features of the Patients with Prediabetes and Diabetes
In this study, we included 100 patients diagnosed with T2DM, aged between 31 and 75 years, with a mean ± standard deviation (SD) age of 54.11 ± 6.33, consisting of 43 women and 57 men. In the control group, the preDM group, we found that the mean ± SD age was 50.08 ± 7.46, and women predominated with a percentage of 56. Thus, a statistically significant difference was observed regarding age (p = 0.003), but not in the case of gender (χ2(1) = 3.38, p = 0.066). In terms of where the patients lived, we saw that most of the people in both the T2DM and preDM groups (65 and 61 patients, respectively) were from rural areas. There was no statistically significant difference between the two groups (χ2(1) = 0.34, p = 0.560).
Analyzing the lifestyle factors of smoking and drinking histories, we identified that more patients with T2DM are smokers and alcohol consumers, with the differences being statistically significant (p = 0.007 and, respectively, p < 0.0001).
Personal history was another important difference between the T2DM and the preDM group based on statistics; diseases such as having hypertension, dyslipidemia, or hepatosteatosis were found in more than 55% of patients. In the same context, systolic blood pressure (SBP), and diastolic blood pressure (DBP) had statistically significantly higher mean values in the T2DM group.
Regarding the measured anthropometric parameters (height, weight, waist circumference (WC), and hip circumference (HC)) and different obesity-related indices (WHR, WHtR, BAI, and BMI), there were statistically significant differences between the two groups for the mean values of height (p = 0.009) and the medians for WHR (p = 0.018), while the mean values of weight and BMI reached the significance limit (p = 0.059 and p = 0.055, respectively).
The analysis of laboratory parameters highlighted that both the parameters determined for diagnosing T2DM and those of the lipid profile showed statistically significant higher mean values compared to the means of the preDM group, as can be seen in Table 1.
Table 1.
Clinical and demographic features of the patients with prediabetes and diabetes.
| Characteristics | preDM Group (n = 100) |
T2DM Group (n = 100) |
p-Value from Pearson’s Chi-Squared/ Student’s t-Test |
|---|---|---|---|
| Demographic characteristics | |||
| Age (yrs) (mean ± SD) | 50.08 ± 7.46 | 54.11 ± 6.33 | 0.003 * |
| Gender, Female/Male (n) | 56/44 | 43/57 | 0.066 |
| Area of residence, Rural/Urban (n) | 61/39 | 65/35 | 0.560 |
| Medical history and clinical condition | |||
| Smoking history, No/Yes (n) | 57/43 | 38/62 | 0.007 * |
| Drinking history, No/Yes (n) | 72 | 44/56 | <0.0001 * |
| Education, No/Yes (n) | 63/37 | 75/25 | 0.034 * |
| Hypertension, n (%) | 47 (47%) | 78 (78%) | <0.0001 * |
| Dyslipidemia, n (%) | 43 (48%) | 68 (68%) | 0.0004 * |
| Hepatosteatosis, n (%) | 34 (34%) | 55 (55%) | 0.003 * |
| SBP (mmHg) (mean ± SD) | 132.20 ± 16.70 | 137.40 ± 15.67 | 0.018 * |
| DBP (mmHg) (mean ± SD) | 77.81 ± 12.11 | 83.86 ± 10.65 | 0.0006 * |
| Height (cm) (mean ± SD) | 1.64 ± 0.15 | 1.69 ± 0.11 | 0.009 * |
| Weight (kg) [median (range)] |
83.00 (56.00–140.50) |
89.00 (17.00–122.00) |
0.059 ** |
| WC (cm) [median (range)] |
100.00 (58.00–171.00) |
105.00 (80.00–143.00) |
0.209 |
| HC (cm) [median (range)] |
106.00 (65.00–195.00) |
106.50 (76.00–146.00) |
0.263 |
| WHR [median (range)] |
0.94 (0.59–1.68) |
0.98 (0.86–1.18) |
0.018 * |
| WHtR [median (range)] |
61.02 (33.92–95.00) |
61.70 (44.20–87.50) |
0.715 |
| BMI (kg/m2) (mean ± SD) | 28.18 ± 6.09 | 32.01 ± 5.24 | 0.055 ** |
| BMI category (n) | |||
| Normal (18.5–24.9 kg/m2) | 28 | 15 | 0.031 * |
| Overweight (25–29.9 kg/m2) | 29 | 25 | 0.256 |
| Obese (≥30 kg/m2) | 43 | 60 | 0.010 * |
| BAI [median (range)] |
30.95 (10.08–67.70) |
29.93 (16.29–62.27) |
0.197 |
| Laboratory examination | |||
| FPG (mmol/L) (mean ± SD) | 108.50 ± 6.12 | 210.20 ± 27.83 | <0.0001 * |
| 2hPG (mmol/L) (mean ± SD) | 167.20 ± 14.14 | 332.30 ± 40.23 | <0.0001 * |
| HbA1c (%) (mean ± SD) | 5.82 ± 0.52 | 10.17 ± 1.98 | <0.0001 * |
| TC (mmol/L) (mean ± SD) | 183.30 ± 47.33 | 220.20 ± 54.43 | <0.0001 * |
| TG (mmol/L) (mean ± SD) | 125.50 ± 65.81 | 188.00 ± 108.60 | <0.0001 * |
| LDL-C (mmol/L) (mean ± SD) | 103.70 ± 42.13 | 136.50 ± 48.70 | <0.0001 * |
| HDL-C (mmol/L) (mean ± SD) | 53.64 ± 12.78 | 43.28 ± 13.38 | <0.0001 * |
| eGFR (mL/min/1.73 m2) | |||
| CKD-EPI [median (range)] |
87.50 (38.00–117.00) |
90.50 (29.00–129.00) |
0.993 |
| MDRD-STUDY [median (range)] |
83.50 (38.00–147.00) |
89.50 (29.00–147.00) |
0 |
| BUN (mg/dL) (mean ± SD) | 41.62 ± 17.07 | 40.79 ± 14.81 | 0.718 |
| Crea (mg/dL) (mean ± SD) | 0.87 ± 0.37 | 0.91 ± 0.24 | 0.454 |
| UA (mg/dL) (mean ± SD) | 5.61 ± 1.46 | 4.98 ± 1.61 | 0.006 * |
| Hb (g/dL) (mean ± SD) | 13.50 ± 1.86 | 14.55 ± 1.73 | 0.0002 * |
| WBC (×103/μL) (mean ± SD) | 7.92 ± 1.93 | 8.68 ± 2.83 | 0.026 * |
| NEU (×103/μL) (mean ± SD) | 4.87 ± 1.47 | 5.29 ± 3.02 | 0.211 |
| LYM (×103/μL) (mean ± SD) | 2.27 ± 0.71 | 2.67 ± 0.89 | 0.0001 * |
| MON (×103/μL) (mean ± SD) | 0.53 ± 0.16 | 0.55 ± 0.18 | 0.298 |
| PLT (×103/μL) (mean ± SD) | 248.30 ± 75.02 | 233.10 ± 71.48 | 0.177 |
| Malnutrition | |||
| ALB (g/dL) (mean ± SD) | 6.19 ± 0.37 | 3.89 ± 0.78 | <0.0001 * |
| CRP (mg/dL) (mean ± SD) | 27.46 ± 19.65 | 47.91 ± 36.27 | <0.0001 * |
| ESR (mm/1st h) (mean ± SD) | 35.01 ± 25.42 | 38.43 ± 16.99 | 0.146 |
| PNI | 61.87 ± 3.67 | 38.87 ± 7.80 | <0.0001 * |
| GPS, n | |||
| 0 | 21 | - | - |
| 1 | 79 | 61 | - |
| 2 | - | 39 | - |
SBP: systolic blood pressure; DBP: diastolic blood pressure; WC: waist circumference; HC: hip circumference; WHR: waist-to-hip ratio; WHtR: waist-to-height ratio; BMI: body mass index; BAI: body adiposity index; FPG: fasting plasma glucose; 2hPG: two-hour plasma glucose after a 75 g oral glucose tolerance test; HbA1c: glycosylated hemoglobin A1c; TC: total cholesterol; TG: total triglycerides; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; e-GFR: estimated glomerular filtration rate; CKD-EPI: chronic kidney disease epidemiology collaboration; MDRD-Study: modification of diet in renal disease study; BUN: blood urea nitrogen; CREA: creatinine; UA: uric acid; Hb: hemoglobin; WBC: white blood cells/leukocytes; NEU: neutrophils; LYM: lymphocytes; MON: monocytes; PLT: platelets; ALB: albumin; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; PNI, prognostic nutritional index; GPS: Glasgow Prognostic Score; SD: standard deviation; * p < 0.05: statistically significant; **: reached the significance limit.
We looked at nutritional status and systemic inflammation in our study. We discovered that levels of the CRP (47.91 ± 36.27 vs. 27.46 ± 19.65, p < 0.0001), ALB (3.89 ± 0.78 vs. 6.19 ± 0.37, p < 0.0001), white blood cells (WBC) (8.68 ± 2.83 vs. 7.92 ± 1.93, p = 0.026), and LYM (2.67 ± 0.89 vs. 2.27 ± 0.71, p < 0.0001) were significantly different between the two groups (T2DM vs. preDM). PNI determined by the number of LYM in peripheral blood and serum ALB showed statistically significantly different values between the preDM and T2DM group, as well as the fact that patients with T2DM had a moderate-to-severe malnutrition status at diagnosis. Based on the GPS value, which reflects both the inflammatory and nutritional status using ALB and CRP levels, we found that 39% of T2DM patients had moderate-to-severe malnutrition status. This was in contrast to 79% of preDM patients who had mild-to-moderate malnutrition status.
3.2. Comparing the PNI and GPS Groups’ Clinical Features Between the preDM and T2DM Groups
Utilizing PNI cut-off values of 61.0 and 38.0, we categorized both groups into two subgroups: low PNI (<61.00 and <38.00, respectively) and high PNI (≥61.00 and ≥38.00, respectively) (Table 2). Additionally, the GPS data were categorized into two subgroups: GPS1 as subgroup 1, and GPS2 as subgroup 2, in the T2DM group, and GPS0 as subgroup 1, and GPS1 as subgroup 2, in the preDM, respectively.
Table 2.
Comparing the PNI and GPS groups’ clinical features between the preDM and T2DM groups.
| Characteristics | preDM Group (n = 100) |
T2DM Group (n = 100) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNI | GPS | PNI | GPS | |||||||||||
| All Patients |
PNI < 61 | PNI ≥ 61 | p-Value | 1 | 2 | p-Value | All Patients |
PNI < 38 | PNI ≥ 38 | p-Value | 1 | 2 | p-Value | |
| Patients (n) | 100 | 33 | 67 | 21 | 79 | 100 | 44 | 56 | 61 | 39 | ||||
| Demographic characteristics | ||||||||||||||
| Age (yrs) (mean ± SD) |
50.08 ± 7.46 | 48.58 ± 7.46 | 50.82 ± 7.41 | 0.065 | 50.67 ± 8.24 | 49.92 ± 7.29 | 0.724 | 54.11 ± 6.33 | 52.91 ± 6.36 | 53.27 ± 6.67 | 0.814 | 53.21 ± 6.55 | 52.95 ± 6.05 | 0.619 |
| Gender, Female/Male (n) | 56/44 | 21/12 | 35/32 | 0.279 | 10//11 | 46/33 | 0.383 | 43/57 | 16/28 | 27/29 | 0.235 | 29/32 | 14/25 | 0.251 |
| Area of residence, Rural/Urban (n) | 61/39 | 20/13 | 41/26 | 0.862 | 12/9 | 49/30 | 0.680 | 65/35 | 25/19 | 40/16 | 0.128 | 42/19 | 23/16 | 0.312 |
| Medical history and clinical condition | ||||||||||||||
| Smoking history, Yes (n) | 43 | 23 | 20 | 0.0001 * | 13 | 30 | 0.049 * | 62 | 35 | 27 | 0.001 * | 23 | 39 | <0.0001 * |
| Drinking history, Yes (n) | 28 | 18 | 10 | <0.0001 * | 8 | 20 | 0.247 | 56 | 32 | 24 | 0.003 * | 20 | 36 | <0.0001 * |
| Education, No (n) | 63 | 33 | 30 | <0.0001 * | 11 | 52 | 0.256 | 75 | 38 | 37 | 0.020 * | 43 | 32 | 0.192 |
| Hypertension, Yes (n) | 47 | 29 | 18 | <0.0001 * | 5 | 42 | 0.017 * | 78 | 24 | 54 | <0.0001 * | 58 | 20 | <0.0001 * |
| Dyslipidemia, Yes (n) | 43 | 24 | 19 | <0.0001 * | 5 | 38 | 0.046 * | 68 | 24 | 44 | 0.011 * | 48 | 20 | 0.004 * |
| Hepatosteatosis, Yes (n) | 34 | 17 | 17 | 0.009 * | 3 | 31 | 0.032 * | 55 | 19 | 36 | 0.035 * | 40 | 15 | 0.008 * |
| SBP (mmHg) (mean ± SD) |
132.20 ± 16.70 | 130.88 ± 15.07 | 132.79 ± 17.53 | 0.306 | 133.10 ± 16.17 | 131.91 ± 16.94 | 0.891 | 137.40 ± 15.67 | 140.2 ± 15.99 | 135.2 ± 15.19 | 0.038 * | 135.62 ± 14.80 | 140.10 ± 16.77 | 0.082 |
| DBP (mmHg) (mean ± SD) |
77.81 ± 12.11 | 79.09 ± 9.42 | 77.18 ± 13.26 | 0.661 | 78.19 ± 14.80 | 77.71 ± 11.40 | 0.909 | 83.86 ± 10.65 | 85.0 ± 8.86 | 82.96 ± 11.87 | 0.133 | 87.78 ± 11.61 | 85.54 ± 8.83 | 0.130 |
| Height (m) (mean ± SD) |
1.64 ± 0.15 | 1.64 ± 0.09 | 1.68 ± 0.10 | 0.441 | 1.69 ± 0.10 | 1.68 ± 0.08 | 0.664 | 1.69 ± 0.11 | 1.69 ± 0.08 | 1.68 ± 0.10 | 0.825 | 1.69 ± 0.10 | 1.68 ± 0.08 | 0.765 |
| Weight (kg) [median (range)] |
83.0 (56.0–140.5) |
87.0 (56.0–140.5) |
73.0 (56.0–101.6) |
0.038 * | 82.0 (56.0–140.5) |
99.0 (60.0–132.0) |
0.067 | 89.0 (17.0–122.0) |
97.45 (17.0–122.0) |
82.15 (53.0–115.0) |
0.023 * | 80.0 (53.0–115.0) |
97.50 (17.0–122.0) |
0.084 |
| Waist circumference (cm) [median (range)] |
100.0 (58.0–171.0) |
108.0 (58.0–171.0) |
92.0 (69.0–138.0) |
0.032 * | 93.0 (58.0–125.0) |
106.0 (69.0–171.0) |
0.102 | 105.0 (80.0–143.0) |
115.10 (80.0–143.0) |
94.75 (80.0–140.0) |
0.029 * | 101.50 (80.0–140.0) |
109.50 (80.0–143.0) |
0.076 |
| Hip circumference (cm) [median (range)] |
106.0 (65.0–195.0) |
118.0 (75.0–133.0) |
97.0 (65.0–195.0) |
0.022 * | 103.0 (65.0–147.0) |
110.2 (86.0–195.0) |
0.073 | 106.5 (76.0–146.0) |
112.35 (76.0–146.0) |
98.25 (87.0–130.0) |
0.035 * | 99.75 (76.0–130.0) |
113.25 (87.0–146.0) |
0.058 ** |
| WHR [median (range)] |
0.94 (0.59–1.68) |
0.91 (0.59–1.64) |
0.96 (0.64–1.68) |
0.232 | 0.85 (0.59–1.11) |
0.94 (0.77–1.68) |
0.113 | 0.98 (0.86–1.18) |
1.05 (0.86–1.18) |
0.95 (0.88–1.18) |
0.055 ** | 1.08 (0.88–1.18) |
0.97 (0.86–1.18) |
0.101 |
| WHtR [median (range)] |
61.02 (33.92–95.00) |
64.84 (33.92–95.00) |
56.35 (44.92–88.46) |
0.041 * | 58.49 (33.92–74.10) |
61.35 (42.94–95.00) |
0.187 | 61.7 (44.2–87.5) |
67.3 (49.4–87.5) |
56.7 (44.2–87.5) |
0.034 * | 60.50 (49.4–87.5) |
65.15 (44.2–87.5) |
0.069 |
| BMI (kg/m2) (mean ± SD) |
28.18 ± 6.09 | 32.54 ± 6.12 | 26.17 ± 5.79 | 0.016 * | 28.5 ± 5.90 | 30.7 ± 6.81 | 0.082 | 32.01 ± 5.24 | 34.06 ± 3.44 | 29.41 ± 5.58 | 0.041 * | 28.55 ± 3.15 | 35.05 ± 1.90 | 0.035 * |
| BMI category (n) | ||||||||||||||
| Normal (18.5–24.9 kg/m2) | 28 | 12 | 16 | 18 | 10 | 15 | 7 | 8 | 9 | 6 | ||||
| Overweight (25–29.9 kg/m2) | 29 | 12 | 17 | 13 | 16 | 25 | 9 | 16 | 16 | 9 | ||||
| Obese (≥30 kg/m2) | 43 | 9 | 34 | 27 | 16 | 60 | 28 | 32 | 37 | 23 | ||||
| BAI [median (range)] |
30.95 (10.08–67.70) |
33.78 (14.12–66.23) |
30.63 (15.84–44.60) |
0.074 | 36.85 (24.37–66.23) |
30.87 (14.12–48.93) |
0.088 | 29.93 (16.29–62.27) |
35.18 (21.36–62.27) |
28.92 (16.29–46.23) |
0.057 ** | 27.45 (16.29–46.23) |
32.88 (21.36–62.27) |
0.099 |
| Laboratory examination | ||||||||||||||
| FPG (mmol/L) (mean ± SD) |
108.50 ± 6.12 | 109.07 ± 6.22 | 107.45 ± 5.87 | 0.334 | 108.48 ± 4.62 | 108.56 ± 6.49 | 0.883 | 210.20 ± 27.83 | 213.1 ± 25.63 | 208.0 ± 29.48 | 0.049 * | 209.03 ± 29.39 | 212.1 ± 25.45 | 0.081 |
| 2hPG (mmol/L) (mean ± SD) |
167.20 ± 14.14 | 167.97 ± 15.65 | 166.79 ± 13.45 | 0.145 | 166.1 ± 12.02 | 167.47 ± 14.71 | 0.001 * | 332.30 ± 40.23 | 339.2 ± 37.93 | 330.4 ± 42.28 | 0.146 | 330.11 ± 41.60 | 336.13 ± 38.48 | 0.594 |
| HbA1c (%) (mean ± SD) |
5.82 ± 0.52 | 5.86 ± 0.55 | 5.72 ± 0.49 | 0.748 | 5.80 ± 0.45 | 5.84 ± 0.52 | 0.334 | 10.17 ± 1.98 | 11.45 ± 1.19 | 8.81 ± 2.65 | 0.032 * | 8.60 ± 1.62 | 11.61 ± 1.43 | 0.024 * |
| TC (mmol/L) (mean ± SD) |
185.30 ± 56.18 | 188.52 ± 55.22 | 180.66 ± 43.14 | 0.262 | 182.34 ± 45.50 | 186.67 ± 54.74 | 0.979 | 220.20 ± 54.43 | 231.3 ± 43.46 | 218.9 ± 40.83 | 0.015 * | 213.9 ± 43.08 | 229.1 ± 60.92 | 0.041 * |
| TG (mmol/L) (mean ± SD) |
125.50 ± 65.81 | 130.91 ± 87.35 | 122.76 ± 52.68 | 0.958 | 112.0 ± 31.71 | 129.03 ± 71.96 | 0.129 | 188.00 ± 108.60 | 199.4 ± 114.2 | 180.3 ± 102.3 | 0.089 | 181.28 ± 112.12 | 192.9 ± 104.03 | 0.166 |
| LDL-C (mmol/L) (mean ± SD) |
103.70 ± 42.13 | 104.21 ± 40.83 | 102.80 ± 45.30 | 0.394 | 101.66 ± 40.56 | 111.6 ± 47.84 | 0.159 | 136.50 ± 48.70 | 142.2 ± 55.82 | 129.6 ± 38.38 | 0.021 * | 132.08 ± 53.62 | 139.57 ± 40.46 | 0.205 |
| HDL-C (mmol/L) (mean ± SD) |
53.64 ± 12.78 | 50.39 ± 12.18 | 55.24 ± 12.85 | 0.220 | 57.54 ± 12.08 | 52.61 ± 12.83 | 0.243 | 43.28 ± 13.38 | 40.53 ± 14.71 | 47.24 ± 11.56 | 0.496 | 42.40 ± 12.19 | 46.65 ± 12.05 | 0.119 |
| eGFR (mL/min/1.73 m2) | ||||||||||||||
| CKD-EPI [median (range)] |
87.5 (38.0–117.0) |
86.0 (38.0–117.0) |
89.0 (45.0–110.0) |
0.766 | 85.0 (61.0–108.0) |
88.0 (38.0–117.0) |
0.221 | 90.5 (29.0–129.0) |
85.0 (29.0–117.0) |
95.0 (45.0–129.0) |
0.001 * | 95.0 (45.0–129.0) |
85.0 (29.0–117.0) |
0.005 * |
| MDRD-STUDY [median (range)] |
83.5 (38.0–147.0) |
81.0 (55.0–119.0) |
88.0 (38.0–147.0) |
0.693 | 82.0 (38.0–142.0) |
86.0 (58.0–147.0) |
0.805 | 89.5 (29.0–147.0) |
79.0 (29.0–141.0) |
95.5 (45.0–147.0) |
0.026 * | 96.0 (45.0–147.0) |
77.0 (29.0–141.0) |
0.033 * |
| BUN (mg/dL) (mean ± SD) |
41.62 ± 17.07 | 42.21 ± 17.40 | 40.42 ± 16.56 | 0.788 | 41.90 ± 19.7 | 41.78 ± 16.43 | 0.854 | 40.79 ± 14.81 | 37.76 ± 12.94 | 43.18 ± 14.71 | 0.170 | 38.67 ± 13.35 | 42.15 ± 15.63 | 0.130 |
| Crea (mg/dL) (mean ± SD) |
0.87 ± 0.37 | 0.88 ± 0.37 | 0.87 ± 0.38 | 0.947 | 0.94 ± 0.43 | 0.86 ± 0.35 | 0.660 | 0.91 ± 0.24 | 0.87 ± 0.19 | 0.94 ± 0.28 | 0.241 | 0.93 ± 0.27 | 0.88 ± 0.19 | 0.170 |
| UA (mg/dL) (mean ± SD) |
5.61 ± 1.46 | 5.29 ± 1.82 | 4.76 ± 1.36 | 0.453 | 5.23 ± 1.60 | 5.16 ± 1.57 | 0.526 | 4.98 ± 1.61 | 4.76 ± 1.46 | 5.16 ± 1.71 | 0.203 | 5.11 ± 1.70 | 4.78 ± 1.44 | 0.507 |
| Hb (g/dL) (mean ± SD) |
13.50 ± 1.86 | 13.24 ± 1.64 | 13.63 ± 1.96 | 0.615 | 13.89 ± 1.61 | 13.40 ± 1.92 | 0.157 | 14.55 ± 1.73 | 14.72 ± 1.79 | 13.21 ± 2.68 | 0.154 | 14.47 ± 1.68 | 14.67 ± 1.81 | 0.185 |
| WBC (×103/μL) (mean ± SD) |
7.92 ± 1.93 | 8.02 ± 1.93 | 7.32 ± 1.64 | 0.038 * | 7.73 ± 1.95 | 8.08 ± 1.98 | 0.141 | 8.68 ± 2.83 | 9.25 ± 1.56 | 8.12 ± 3.58 | 0.035 * | 8.42 ± 3.50 | 9.13 ± 2.32 | 0.056 ** |
| NEU (×103/μL) (mean ± SD) |
4.87 ± 1.47 | 5.05 ± 1.46 | 4.22 ± 1.37 | 0.018 * | 4.70 ± 1.33 | 4.95 ± 1.54 | 0.488 | 5.29 ± 3.02 | 5.85 ± 2.83 | 4.93 ± 4.19 | 0.026 * | 5.46 ± 2.99 | 5.10 ± 3.07 | 0.141 |
| LYM (×103/μL) (mean ± SD) |
2.27 ± 0.71 | 2.29 ± 0.67 | 2.24 ± 0.79 | 0.915 | 2.26 ± 0.36 | 2.28 ± 0.78 | 0.926 | 2.67 ± 0.89 | 2.55 ± 0.83 | 2.77 ± 0.94 | 0.274 | 2.52 ± 0.83 | 2.77 ± 0.93 | 0.335 |
| MON (×103/μL) (mean ± SD) |
0.53 ± 0.16 | 0.55 ± 0.18 | 0.52 ± 0.15 | 0.220 | 0.52 ± 0.15 | 0.53 ± 0.16 | 0.454 | 0.55 ± 0.18 | 0.55 ± 0.21 | 0.56 ± 0.15 | 0.799 | 0.55 ± 0.16 | 0.56 ± 0.21 | 0.920 |
| PLT (×103/μL) (mean ± SD) |
248.30 ± 75.02 | 264.18 ± 57.98 | 235.41 ± 82.38 | 0.066 | 222.38 ± 66.96 | 259.54 ± 76.86 | 0.130 | 233.10 ± 71.48 | 218.2 ± 78.12 | 254.5 ± 66.48 | 0.085 | 227.12 ± 64.23 | 244.59 ± 82.42 | 0.108 |
| Malnutrition | ||||||||||||||
| ALB (g/dL) (mean ± SD) |
6.19 ± 0.37 | 5.74 ± 0.19 | 6.41 ± 0.18 | <0.0001 * | 6.30 ± 0.30 | 6.16 ± 0.38 | 0.230 | 3.89 ± 0.78 | 3.13 ± 0.34 | 4.49 ± 0.42 | <0.0001 * | 4.41 ± 0.48 | 3.07 ± 0.32 | <0.0001 * |
| CRP (mg/dL) (mean ± SD) |
27.46 ± 19.65 | 29.94 ± 20.44 | 26.24 ± 19.28 | 0.551 | 11.77 ± 10.76 | 31.63 ± 19.25 | 0.0002 * | 47.91 ± 36.27 | 50.65 ± 37.74 | 44.42 ± 34.41 | 0.130 | 45.87 ± 35.92 | 49.21 ± 36.73 | 0.289 |
| ESR (mm/1st h) (mean ± SD) |
35.01 ± 25.42 | 36.31 ± 31.01 | 34.36 ± 22.35 | 0.885 | 34.43 ± 25.96 | 34.70 ± 24.98 | 0.976 | 38.43 ± 16.99 | 41.91 ± 16.70 | 35.05 ± 17.36 | 0.063 | 36.03 ± 18.91 | 42.05 ± 14.33 | 0.092 |
| PNI (mean ± SD) |
61.87 ± 3.67 | 57.44 ± 1.87 | 64.06 ± 1.93 | <0.0001 * | 63.06 ± 3.01 | 61.57 ± 3.78 | 0.231 | 38.87 ± 7.80 | 31.27 ± 3.37 | 44.86 ± 4.21 | <0.0001 * | 44.11 ± 4.76 | 30.70 ± 3.15 | <0.0001 * |
| GPS, n | ||||||||||||||
| 1 | 21 | 3 | 18 | - | - | 61 | 5 | 56 | - | - | ||||
| 2 | 79 | 30 | 49 | - | - | 39 | 39 | 0 | - | - | ||||
SBP: systolic blood pressure; DBP: diastolic blood pressure; WC: waist circumference; HC: hip circumference; WHR: waist-to-hip ratio; WHtR: waist-to-height ratio; BMI: body mass index; BAI: body adiposity index; FPG: fasting plasma glucose; 2hPG: two-hour plasma glucose after a 75 g oral glucose tolerance test; HbA1c: glycosylated hemoglobin A1c; TC: total cholesterol; TG: total triglycerides; LDL-C: low-density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; e-GFR: estimated glomerular filtration rate; CKD-EPI: chronic kidney disease epidemiology collaboration; MDRD-Study: modification of diet in renal disease study; BUN: blood urea nitrogen; CREA: creatinine; UA: uric acid; Hb: hemoglobin; WBC: white blood cells/leukocytes; NEU: neutrophils; LYM: lymphocytes; MON: monocytes; PLT: platelets; ALB: albumin; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; PNI, prognostic nutritional index; GPS: Glasgow Prognostic Score; SD: standard deviation; * p < 0.05: statistically significant; **: reached the significance limit.
The majority of patients (67%) in the preDM group had a PNI ≥ 61.00 and a GPS2 score (79 patients). Additionally, in the T2DM group, just over 50 percent of patients had a PNI ≥ 38.00, but 61% of them recorded a GPS1 score.
There were no differences in age, gender, and area of residence (p ≥ 0.05), both in the preDM subgroups (PNI < 61.00 and PNI ≥ 61.00, and GPS1 and GPS2, respectively) and in the T2DM subgroups (PNI < 38.00 and PNI ≥ 38.00, and GPS1 and GPS2, respectively).
In the preDM group, we saw that PNI values below 61.00, which means a moderate nutritional and inflammatory status, were strongly linked (p < 0.0001) to lifestyle factors like smoking and drinking, education, and the main risk factors that can lead to T2DM, hypertension, dyslipidemia, and hepatosteatosis. Additionally, in the preDM group, over 38% of patients with a GPS2 score presented smoking, hypertension, dyslipidemia, and hepatosteatosis as risk factors. Thus, we can consider that these risk factors can influence and induce a moderate-to-severe nutritional and inflammatory status. This nutritional and inflammatory status is also supported by ALB, which presented significantly lower mean values (p < 0.0001). In the PNI < 61.00 subgroups, the patient’s mean values of WBC (p = 0.038) and neutrophils (NEU) (p = 0.018) were significantly different compared to those in the PNI ≥ 61.00 subgroup.
Analyzing the association between the PNI index and different obesity-related indices, it was observed that patients in the PNI < 61.00 subgroups showed significant changes only for the median values of weight (p = 0.038), WC (p = 0.032), HC (p = 0.022), WHtR (p = 0.041), and BMI (p = 0.016) compared to those in the PNI ≥ 61.00 subgroups.
In the T2D group, we observed that lifestyle factors (smoking and drinking), education, and the main risk factors of T2DM (hypertension, dyslipidemia, hepatosteatosis, and SBP) were present in more than 54.54% of patients with PNI < 38.00, or patients with a GPS2 score.
Anthropometric parameters, such as weight, WC (p = 0.023), HC (p = 0.035), and obesity-related indices, WHtR (p = 0.034) and BMI (p = 0.041), presented significantly higher median values in the PNI < 38.00 subgroups, and the WHR index (p = 0.055) and BAI (p = 0.057), reached the significance limits, compared to those in the PNI ≥ 38.00 subgroup.
Among patients diagnosed with T2DM, those in the PNI < 38.00 subgroups had a moderate-to-severe nutritional and inflammatory status, associating significantly modified WBC, NEU, and ALB mean values comparable to those in the PNI ≥ 38.00 subgroups.
Patients in PNI < 38.00 subgroups had mean values of fasting plasma glucose (FPG) (p = 0.049), HbA1c (p = 0.032), total cholesterol (TC) (p = 0.015), and low-density lipoprotein cholesterol (LDL-C) (p = 0.021) significantly different from those in the PNI ≥ 38.00 subgroups. Also, patients who had a GPS2 score were associated with higher values of HbA1c and TC.
3.3. Associations of PNI with BMI, WHR, WHtR, and BAI in the preDM and T2DM Groups
Table 3 and Figure 1 display how PNI and BMI, WHR, WHtR, and BAI relate to each other in the preDM and T2DM groups. BMI was categorized into normal weight (18.5–22.9 kg/m2), overweight (23.0–25.0 kg/m2), and obese (>25.0 kg/m2), according to the World Health Organization (WHO) [39]. Due to the lack of standard categories, we classified WHR, WHtR, and BAI into quarters.
Table 3.
Associations of PNI with BMI, WHR, WHtR, and BAI in the preDM and T2DM groups.
| Variables (Mean ± SD) |
preDM Group (n = 100) |
T2DM Group (n = 100) |
||
|---|---|---|---|---|
| PNI |
p-Value from Kruskal–Wallis/ One-Way ANOVA |
PNI |
p-Value from Kruskal–Wallis/ One-Way ANOVA |
|
| BMI category (kg/m2) | ||||
| Normal weight | 63.03 ± 3.30 | 0.019 * | 40.44 ± 9.28 | 0.442 |
| Over weight | 61.05 ± 3.96 | 39.87 ± 8.13 | ||
| Obese | 60.88 ± 3.49 | 38.57 ± 7.27 | ||
| WHR | ||||
| Quarter 1 | 62.88 ± 3.37 | 0.059 ** | 39.89 ± 7.40 | 0.782 |
| Quarter 2 | 61.55 ± 4.61 | 39.16 ± 8.44 | ||
| Quarter 3 | 62.64 ± 3.09 | 38.66 ± 7.28 | ||
| Quarter 4 | 60.44 ± 3.06 | 37.80 ± 8.31 | ||
| WHtR | ||||
| Quarter 1 | 61.77 ± 3.30 | 0.845 | 41.71 ± 7.01 | 0.109 |
| Quarter 2 | 61.41 ± 3.49 | 38.59 ± 8.30 | ||
| Quarter 3 | 62.03 ± 4.66 | 37.67 ± 7.98 | ||
| Quarter 4 | 62.31 ± 3.20 | 36.55 ± 7.28 | ||
| BAI | ||||
| Quarter 1 | 60.57 ± 3.48 | 0.053 ** | 40.21 ± 7.58 | 0.695 |
| Quarter 2 | 61.74 ± 4.33 | 38.77 ± 8.75 | ||
| Quarter 3 | 61.95 ± 3.18 | 38.74 ± 7.23 | ||
| Quarter 4 | 63.10 ± 3.23 | 37.79 ± 7.82 | ||
BMI: body mass index; WHR: waist-to-hip ratio; WHtR: waist-to-height ratio; BAI: body adiposity index; * p < 0.05: statistically significant; **: reached the significance limit.
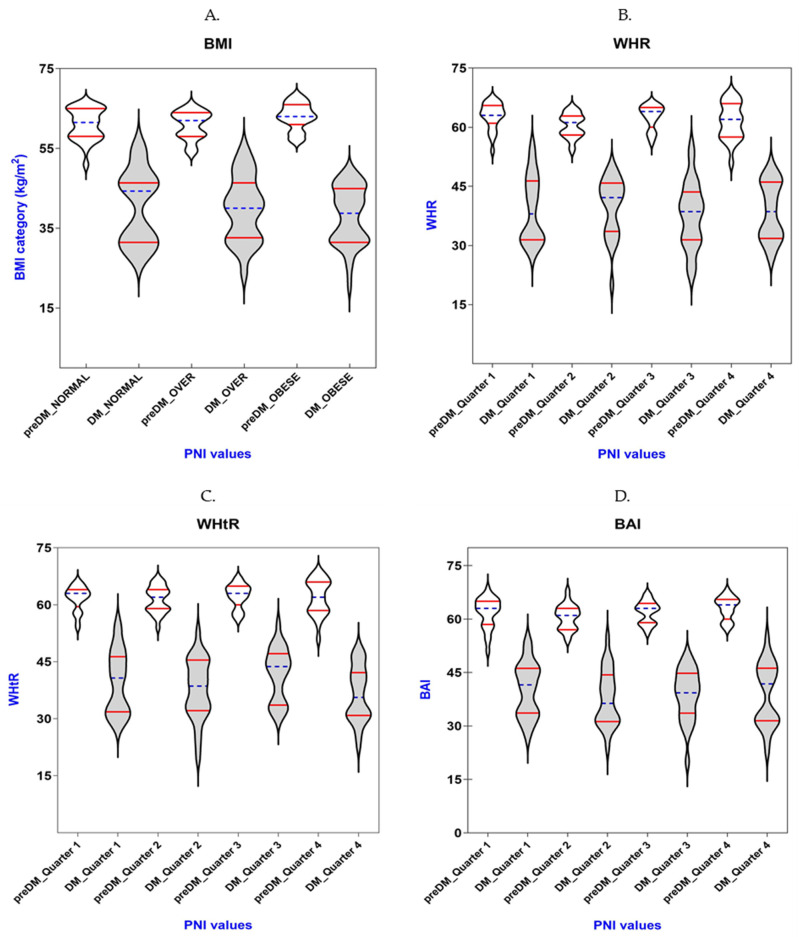
Figure 1.
The PNI levels for patients with prediabetes (white color) or diabetes (gray color) vary in different quarters of the obesity-related indices: (A) BMI; (B) WHR; (C) WHtR; (D) BAI. Violin plot represents values of indices; horizontal blue lines represent median values accompanied by the quartiles represented by horizontal red lines.
Using the one-way ANOVA test, we obtained that preDM patients had statistically significant differences in values between BMI categories (p = 0.019). On the other hand, the Kruskal–Wallis test highlighted that in the case of preDM patients, the differences between the quarters of WHR and BAI reached the significance limits (p = 0.059 and p = 0.053, respectively).
There were no statistically significant differences in values between BMI categories and differences between the quarters of WHR, WHtR, and BAI, respectively, in the T2DM group.
3.4. Correlations Between PNI and the Obesity-Related Indices in the preDM and T2DM Groups
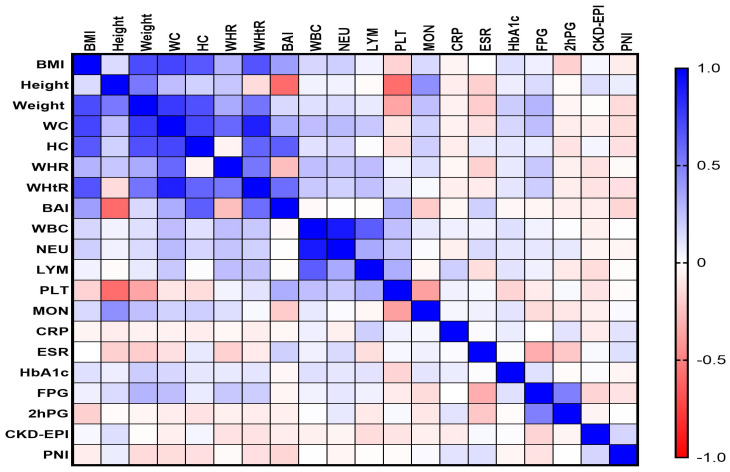
Spearman’s correlation analysis revealed that the values of PNI correlated much better with the obesity-related indices in the T2DM group (Figure 2).
Figure 2.
Correlations matrix between PNI and the obesity-related indices in the T2DM group. The correlation heatmap shows how the measured indicators relate to one another. Strong positive correlations are indicated by bright blue, whereas strong negative correlations are indicated by bright red.
In the T2DM group, our study revealed a moderate and statistically significant negative correlation between PNI and weight (rho = −0.322, p-value = 0.035), WC (rho = −0.308, p-value = 0.042), HC (rho = −0.338, p-value = 0.039), WHtR (rho = −0.341, p-value = 0.022), BAI (rho = −0.312, p-value = 0.032), and FPG (rho = −0.318, p-value = 0.029). Additionally, the PNI values expressed a negative weak correlation with BMI (rho = −0.279, p-value = 0.015) and HbA1c (rho = −0.245, p-value = 0.025). We found a single positive correlation, weak but statistically significant, between PNI and CKD-EPI (rho = 0.263, p-value = 0.018).
4. Discussion
Globally, 26.6 million people are expected to have diabetes in 2025, whereas 579.9 million people will have the disease [40]. The WHO projects that diabetes’s rising prevalence will make it the seventh-largest cause of death globally by 2030 [41]. Furthermore, it is also expected that the number of people with preDM—a condition marked by blood glucose levels that are higher than normal but fall short of the diagnostic cut-off for T2DM—will increase [4].
Forecasts indicate that by 2030, there will be more than 470 million people living with preDM worldwide [42]. Importantly, one should not undervalue preDM. People with preDM are associated with an increased risk of cardiovascular disease and diabetic microangiopathy as compared to people with normal glucose metabolism [43,44].
Insulin resistance and malfunction of the pancreatic beta cells are important markers of DM and preDM. Given the varying effects of these factors on preDM subgroups across diverse racial and ethnic groups, it is imperative to establish prevention initiatives and effective treatment procedures for DM and preDM [45,46]. Remarkably, the proportion of people with preDM greatly exceeds that of people with DM [47]. Therefore, early diagnosis of preDM and DM is essential for effective care and the prevention of the disease’s progression [48,49].
In this study, we enrolled one-hundred patients with newly diagnosed T2DM and one-hundred patients with preDM and analyzed the association of obesity-related indices with the immunological and nutritional factors, PNI, and GPS, and identified the possible correlation between them.
From our observational study, four findings emerged. As a first observation, 28% of patients with preDM and 15% of patients with T2DM had a normal weight, while up to 43% of patients with preDM and 60% of patients with T2DM were obese. The second important observation was that the PNI of the T2DM patients was significantly lower than the PNI of the patients with preDM. The PNI showed that patients with T2DM had a moderate-to-severe malnutrition status (median value of 38.00). Patients with preDM had a mild-to-moderate malnutrition status (median value of 61.00) at diagnosis. Third, we observed that preDM patients with PNI < 61.00 and T2DM patients with PNI < 38.00 were associated with significantly higher median values of WHtR and BMI. Fourth, in the T2DM group, our study revealed a moderate and statistically significant negative correlation between PNI and weight, WC, HC, WHtR, BAI, and FP. Additionally, the PNI values expressed a weak negative correlation with BMI and HbA1c. The PNI levels exhibited a single positive correlation, weak but statistically significant, with CKD-EPI values.
Obesity and type 2 diabetes have a lot of complicated connections and shared pathophysiological pathways. This makes it more likely for obese people to have insulin resistance, dyslipidemia, non-alcoholic fatty liver disease, and many other metabolic problems [50,51]. Due to changes in adipose tissue biology that link obesity with insulin resistance and beta cell dysfunction, an increased BMI and the distribution of fat around the abdomen linearly increase the risk of type 2 diabetes [52]. Obesity leads to chronic, systemic inflammation [53]. Like in endometriosis, the appearance of cellular atypia or malignant transformation can occur under the influence of the proinflammatory microenvironment, particularly in inflammatory cells, which provides a favorable environment for neovascularization and the presence of mutations in tumor suppressor proteins or oncoproteins, with an associated increase in cell proliferation and tumor growth [54].
To lower the frequency of complications, weight control is typically advised in the treatment of diabetes and several systemic disorders [55]. Even when the overweight person with a predominance of abdominal fat does not match the BMI requirements for obesity, abdominal obesity, which is frequently measured by indices such as the WHR, BAI, or visceral adiposity index, is an independent predictor for the development of hypertension and high fasting glucose [52,56]. The BMI is not a reliable indicator of adiposity; De Lorenzo has demonstrated that a more accurate measure could be the anthropometric measurement of body fat percentage [57]. A study published in 2020 in the United States reported that approximately two-thirds of the analyzed adult population was obese or overweight [58].
Referring to the patients included in our study, we observed the same findings: 25% of patients with T2DM were overweight and 60% were obese. The observation can be explained by the fact that Romania has quickly adopted a relatively high socioeconomic status, with an increase in income per capita, circumstances that have led to a higher prevalence of obesity.
Li et al. [34] found that the three obesity-related indices, BMI, WHtR, and BAI, were negatively associated with the development of diabetic retinopathy (DR). According to the authors, there might be a lack of differentiation between general obesity and centripetal obesity using the BMI, and the two may have differential effects on the development of diabetes [57]. Regarding this aspect, Man et al. [59] found a positive correlation between centripetal obesity, indicated by a higher WHR, and the progression of diabetes. In another study, it was shown that WHR should be considered to assess centripetal obesity, while it was established that BAI has a significant linear relationship with body fat percentage [60]. They have also shown that abdominal obesity can be a more critical factor in diabetic retinopathy than generalized obesity.
In our study, we observed patients with T2DM-associated centripetal obesity, indicated by elevated values of WHR and WHtR. Additionally, nearly half of these patients, who had a moderate-to-severe nutritional and inflammatory status, were significantly associated with centripetal obesity. In contrast, only 33% of the preDM group exhibited a moderate-to-severe nutritional and inflammatory status, which was also associated with centripetal obesity.
As far as we know, there have been no population-based studies reporting the association between WHR, WHtR, or BAI measures and PNI index in patients with newly diagnosed T2DM or preDM.
Recent investigations have established the role of PNI as an emerging clinical marker of diagnostic and prognostic significance in patients with diabetes who present microvascular complications [61,62,63,64,65,66].
Aktas et al. discovered that the PNI of the type 2 diabetes mellitus patients was significantly lower than the PNI of the healthy controls [61]. Additionally, they noticed that the PNI of the diabetic subjects with microvascular complications (diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy) was considerably lower than the PNI of the healthy controls and the diabetic patients without microvascular complications.
We obtained similar results in our study with newly diagnosed patients with T2DM. The PNI of the T2DM patients was significantly lower than the PNI of the patients with preDM, and the PNI showed that patients with T2DM had a moderate-to-severe malnutrition status, and patients with preDM had a mild-to-moderate malnutrition status at diagnosis.
Zhang et al. hypothesized that the PNI, which reflects nutrition, immunology, and inflammation—all of which are closely related to diabetic nephropathy (DN)—may be a more reliable predictor of end-stage renal disease (ESRD) in patients with DN than serum albumin, the inflammation index, or the lymphocyte count [62]. The same author found in another study that there was a significant correlation between all participants’ greater PNI and their risk of mortality and prevalence of CKD [63].
Kurtul et al. [64] found an independent and negative correlation between PNI and the presence of diabetic retinopathy (DR). Using PNI to examine a patient’s inflammatory–nutritional balance may help doctors identify T2DM patients who are at heightened risk for developing DR. According to Wei et al. [66], patients with DR also tended to be malnourished compared to non-DR patients, and malnourished patients had a greater incidence of DR than normal nutritional status patients; there was an independent correlation between the intensity and existence of DR and malnutrition. Yang et al. observed a strong correlation between a lower incidence of DR and greater PNI levels in hospitalized T2DM patients. The severity and prevalence of DR were found to be inversely and independently correlated with PNI, indicating that PNI may be utilized to predict the prognosis of DR in clinical practice [65].
Our study also revealed, in the T2DM group, a moderate and statistically significant negative correlation between PNI and weight, WC, HC, WHtR, BAI, and FPG. Additionally, the PNI values expressed a weak negative correlation with BMI and HbA1c. The PNI levels exhibited a single positive correlation, weak but statistically significant, with CKD-EPI values. Our findings were in agreement with those mentioned in other studies. PNI was found to be positively connected with eGFR and negatively correlated with BMI, HbA1c, and fasting blood glucose, according to Aktas et al. [61]. Additionally, Zhang et al. [62,63] found that the PNI was positively correlated with eGFR and discovered a negative correlation between the PNI and the following: red cell distribution width, HbA1c, urine albumin-to-creatinine ratio, neutrophil-to-lymphocyte ratio, glomerular injury, and high-sensitivity C-reactive protein.
The PNI is made up of serum albumin and blood lymphocyte count, both of which are easily obtained in the laboratory of practically every medical facility. The PNI is therefore a marker that is simple to evaluate and is a cheap marker since it is economical to study serum albumin and lymphocyte count.
Since this study was restricted to two university clinical hospitals representative of Dolj County, we acknowledge that it has inherent limitations. In addition, as we lacked a pilot study and prior data that were referenced in the literature, we were unable to determine the effect size and hence did not compute the simulation of sample size.
Patients with serious complications, a low willingness to seek medical attention, or mobility issues would be few, which would bias the study’s selection process. However, the limited number of patients in certain categories following their grouping might have an impact on the outcomes.
Therefore, more studies should be designed to investigate the association of obesity-related indices with the PNI, regarding different categories of patients with diabetes and prediabetes.
5. Conclusions
From our observational study, four conclusions emerged. As a first observation, up to 43% of patients with preDM and 60% of patients with T2DM were obese. The PNI showed that patients with T2DM had a moderate-to-severe malnutrition status, and patients with preDM had a mild-to-moderate malnutrition status at diagnosis. We observed that preDM and T2DM patients with low PNI were significantly associated with higher median values of WHtR and BMI. In the T2DM group, our study revealed a moderate and statistically significant negative correlation between PNI and weight, WC, HC, WHtR, BAI, and FP. Additionally, the PNI values expressed a weak negative correlation with BMI and HbA1c. The PNI levels exhibited a single positive correlation, weak but statistically significant, with CKD-EPI values. Our results can undoubtedly serve as a springboard for additional research and multicentric, long-term investigations. The findings of this study regarding the correlations between PNI, GPS, and different obesity-related indices in people with diabetes or prediabetes suggest that these indices, which assess nutritional and inflammatory status, can be used as independent predictor factors associated with the four pillars of DM management (glucose, blood pressure, lipids, and weight control) recommended by the ADA.
Acknowledgments
This study is part of the PhD thesis of Roxana-Viorela Ahrițculesei from the University of Medicine and Pharmacy of Craiova, Craiova, Romania.
Author Contributions
Conceptualization, R.-V.A., M.V.B., and L.B.; methodology, I.M.V., R.C.C., D.C., and A.M.; validation, M.V.B., I.S., and C.C.V.; investigation, R.-V.A., R.C.C., D.C., A.M., M.-L.M., and I.S.; resources, R.-V.A., D.C., A.M., and I.M.V.; data curation, I.S., L.B., M.-L.M., and C.C.V.; writing—R.-V.A., R.C.C., D.C., A.M., and M.-L.M.; writing—review and editing, M.V.B., I.M.V., and C.C.V.; supervision, M.V.B., I.M.V., and C.C.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted by the Declaration of Helsinki, and approved by the Ethics Committee of the Filantropia Municipal Clinical Hospital (no. 886/15 January 2024) and Emergency County Clinical Hospital of Craiova (no. 2371/14 January 2022), Dolj, Romania.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) to publish this paper.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The Article Processing Charges were funded by the University of Medicine and Pharmacy of Craiova, Romania.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization (WHO) Diabetes. [(accessed on 30 August 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- 2.IDF Diabetes Atlas, 10th Edition. [(accessed on 30 August 2024)]. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf.
- 3.Watson C.S. Screening, diagnosis, and intervention. J. Nurse. Pract. 2017;13:216–221. doi: 10.1016/j.nurpra.2016.08.005. [DOI] [Google Scholar]
- 4.Bansal N. Prediabetes diagnosis and treatment: A review. World J. Diabetes. 2015;6:296–303. doi: 10.4239/wjd.v6.i2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colagiuri S. Epidemiology of prediabetes. Med. Clin. N. Am. 2011;95:299–307. doi: 10.1016/j.mcna.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Bahijri S.M., Jambi H.A., Al Raddadi R.M., Ferns G., Tuomilehto J. The prevalence of diabetes and prediabetes in the adult population of Jeddah, Saudi Arabia—A community-based survey. PLoS ONE. 2016;11:e0152559. doi: 10.1371/journal.pone.0152559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindström J., Peltonen M., Eriksson J.G., Ilanne-Parikka P., Aunola S., Keinänen-Kiukaanniemi S., Uusitupa M., Tuomilehto J., Finnish Diabetes Prevention Study (DPS) Improved lifestyle and decreased diabetes risk over 13 years: Long-term follow-up of the randomized Finnish Diabetes Prevention Study (DPS) Diabetologia. 2013;56:284–293. doi: 10.1007/s00125-012-2752-5. [DOI] [PubMed] [Google Scholar]
- 8.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert A.A., Al Dawish M.A., Braham R., Musallam M.A., Al Hayek A.A., Al Kahtany N.H. Type 2 Diabetes Mellitus in Saudi Arabia: Major Challenges and Possible Solutions. Curr. Diabetes Rev. 2017;13:59–64. doi: 10.2174/1573399812666160126142605. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Geiss L.S., Burrows N.R., Rolka D.B., Albright A. Awareness of prediabetes—United States, 2005–2010. MMWR Morb. Mortal. Wkly. Rep. 2013;62:209–212. [PMC free article] [PubMed] [Google Scholar]
- 11.Federation H. In: Global Atlas on Cardiovascular Disease Prevention and Control. Mendis S., editor. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- 12.American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2014;37((Suppl. S1)):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 13.Mainous A.G., Tanner R.J., Jo A., Anton S.D. Prevalence of prediabetes and abdominal obesity among healthy-weight adults: 18-year trend. Ann. Fam. Med. 2016;14:304–310. doi: 10.1370/afm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berceanu C., Mehedinţu C., Berceanu S., Voicu N.L., Brătilă E., Istrate-Ofiţeru A.M., Navolan D.B., Niculescu M., Szasz F.A., Căpitănescu R.G., et al. Morphological and ultrasound findings in multiple pregnancy placentation. Rom. J. Morphol. Embryol. 2018;59:435–453. [PubMed] [Google Scholar]
- 15.Hao Z., Huang X., Qin Y., Li H., Tian F., Xu R., Chang B., Shao H. Analysis of factors related to diabetic retinopathy in patients with newly diagnosed type 2 diabetes: A cross-sectional study. BMJ Open. 2020;10:e32095. doi: 10.1136/bmjopen-2019-032095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madsen L.R., Bek T., Richelsen B. Diabetic retinopathy in people with Type 2 diabetes and obesity treated by Roux-en-Y gastric bypass compared with non-operated controls: With focus on the role of diabetes remission in a cross-sectional and a 6-year follow-up study. Diabet. Med. 2019;36:457–464. doi: 10.1111/dme.13876. [DOI] [PubMed] [Google Scholar]
- 17.Raum P., Lamparter J., Ponto K.A., Peto T., Hoehn R., Schulz A., Schneider A., Wild P.S., Pfeiffer N., Mirshahi A. Prevalence and cardiovascular associations of diabetic retinopathy and maculopathy: Results from the Gutenberg health study. PLoS ONE. 2015;10:e127188. doi: 10.1371/journal.pone.0127188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onodera T., Goseki N., Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 19.Yang Y., Gao P., Chen X., Song Y., Shi J., Zhao J., Sun J., Xu Y., Wang Z. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: Results from a retrospective cohort study and a meta-analysis. Oncotarget. 2016;7:58543–58552. doi: 10.18632/oncotarget.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soh J., Suzawa K., Shien K., Otani S., Yamamoto H., Okazaki M., Sugimoto S., Katsui K., Yamane M., Kiura K., et al. Prognostic nutrition index affects the prognosis of patients undergoing trimodality therapy for locally advanced non-small cell lung cancer. Surg. Today. 2020;50:1610–1618. doi: 10.1007/s00595-020-02067-7. [DOI] [PubMed] [Google Scholar]
- 21.Cîmpeanu R.C., Boldeanu M.V., Ahrițculesei R.V., Ciobanu A.E., Cristescu A.M., Forțofoiu D., Siloși I., Pirici D.N., Cazacu S.M., Boldeanu L., et al. Correlation between Neurotransmitters (Dopamine, Epinephrine, Norepinephrine, Serotonin), Prognostic Nutritional Index, Glasgow Prognostic Score, Systemic Inflammatory Response Markers, and TNM Staging in a Cohort of Colorectal Neuroendocrine Tumor Patients. Int. J. Mol. Sci. 2024;25:6977. doi: 10.3390/ijms25136977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zencirkiran Agus H., Kahraman S. Prognostic nutritional index predicts one-year outcome in heart failure with preserved ejection fraction. Acta Cardiol. 2020;75:450–455. doi: 10.1080/00015385.2019.1661139. [DOI] [PubMed] [Google Scholar]
- 23.Keskin M., Hayıroğlu M., Keskin T., Kaya A., Tatlısu M., Altay S., Uzun A., Börklü E., Güvenç T., Avcı I.I., et al. A novel and useful predictive indicator of prognosis in ST-segment elevation myocardial infarction, the prognostic nutritional index. Nutr. Metab. Cardiovasc. Dis. 2017;27:438–446. doi: 10.1016/j.numecd.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y., Sung S., Cheng H., Hsu P., Guo C., Yu W., Chen C. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J. Am. Heart Assoc. 2017;6:e004876. doi: 10.1161/JAHA.116.004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tak B.T., Cay S., Pamukcu H.E., Ekizler F.A., Kafes H., Cetin E.H.O., Ulvan N., Ozeke O., Ozcan F., Topaloglu S., et al. Prognostic nutritional index as a novel marker for prediction of prognosis in patients with peripartum cardiomyopathy. Medicine. 2020;99:e19524. doi: 10.1097/MD.0000000000019524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei W., Wu X., Jin C., Mu T., Gu G., Min M., Mu S., Han Y. Predictive Significance of the Prognostic Nutritional Index (PNI) in Patients with Severe COVID-19. J. Immunol. Res. 2021;2021:9917302. doi: 10.1155/2021/9917302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adam A.M., Popa R.F., Vaduva C., Georgescu C.V., Adam G., Melinte-Popescu A.S., Popa C., Socolov D., Nechita A., Vasilache I.A., et al. Pregnancy Outcomes, Immunophenotyping and Immunohistochemical Findings in a Cohort of Pregnant Patients with COVID-19-A Prospective Study. Diagnostics. 2023;13:1345. doi: 10.3390/diagnostics13071345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popescu M., Terzea D.C., Carsote M., Ghenea A.E., Costache A., Popescu I.A.S., Biciuşcă V., Busuioc C.J., Ghemigian A.M. COVID-19 infection: From stress-related cortisol levels to adrenal glands infarction. Rom. J. Morphol. Embryol. 2022;63:39–48. doi: 10.47162/RJME.63.1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barutcu Atas D., Tugcu M., Asicioglu E., Velioglu A., Arikan H., Koc M., Tuglular S. Prognostic nutritional index is a predictor of mortality in elderly patients with chronic kidney disease. Int. Urol. Nephrol. 2022;54:1155–1162. doi: 10.1007/s11255-021-03002-6. [DOI] [PubMed] [Google Scholar]
- 30.Iwasaki T., Togashi Y., Terauchi Y. Significant association of serum albumin with severity of retinopathy and neuropathy, in addition to that of nephropathy, in Japanese type 2 diabetic patients. Endocr. J. 2008;55:311–316. doi: 10.1507/endocrj.K07-086. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association Professional Practice Committee 12. Retinopa-thy, neuropathy, and foot care: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47((Suppl. S1)):S231–S243. doi: 10.2337/dc24-S012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lameire N.H., Levin A., Kellum J.A., Cheung M., Jadoul M., Winkelmayer W.C., Stevens P.E. Conference Participants Harmonizing acute and chronic kidney disease definition and classification: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021;100:516–526. doi: 10.1016/j.kint.2021.06.028. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association Professional Practice Committee 2. Diagnosis and classification of diabetes: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47((Suppl. S1)):S20–S42. doi: 10.2337/dc24-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W., Gong X., Wang W., Xiong K., Meng J., Li Y., Wang L., Liang X., Jin L., Huang W. Association of different kinds of obesity with diabetic retinopathy in patients with type 2 diabetes. BMJ. 2022;12:e056332. doi: 10.1136/bmjopen-2021-056332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Kidney Foundation CKD-EPI Creatinine Equation 2021. [(accessed on 30 August 2024)]. Available online: https://www.kidney.org/professionals/gfr_calculator.
- 36. [(accessed on 30 August 2024)]. Available online: https://www.mdcalc.com/calc/76/mdrd-gfr-equation.
- 37. [(accessed on 30 August 2024)]. Available online: https://radiologyreviewarticles.com/radiology-calculators/prognostic-nutritional-index/
- 38.Forrest L.M., McMillan D.C., McArdle C.S., Angerson W.J., Dunlop D.J. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br. J. Cancer. 2004;90:1704–1706. doi: 10.1038/sj.bjc.6601789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization . Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation. WHO; Geneva, Switzerland: 2008. [Google Scholar]
- 40.Lin X., Xu Y., Pan X., Xu J., Ding Y., Sun X., Song X., Ren Y., Shan P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020;10:14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabák A.G., Herder C., Rathmann W., Brunner E.J., Kivimäki M. Prediabetes: A highrisk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sánchez E., Betriu À., López-Cano C., Hernández M., Fernández E., Purroy F., Bermúdez-López M., Farràs-Sallés C., Barril S., Pamplona R., et al. Characteristics of atheromatosis in the prediabetes stage: A cross-sectional investigation of the ILERVAS project. Cardiovasc. Diabetol. 2019;18:154. doi: 10.1186/s12933-019-0962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y., Cai X., Mai W., Li M., Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ. 2016;355:i5953. doi: 10.1136/bmj.i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kodama K., Tojjar D., Yamada S., Toda K., Patel C.J., Butte A.J. Ethnic differences in the relationship between insulin sensitivity and insulin response. Diabetes Care. 2013;26:1789–1796. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aoyama-Sasabe S., Fukushima M., Xin X., Taniguchi A., Nakai Y., Mitsui R., Takahashi Y., Tsuji H., Yabe D., Yasuda K., et al. Insulin secretory defect and insulin resistance in isolated impaired fasting glucose and isolated impaired glucose tolerance. J. Diabetes Res. 2016;2016:1298601. doi: 10.1155/2016/1298601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vatcheva K.P., Fisher-Hoch S.P., Reininger B.M., McCormick J.B. Sex and age differences in prevalence and risk factors for prediabetes in Mexican-Americans. Diabetes Res. Clin. Pract. 2020;159:107950. doi: 10.1016/j.diabres.2019.107950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cowie C.C. Diabetes diagnosis and control: Missed opportunities to improve health. Diabetes Care. 2019;42:994–1004. doi: 10.2337/dci18-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimodaira M., Okaniwa S., Hanyu N., Nakayama T. Optimal hemoglobin A1c levels for screening of diabetes and prediabetes in the Japanese population. J. Diabetes Res. 2015;2015:932057. doi: 10.1155/2015/932057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malone J.I., Hansen B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? or is it the opposite? Pediatr. Diabetes. 2019;20:5–9. doi: 10.1111/pedi.12787. [DOI] [PubMed] [Google Scholar]
- 51.Amzolini A.M., Forţofoiu M.C., Barău Abu-Alhija A., Vladu I.M., Clenciu D., Mitrea A., Forţofoiu M., Matei D., Enăchescu V., Predescu O.I., et al. Triglyceride and glucose index: A useful tool for non-alcoholic liver disease as-sessed by liver biopsy in patients with metabolic syndrome? Rom. J. Morphol. Embryol. 2021;62:475–480. doi: 10.47162/RJME.62.2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein S., Gastaldelli A., Yki-Jarvinen H., Scherer P.E. Why does obesity cause diabetes? Cell Metab. 2022;34:11–20. doi: 10.1016/j.cmet.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohm T.V., Meier D.T., Olefsky J.M., Donath M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Istrate-Ofiţeru A.M., Mogoantă C.A., Zorilă G.L., Roşu G.C., Drăguşin R.C., Berbecaru E.I., Zorilă M.V., Comănescu C.M., Mogoantă S.Ș., Vaduva C.C., et al. Clinical Characteristics and Local Histopathological Modulators of Endometriosis and Its Progression. Int. J. Mol. Sci. 2024;25:1789. doi: 10.3390/ijms25031789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Authors/Task Force Members. Rydén L., Grant P.J., Anker S.D., Berne C., Cosentino F., Danchin N., Deaton C., Escaned J., Hammes H.P., et al. Esc guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 56.Socea B., Radu L., Clenciu D., Cojan T.S.T., Baleanu V., Ene C.G., Girgavu S.R., Vladu I.M. The Utility of Visceral Adiposity Index in Prediction of Metabolic Syndrome and Hypercholesterolemia. Rev. Chim. 2018;69:3112–3114. doi: 10.37358/RC.18.11.6693. [DOI] [Google Scholar]
- 57.De Lorenzo A., Soldati L., Sarlo F., Calvani M., Di Lorenzo N., Di Renzo L. New obesity classification criteria as a tool for bariatric surgery indication. World J. Gastroenterol. 2016;22:681–703. doi: 10.3748/wjg.v22.i2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 59.Man R.E.K., Sabanayagam C., Chiang P.P.-C., Li L.-J., Noonan J.E., Wang J.J., Wong T.Y., Cheung G.C.-M., Tan G.S.W., Lamoureux E.L. Differential association of generalized and abdominal obesity with diabetic retinopathy in Asian patients with type 2 diabetes. JAMA Ophthalmol. 2016;134:251–257. doi: 10.1001/jamaophthalmol.2015.5103. [DOI] [PubMed] [Google Scholar]
- 60.Bergman R.N., Stefanovski D., Buchanan T.A., Sumner A.E., Reynolds J.C., Sebring N.G., Xiang A.H., Watanabe R.M. A better index of body adiposity. Obesity. 2011;19:1083–1089. doi: 10.1038/oby.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aktas G. Association between the Prognostic Nutritional Index and Chronic Microvascular Complications in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2023;12:5952. doi: 10.3390/jcm12185952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J., Xiao X., Wu Y., Yang J., Zou Y., Zhao Y., Yang Q., Liu F. Prognostic Nutritional Index as a Predictor of Diabetic Nephropathy Progression. Nutrients. 2022;14:3634. doi: 10.3390/nu14173634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J., Chen Y., Zou L., Gong R. Prognostic nutritional index as a risk factor for diabetic kidney disease and mortality in patients with type 2 diabetes mellitus. Acta Diabetol. 2023;60:235–245. doi: 10.1007/s00592-022-01985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurtul B.E., Koca S., Yilmaz M.O. Prognostic nutritional index as a novel marker for diabetic retinopathy in individuals with type 2 diabetes mellitus. Saudi J. Ophthalmol. 2022;36:322–326. doi: 10.4103/sjopt.sjopt_63_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L., Yu W., Pan W., Chen S., Ye X., Gu X., Hu X. A Clinical Epidemiological Analysis of Prognostic Nutritional Index Associated with Diabetic Retinopathy. Diabetes Metab. Syndr. Obes. 2021;14:839–846. doi: 10.2147/DMSO.S295757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei W., Lin R., Li S., Chen Z., Kang Q., Lv F., Zhong W., Chen H., Tu M. Malnutrition Is Associated with Diabetic Retinopathy in Patients with Type 2 Diabetes. J. Diabetes Res. 2023;2023:1613727. doi: 10.1155/2023/1613727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.