Abstract
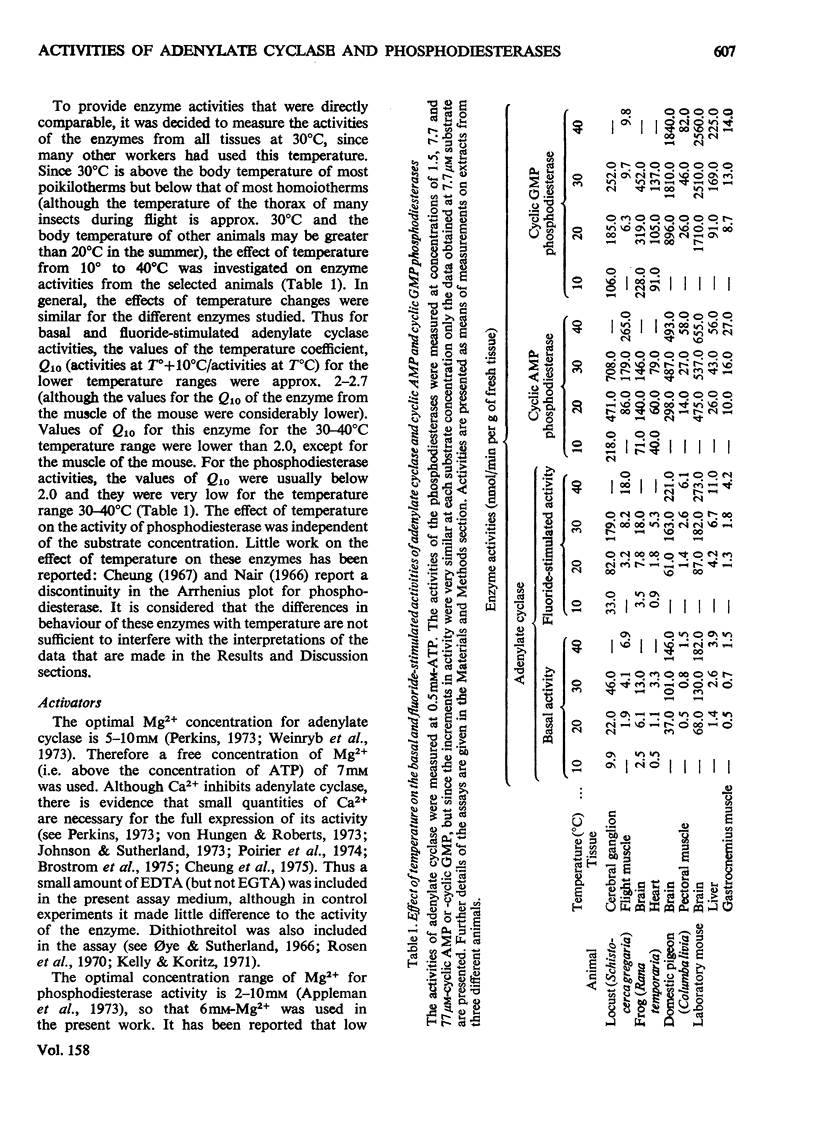
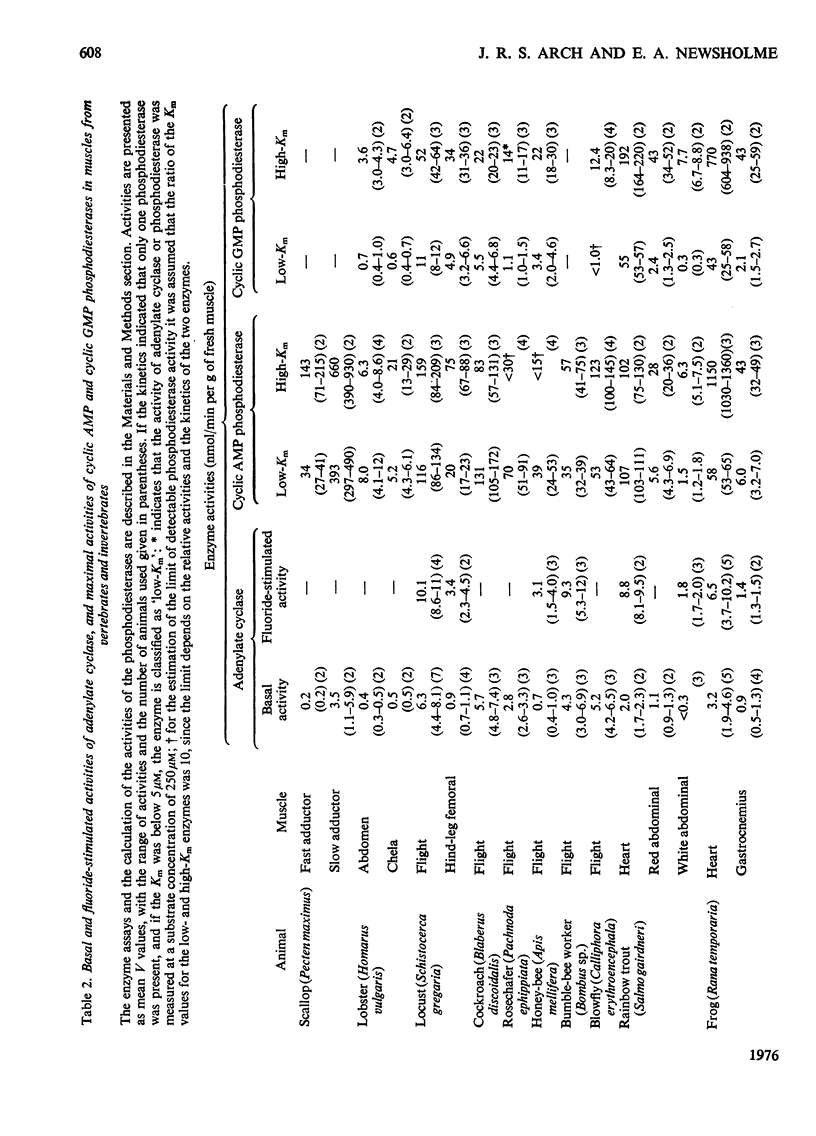
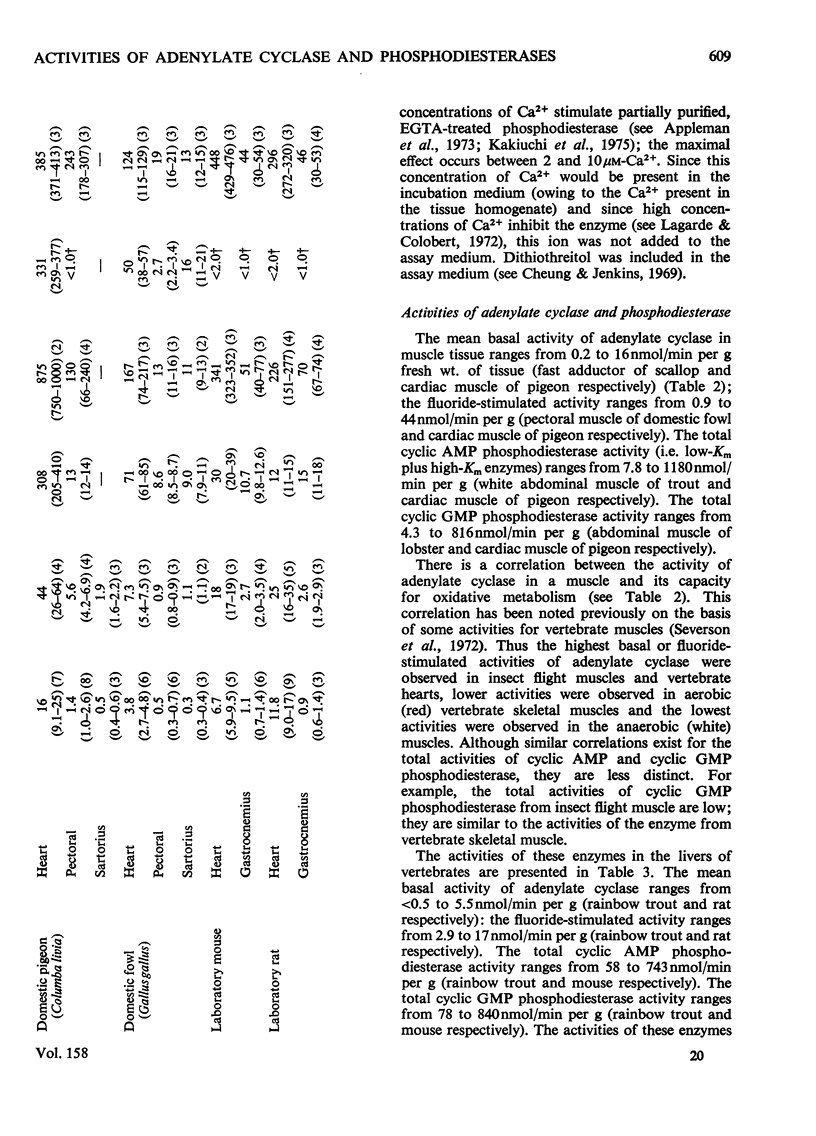
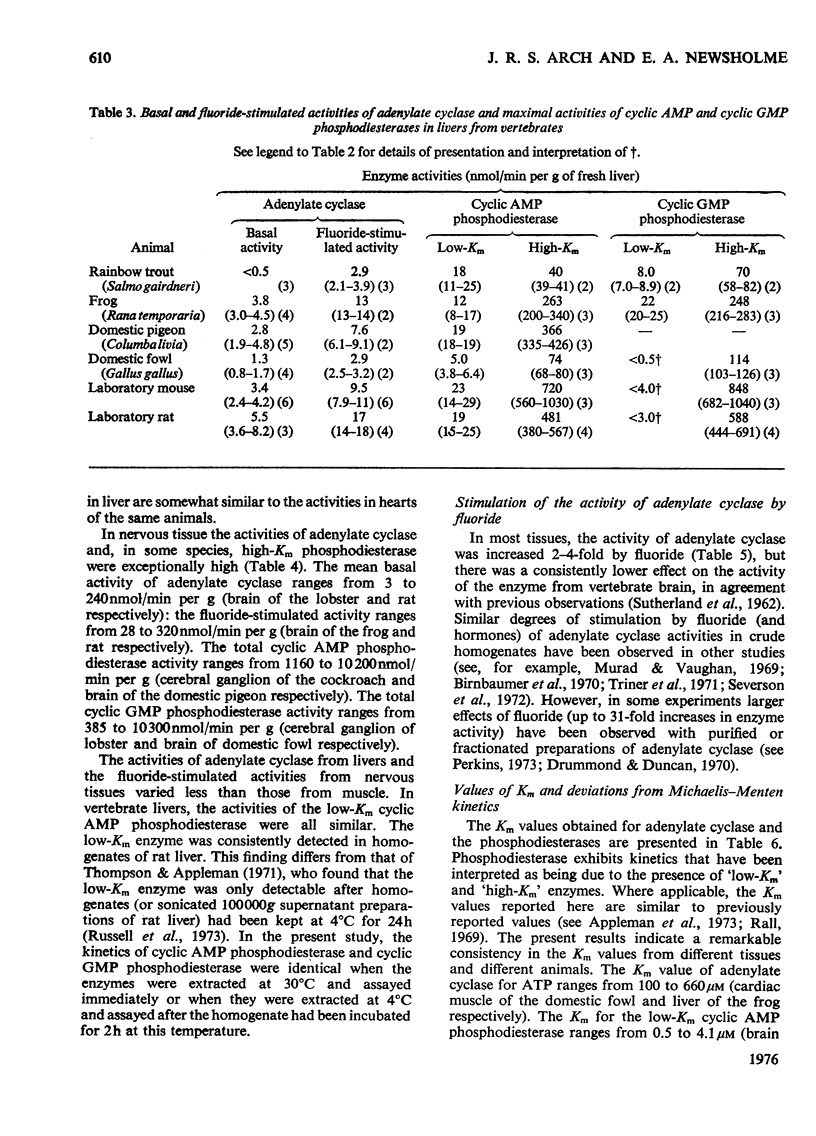
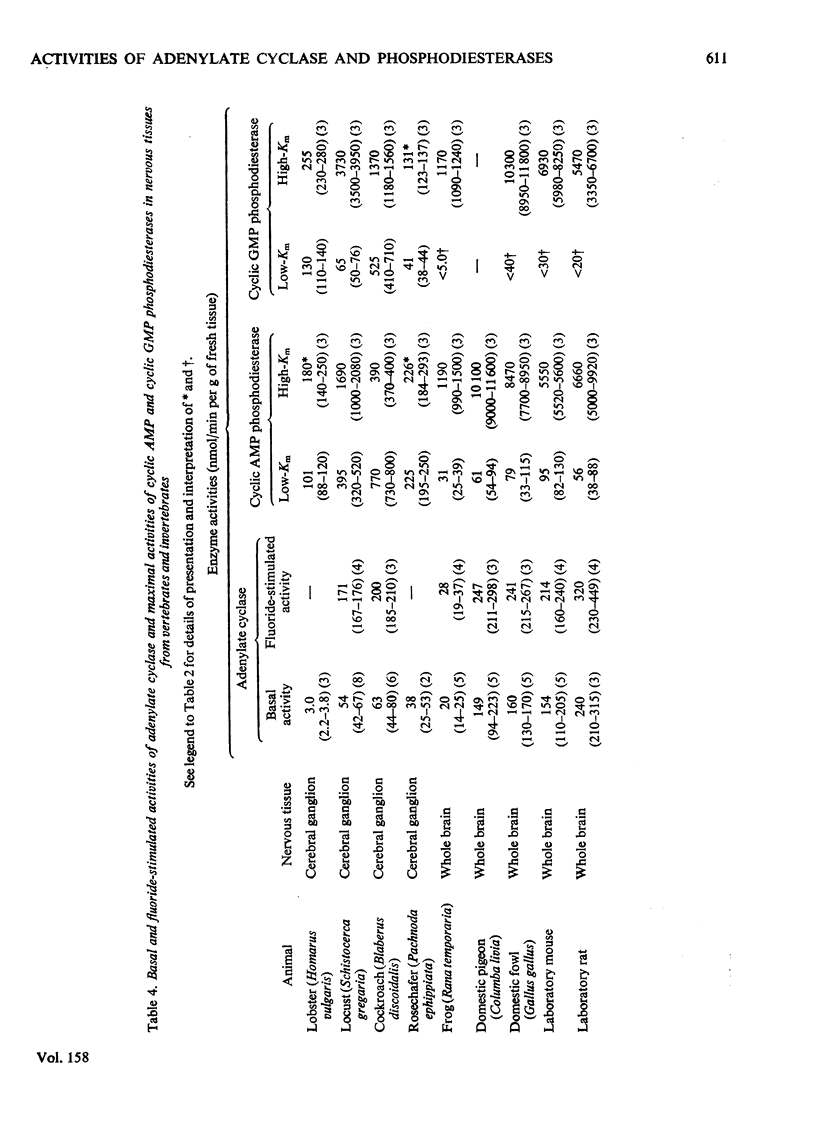
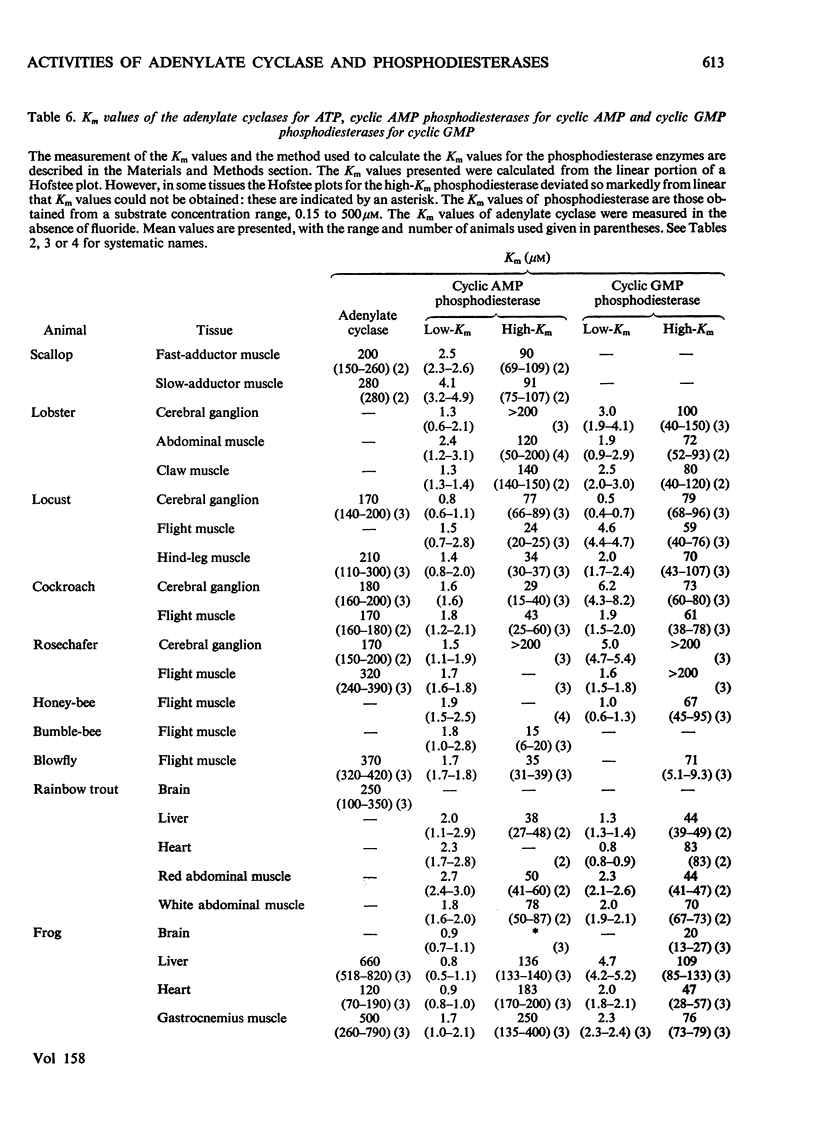
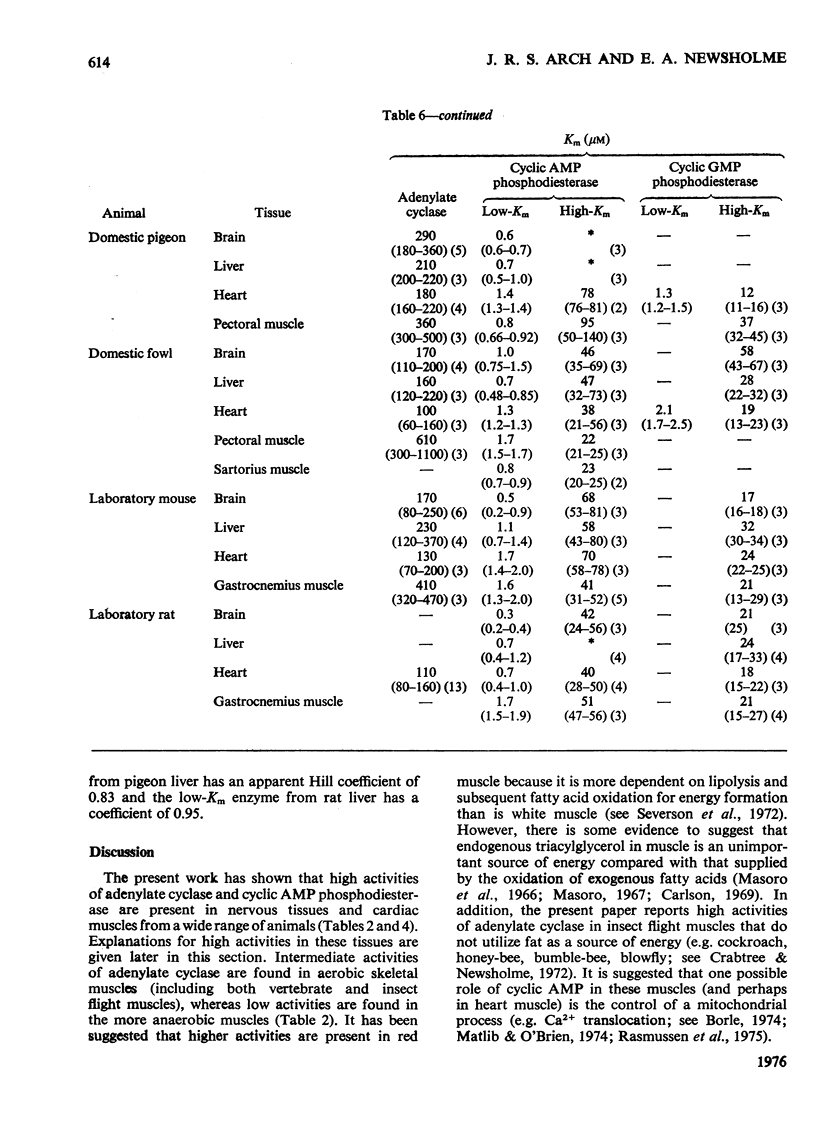
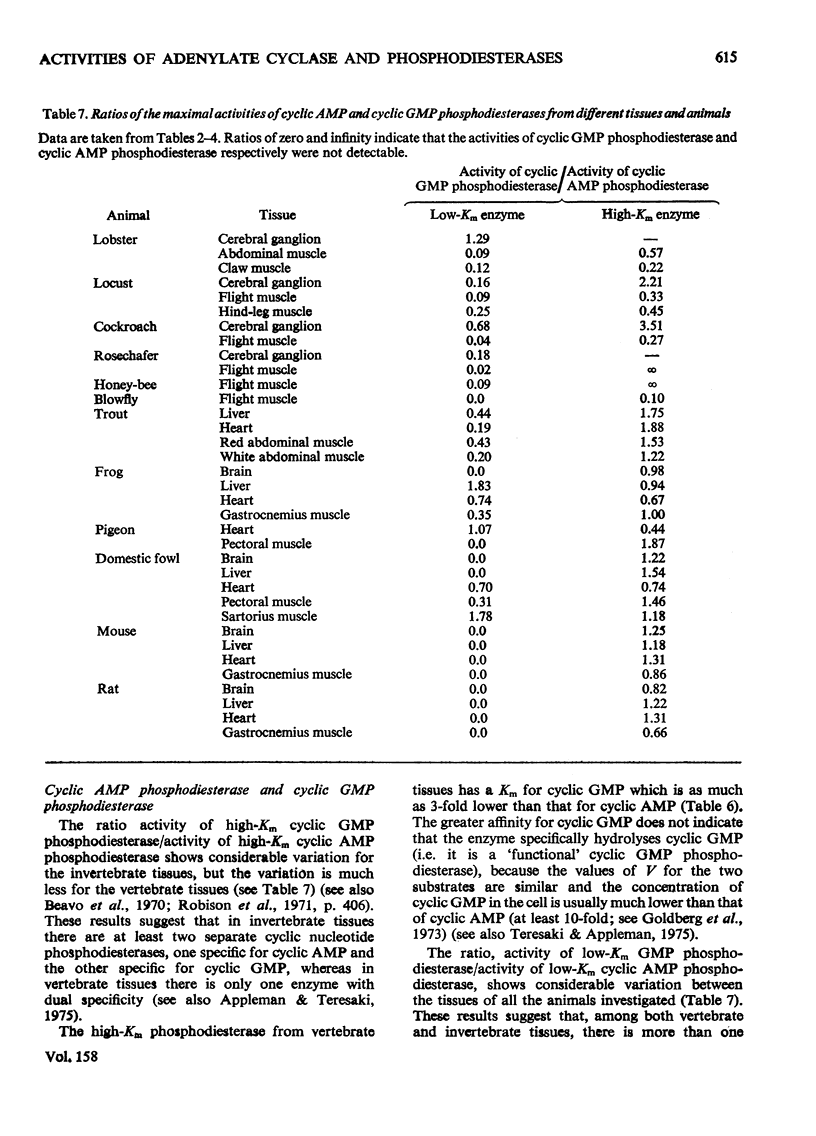
1. The basal and fluoride-stimulated activities of adenylate cyclase, and the maximal activities of 3':5'-cyclic AMP phosphodiesterase and 3':5'-cyclic GMP phosphodiesterase, together with the Km values for their respective substrates, were measured in muscle, liver and nervous tissues from a large range of animals to provide information on the mechanism of control of cyclic AMP concentrations in these tissues. High activities of adenylate cyclase and cyclic AMP diesterase are found in nervous tissues and in the more aerobic muscles (e.g. insect flight muscles, cardiac muscle and some vertebrate skeletal muscles). The activities of these enzymes in liver are similar to those in the heart of the same animal. The Km values for the enzymes from different tissues and animals are remarkably similar. 2. The comparison of cyclic AMP phosphodiesterase and cyclic GMP phosphodiesterase activities suggests that in vertebrate tissues only one enzyme (the high-Km enzyme), which possesses dual specificity, exists, whereas in invertebrate tissues there are at least two phosphodiesterases with separate specificities. 3. A simple quantitative model to explain the control of the steady-state concentrations of cyclic AMP is proposed. The maximum increase in cyclic AMP concentration predicted by comparison of basal with fluoride-stimulated activities of adenylate cyclase is compared with the maximum increases in concentration produced in the intact tissue by hormonal stimulation: reasonable agreement is obtained. The model is also used to predict the actual concentrations and the rates of turnover of cyclic AMP in different tissues and, where possible, these values are compared with reported values. Reasonable agreement is found between predicted and reported values. The possible physiological significances of different rates of turnover of cyclic AMP and the different ratios of high- and low-Km phosphodiesterases in different tissues are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleman M. M., Terasaki W. L. Regulation of cyclic nucleotide phosphodiesterase. Adv Cyclic Nucleotide Res. 1975;5:153–162. [PubMed] [Google Scholar]
- Appleman M. M., Thompson W. J., Russell T. R. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1973;3:65–98. [PubMed] [Google Scholar]
- Armstrong K. J., Stouffer J. E., Van Inwegen R. G., Thompson W. J., Robison G. A. Effects of thyroid hormone deficiency on cyclic adenosine 3':5'-monophosphate and control of lipolysis in fat cells. J Biol Chem. 1974 Jul 10;249(13):4226–4231. [PubMed] [Google Scholar]
- Aurbach G. D., Houston B. A. Determination of 3',5'-adenosine monophosphate with a method based on a radioactive phosphate exchange reaction. J Biol Chem. 1968 Nov 25;243(22):5935–5940. [PubMed] [Google Scholar]
- BRECKENRIDGE B. M. THE MEASUREMENT OF CYCLIC ADENYLATE IN TISSUES. Proc Natl Acad Sci U S A. 1964 Dec;52:1580–1586. doi: 10.1073/pnas.52.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Hydrolysis of cyclic guanosine and adenosine 3',5'-monophosphates by rat and bovine tissues. J Biol Chem. 1970 Nov 10;245(21):5649–5655. [PubMed] [Google Scholar]
- Beis I., Newsholme E. A. The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J. 1975 Oct;152(1):23–32. doi: 10.1042/bj1520023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevers M. M., Smits R. A., van Rijn J., van Wijk R. Heat treatment of rat liver denosine 3':5'-monophosphate phosphodiesterase. Kinetic characterization of the low affinity enzyme. Biochim Biophys Acta. 1974 Mar 21;341(1):120–128. doi: 10.1016/0005-2744(74)90072-2. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Michiel H., Krans M. J., Rodbell M. The actions of hormones on the adenyl cyclase system. Adv Biochem Psychopharmacol. 1970;3:185–208. [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. II. Comparison between glucagon- and fluoride-stimulated activities. J Biol Chem. 1971 Mar 25;246(6):1857–1860. [PubMed] [Google Scholar]
- Bloom F. E., Wedner H. J., Parker C. W. The use of antibodies to study cell structure and metabolism. Pharmacol Rev. 1973 Jun;25(2):343–358. [PubMed] [Google Scholar]
- Boeynaems J. M., Van Sande J., Pochet R., Dumont J. E. The relation between adenylate cyclase activation and cAMP acculumation in the horse thyroid gland stimulated by thyrotropin. Mol Cell Endocrinol. 1974 Apr;1(2):139–155. doi: 10.1016/0303-7207(74)90006-9. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Cyclic AMP stimulation of calcium efflux from kidney, liver and heart mitochondria. J Membr Biol. 1974;16(3):221–236. doi: 10.1007/BF01872416. [DOI] [PubMed] [Google Scholar]
- Brooker G. Oscillation of cyclic adenosine monophosphate concentration during the myocardial contraction cycle. Science. 1973 Nov 20;182(4115):933–934. doi: 10.1126/science.182.4115.933. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard W. P. Catecholamine induced increase of cyclic adenosine 3',5'-monophosphate in rat brain in vivo. J Neurochem. 1972 Nov;19(11):2615–2619. doi: 10.1111/j.1471-4159.1972.tb01320.x. [DOI] [PubMed] [Google Scholar]
- Butcher R. W., Baird C. E., Sutherland E. W. Effects of lipolytic and antilipolytic substances on adenosine 3',5'-monophosphate levels in isolated fat cells. J Biol Chem. 1968 Apr 25;243(8):1705–1712. [PubMed] [Google Scholar]
- Cheung W. Y., Bradham L. S., Lynch T. J., Lin Y. M., Tallant E. A. Protein activator of cyclic 3':5'-nucleotide phosphodiesterase of bovine or rat brain also activates its adenylate cyclase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1055–1062. doi: 10.1016/0006-291x(75)90747-0. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Properties of cyclic 3',5'-nucleotide phosphodiesterase from rat brain. Biochemistry. 1967 Apr;6(4):1079–1087. doi: 10.1021/bi00856a017. [DOI] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972 Jan;126(1):49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W. Cyclic adenosine 3',5'monophosphate role in the physiology and pharmacology of the central nervous system. Biochem Pharmacol. 1975 Jan 15;24(2):159–164. doi: 10.1016/0006-2952(75)90272-5. [DOI] [PubMed] [Google Scholar]
- Davies J. I., Williams P. A. Quantitative aspects of the regulation of cellular cyclic AMP levels. I. Structure and kinetics of a model system. J Theor Biol. 1975 Sep;53(1):1–30. doi: 10.1016/0022-5193(75)90100-9. [DOI] [PubMed] [Google Scholar]
- Drummond G. I., Duncan L. Adenyl cyclase in cardiac tissue. J Biol Chem. 1970 Mar 10;245(5):976–983. [PubMed] [Google Scholar]
- Gilman A. G. Protein binding assays for cyclic nucleotides. Adv Cyclic Nucleotide Res. 1972;2:9–24. [PubMed] [Google Scholar]
- Goldberg N. D., O'Dea R. F., Haddox M. K. Cyclic GMP. Adv Cyclic Nucleotide Res. 1973;3:155–223. [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- House P. D., Poulis P., Weidemann M. J. Isolation of a plasma-membrane subfraction from rat liver containing an insulin-sensitive cyclic-AMP phosphodiesterase. Eur J Biochem. 1972 Jan 21;24(3):429–437. doi: 10.1111/j.1432-1033.1972.tb19703.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Sutherland E. W. Detergent-dispersed adenylate cyclase from rat brain. Effects of fluoride, cations, and chelators. J Biol Chem. 1973 Jul 25;248(14):5114–5121. [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. Studies on adenosine 3',5'-phosphate in rabbit cerebral cortex. Mol Pharmacol. 1968 Jul;4(4):379–388. [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y., Uenishi K., Miyamoto E. Multiple cyclic nucleotide phosphodiesterase activities from rat tissues and occurrence of a calcium-plus-magnesium-ion-dependent phosphodiesterase and its protein activator. Biochem J. 1975 Jan;146(1):109–120. doi: 10.1042/bj1460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L. A., Koritz S. B. Bovine adrenal cortical adenyl cyclase and its stimulation by adrenocorticotropic hormone and NaF. Biochim Biophys Acta. 1971 Apr 20;237(1):141–155. doi: 10.1016/0304-4165(71)90043-2. [DOI] [PubMed] [Google Scholar]
- Kuster J., Zapf J., Jakob A. Effects of hormones on cyclic AMP release in perfused rat livers. FEBS Lett. 1973 May 15;32(1):73–77. doi: 10.1016/0014-5793(73)80740-9. [DOI] [PubMed] [Google Scholar]
- Lagarde A., Colobert L. Cyclic 3',5'-AMP phosphodiesterase of human blood lymphocytes. Biochim Biophys Acta. 1972 Aug 28;276(2):444–453. doi: 10.1016/0005-2744(72)91006-6. [DOI] [PubMed] [Google Scholar]
- Loten E. G., Sneyd J. G. An effect of insulin on adipose-tissue adenosine 3':5'-cyclic monophosphate phosphodiesterase. Biochem J. 1970 Nov;120(1):187–193. doi: 10.1042/bj1200187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon J. B., Jr, Mayer S. E. Epinephrine induced formation of adenosine 3', 5'-monophosphate in mouse skeletal muscle. Biochem Biophys Res Commun. 1969 Feb 21;34(4):459–464. doi: 10.1016/0006-291x(69)90404-5. [DOI] [PubMed] [Google Scholar]
- Masoro E. J., Rowell L. B., McDonald R. M., Steiert B. Skeletal muscle lipids. II. Nonutilization of intracellular lipid esters as an energy source for contractile activity. J Biol Chem. 1966 Jun 10;241(11):2626–2634. [PubMed] [Google Scholar]
- Masoro E. J. Skeletal muscle lipids. 3. Analysis of the functioning of skeletal muscle lipids during fasting. J Biol Chem. 1967 Mar 25;242(6):1111–1114. [PubMed] [Google Scholar]
- Mayer S. E., Namm D. H., Rice L. Effect of glucagon on cyclic 3',5'-AMP, phosphorylase activity and contractility of heart muscle of the rat. Circ Res. 1970 Feb;26(2):225–233. doi: 10.1161/01.res.26.2.225. [DOI] [PubMed] [Google Scholar]
- Minson A. C., Creaser E. H. Purification of a trifunctional enzyme, catalysing three steps of the histidine pathway, from Neurospora crassa. Biochem J. 1969 Aug;114(1):49–56. doi: 10.1042/bj1140049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F., Vaughan M. Effect of glucagon on rat heart adenyl cyclase. Biochem Pharmacol. 1969 May;18(5):1053–1059. doi: 10.1016/0006-2952(69)90109-9. [DOI] [PubMed] [Google Scholar]
- Nair K. G. Purification and properties of 3',5'-cyclic nucleotide phosphodiesterase from dog heart. Biochemistry. 1966 Jan;5(1):150–157. doi: 10.1021/bi00865a020. [DOI] [PubMed] [Google Scholar]
- Namm D. H., Mayer S. E., Maltbie M. The role of potassium and calcium ions in the effect of epinephrine on cardiac cyclic adenosine 3',5'-monophosphate, phosphorylase kinase, and phosphorylase. Mol Pharmacol. 1968 Sep;4(5):522–530. [PubMed] [Google Scholar]
- Newsholme E. A., Taylor K. Glycerol kinase activities in muscles from vertebrates and invertebrates. Biochem J. 1969 May;112(4):465–474. doi: 10.1042/bj1120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oye I., Sutherland E. W. The effect of epinephrine and other agents on adenyl cyclase in the cell membrane of avian erythrocytes. Biochim Biophys Acta. 1966 Oct 31;127(2):347–354. doi: 10.1016/0304-4165(66)90389-8. [DOI] [PubMed] [Google Scholar]
- POSNER J. B., STERN R., KREBS E. G. EFFECTS OF ELECTRICAL STIMULATION AND EPINEPHRINE ON MUSCLE PHOSPHORYLASE, PHOSPHORYLASE B KINASE, AND ADENOSINE 3',5'-PHOSPHATE. J Biol Chem. 1965 Mar;240:982–985. [PubMed] [Google Scholar]
- Pawlson L. G., Lovell-Smith C. J., Manganiello V. C., Vaughan M. Effects of epinephrine, adrenocorticotrophic hormone, and theophylline on adenosine 3', 5'-monophosphate phosphodiesterase activity in fat cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1639–1642. doi: 10.1073/pnas.71.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J. P. Adenyl cyclase. Adv Cyclic Nucleotide Res. 1973;3:1–64. [PubMed] [Google Scholar]
- Perkins J. P., Moore M. M. Adenyl cyclase of rat cerebral cortex. Activation of sodium fluoride and detergents. J Biol Chem. 1971 Jan 10;246(1):62–68. [PubMed] [Google Scholar]
- Pilkis S. J., Exton J. H., Johnson R. A., Park C. R. Effects of glucagon on cyclic AMP and carbohydrate metabolism in livers from diabetic rats. Biochim Biophys Acta. 1974 Mar 20;343(1):250–267. doi: 10.1016/0304-4165(74)90258-x. [DOI] [PubMed] [Google Scholar]
- Poirier G., De Lean A., Pelletier G., Lemay A., Labrie F. Purification of adenohypophyseal plasma membranes and properties of associated adenylate cyclase. J Biol Chem. 1974 Jan 10;249(1):316–322. [PubMed] [Google Scholar]
- Rasmussen H., Jensen P., Lake W., Friedmann N., Goodman D. B. Cyclic nucleotides and cellular calcium metabolism. Adv Cyclic Nucleotide Res. 1975;5:375–394. [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci. 1967 Feb 10;139(3):703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Goren E. N., Erlichman J., Rosen S. M. Synthesis and degradation of cyclic 3',5'-adenosine monophosphate in frog erythrocytes. Adv Biochem Psychopharmacol. 1970;3:31–50. [PubMed] [Google Scholar]
- Russell T. R., Terasaki W. L., Appleman M. M. Separate phosphodiesterases for the hydrolysis of cyclic adenosine 3',5'-monophosphate and cyclic guanosine 3',5'-monophosphate in rat liver. J Biol Chem. 1973 Feb 25;248(4):1334–1340. [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Sakai T., Thompson W. J., Lavis V. R., Williams R. H. Cyclic nucleotide phosphodiesterase activities from isolated fat cells: correlation of subcellular distribution with effects of nucleotides and insulin. Arch Biochem Biophys. 1974 Jun;162(2):331–339. doi: 10.1016/0003-9861(74)90190-8. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Solomon B. Cyclic 3',5'-AMP phosphodiesterase of Neurospora crassa. Biochem Biophys Res Commun. 1973 Aug 6;53(3):1024–1030. doi: 10.1016/0006-291x(73)90194-0. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Drummond G. I., Sulakhe P. V. Adenylate cyclase in skeletal muscle. Kinetic properties and hormonal stimulation. J Biol Chem. 1972 May 10;247(9):2949–2958. [PubMed] [Google Scholar]
- Sherline P., Lynch A., Glinsmann W. H. Cyclic AMP and adrenergic receptor control of rat liver glycogen metabolism. Endocrinology. 1972 Sep;91(3):680–690. doi: 10.1210/endo-91-3-680. [DOI] [PubMed] [Google Scholar]
- Spears G., Sneyd J. G., Loten E. G. A method for deriving kinetic constants for two enzymes acting on the same substrate. Biochem J. 1971 Dec;125(4):1149–1151. doi: 10.1042/bj1251149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Newsholme E. A. Activities of hexokinase, phosphofructokinase, 3-oxo acid coenzyme A-transferase and acetoacetyl-coenzyme A thiolase in nervous tissue from vertebrates and invertebrates. Biochem J. 1973 May;134(1):97–101. doi: 10.1042/bj1340097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillens S., Paiva M., Dumont J. E. Consequences of the intracellular distribution of cyclic 3',5'-nucleotides phosphodiesterases. FEBS Lett. 1974 Dec 1;49(1):92–95. doi: 10.1016/0014-5793(74)80639-3. [DOI] [PubMed] [Google Scholar]
- Terasaki W. L., Appleman M. M. The role of cyclic GMP in the regulation of cyclic AMP hydrolysis. Metabolism. 1975 Mar;24(3):311–319. doi: 10.1016/0026-0495(75)90112-2. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Characterization of cyclic nucleotide phosphodiesterases of rat tissues. J Biol Chem. 1971 May 25;246(10):3145–3150. [PubMed] [Google Scholar]
- Triner L., Nahas G. G., Vulliemoz Y., Overweg N. T., Verosky M., Habif D. V., Ngai S. H. Cyclic AMP and smooth muscle function. Ann N Y Acad Sci. 1971 Dec 30;185:458–476. doi: 10.1111/j.1749-6632.1971.tb45273.x. [DOI] [PubMed] [Google Scholar]
- Van Inwegen R. G., Robison G. A., Thompson W. J. Cyclic nucleotide phosphodiesterases and thyroid hormones. J Biol Chem. 1975 Apr 10;250(7):2452–2456. [PubMed] [Google Scholar]
- Von Hungen K., Roberts S. Catecholamine and Ca 2+ activation of adenylate cyclase systems in synaptosomal fractions from rat cerebral cortex. Nat New Biol. 1973 Mar 14;242(115):58–60. doi: 10.1038/newbio242058a0. [DOI] [PubMed] [Google Scholar]
- Weinryb I., Michel I. M., Hess S. M. Adenylate cyclase from guinea pig lung: further characterization and inhibitory effects of substrate analogs and cyclic nucleotides. Arch Biochem Biophys. 1973 Jan;154(1):240–249. doi: 10.1016/0003-9861(73)90054-4. [DOI] [PubMed] [Google Scholar]
- Williams M., Rodnight R. Protein phosphorylation in respiring slices of guinea-pig cerebral cortex. Evidence for a role for noradrenaline and adenosine 3':5'-cyclic monophosphate in the increased phosphorylation observed on application of electrical pulses. Biochem J. 1976 Jan 15;154(1):163–170. doi: 10.1042/bj1540163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberger A., Babskii E. B., Krause E. G., Genz S., Blohm D., Bogdanova E. V. Cyclic changes in levels of cyclic AMP and cyclic GMP in frog myocardium during the cardiac cycle. Biochem Biophys Res Commun. 1973 Nov 16;55(2):446–452. doi: 10.1016/0006-291x(73)91107-8. [DOI] [PubMed] [Google Scholar]
- Woods W. D., Waitzman M. B. Isolation of cyclic 3',5'-adenosine monophosphate on a neutral silicic acid-glass microfiber matrix. J Chromatogr. 1970 Mar 31;47(3):536–542. doi: 10.1016/0021-9673(70)80087-5. [DOI] [PubMed] [Google Scholar]


