Simple Summary
Prophylactic cranial irradiation (PCI) is recommended to decrease the incidence of brain metastases (BM) in extensive stage small cell lung cancer (ESSCLC) without BM after response to chemotherapy while it is known also to be associated with significant neurocognitive effects. In recent years, advancements in radiotherapy technologies and the availability of MRI have led to stereotactic radiotherapy being a preferred option for brain metastases in various primary cancers, including non-small cell lung cancer (NSCLC). Several studies about BM in NSCLC have reported intracranial activity signal and significant improvement of intracerebral disease control rates with immunotherapy treatment However the brain effetc of immunothrapy remains unclear. We report a single-center retrospective study evaluating the impact of omitting. PCI from real-life treatment, including immunotherapy, of patients with ES-SCLC. About a cohort of 56 patients, A recursive partitioning analysis (RPA) found PS, immunotherapy and age to be influential factors for OS but not PCI.
Keywords: extensive-stage small-cell lung cancer, prophylactic cranial irradiation, immunotherapy
Abstract
Background: Prophylactic cranial irradiation (PCI) is recommended to decrease the incidence of brain metastases (BM) in extensive-stage small-cell lung cancer (ESSCLC) without BM after response to chemotherapy. However, PCI is associated with significant neurocognitive effects, and new studies are debating its benefits. Moreover, the introduction of immunotherapy in the management of the disease has raised new questions, and there is a lack of data on PCI and immunotherapy. We report a single-center retrospective study evaluating the impact of omitting PCI from real-life treatment, including immunotherapy, of patients with ES-SCLC. Methods: We identified patients followed at APHM between January 2014 and January 2021 for ES-SCLC without BM with an indication for PCI. The main assessment criteria considered in this study were overall survival (OS) and brain metastasis-free survival (BMFS) between patients who received PCI and those who did not. Results: 56 patients were included, 25 receiving PCI and 31 without PCI. The median follow-up was 16 months. Eighteen patients received immunotherapy, mostly in the group without PCI (p = 0.024). The median OS and BMFS were, respectively, 11.7 and 13.4 months in patients with PCI, and 20.3 and 10.7 months in patients without PCI, without any significant statistical difference (p = 0.412, p = 0.336). The prognostic factors highlighted in multivariate analysis were initial performance status (PS) < 2 for OS (HR = 2.74 (IC95% [1.23; 6.13])) and monocyte lymphocyte ratio (MLR) < 0.12 for BMFS (HR = 1.21 (IC95% [1.01; 1.45])). A recursive partitioning analysis (RPA) found PS, immunotherapy, and age to be influential factors for OS but not PCI. Conclusions: The clinical results of our study showed no benefit of PCI in terms of OS and BMFS for patients with ES-SCLC. This can be explained by the lack of benefit of PCI or by the introduction of immunotherapy.
1. Introduction
Small-cell lung cancer (SCLC) accounts for approximately 15% of all lung cancers. During follow-up, brain metastases (BM) occur in 70 to 80% of cases, resulting in a poor prognosis with a 5-year survival rate of 15–25% regardless of the initial stage [1,2]. The median survival for extensive-stage SCLC (ES-SCLC) is around 7 to 11 months. Until recently, the standard of care was a systemic treatment based on a combination of platinum-based chemotherapy [3]. This treatment was followed by prophylactic cranial irradiation (PCI) for patients with a partial or complete response without BM. These recommendations for PCI are based on the findings of two meta-analyses, which reported a benefit of PCI on overall survival (OS) compared to no irradiation after complete response to radio chemotherapy for limited or chemotherapy for disseminated SCLC [4,5]. These results were further supported by a randomized phase 3 EORTC trial published by Slotman et al. [6] in 2007. The study focused on ES-SCLC patients with a partial or complete response and reported a significant increase in both brain metastasis-free survival (BMFS) and OS (hazard ratio (HR) of 0.68 (IC95% [0.52; 0.88] for OS) in the PCI group. Nevertheless, patients did not undergo brain magnetic resonance imaging (MRI) before radiotherapy, so the evaluation of cerebral disease before PCI may have been underestimated [7]. In addition, in 2017, Takahashi et al. [8] reported the results of a study comparing PCI with an MRI follow-up every 3 months vs. an MRI follow-up only in patients with ES-SCLC. The study found no statistically significant difference in OS (13.7 months vs. 11.6 months, HR = 1.27 (CI95% [0.96; 1.68]) between the two populations with an increase in side effects in the PCI group. Despite this trial, European recommendations still support the use of PCI for patients under 75 years old, with a performance status (PS) from 0 to 2, with no progression after chemotherapy [3]. A recently published meta-analysis found that PCI was associated with improved OS and a reduced cumulative incidence of BM in patients with SCLC, regardless of disease stage. But in a subgroup analysis limited to studies in which all patients underwent MRI, excluding patients with BM, PCI was associated with a reduced incidence of BM without an effect on OS [9]. From different adult and pediatric studies, it is known that whole-brain irradiation induces major neurocognitive side effects [10,11,12]. In recent years, advancements in radiotherapy technologies and the availability of MRI have led to stereotactic radiotherapy being a preferred option for brain metastases in various primary cancers, including non-small-cell lung cancer (NSCLC). According to the ASTRO 2021 recommendations [13], whole-brain radiotherapy (WBRT) has largely been replaced by focal treatment whenever possible, without compromising OS [14,15,16] in other cancers. In BM of SCLC, a retrospective study published in 2020 found no difference in OS between radiosurgery and WBRT [17], and a phase 3 trial (NRG CC009) is currently recruiting to answer this question.
Furthermore, the emergence of immunotherapy against SCLC [18,19] with controversial effects on BM raises questions about the role of PCI. Several studies about BM in NSCLC have reported intracranial activity signal and a significant improvement in intracerebral disease control rates with immunotherapy treatment [20,21,22]. Since the ESMO 2021 recommendations [3], the new standard of care in ES-SCLC is a combination of immunotherapy and chemotherapy using atezolizumab or durvalumab in concomitant and maintenance treatment, but there are very limited data on immunotherapy and PCI. The IM-Power 133 study showed that the monoclonal antibody atezolizumab significantly delayed the time to intracranial progression (20.2 months vs. 10.5 months, p = 0.046) compared to a placebo. Although the PCI was allowed in both groups in this trial, only a few patients actually received it [18]. Likewise, in the CASPIAN Trial [19], PCI was allowed only in the standard chemotherapy arm, and only 21 patients out of 269 received it.
In this context of lack of data including immunotherapy in the treatment schedule, we report here a single-center retrospective study evaluating the impact of omitting PCI from real-life treatment, including immunotherapy, on OS and BMFS in patients with ES-SCLC, over the period January 2014 to January 2021.
2. Materials and Methods
Population: This is a retrospective, single-center study of patients treated for ES-SCLC with chemotherapy, at Assistance Publique des Hôpitaux de Marseille (APHM) between January 2014 and January 2021, with an indication of PCI after partial response or stable disease. Inclusion criteria were histologically proven ES-SCLC, PS ≤ 2, age > 18 years, diagnosis between January 2014 and January 2021, and follow-up at APHM. Non-inclusion criteria were the presence of initial brain metastases, and extra-cerebral or cerebral progression either ongoing or immediately after the first treatment, PS ≥ 3. Initial patient characteristics, details of initial treatments, and clinical data were extracted from medical records. The date of diagnosis was the date of histological sampling. The date of last treatment was the date of the last cycle of chemotherapy. Chemotherapy treatment was platinum- and etoposide-based. A reevaluation by a CT scan of chest/abdomen/pelvis and brain imaging (CT or MRI) was scheduled less than 2 months after the last treatment date. Complete or partial response and stability were defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The performance of PCI was modulated according to the patient’s neurocognitive status and was not performed if the patient refused. PCI was delivered using linear accelerators with a mean total dose of 24 Gy. All patients signed an informed consent form. The data used were anonymized, collected from the APHM computer file, and declared to the APHM health data access portal. The study received approval from the Institutional Review Board of the French learned society for respiratory medicine, Société de Pneumologie de Langue Française.
The primary endpoint of the study was OS, while secondary endpoints were BMFS, extra- cerebral metastasis-free survival (ECMFS), and survival without a second cerebral event. A cerebral event was defined as the appearance or progression of one or more brain metastases. The first cerebral event was defined as the date of the first imaging (CT or MRI) showing a suspicious brain lesion. Overall, survival was defined as the time between last treatment and death from any cause, censored at the date of last follow-up. BMFS and ECMFS were defined by the time between the date of inclusion (date of last treatment) and the occurrence of the respective specific events, censored at the date of last follow-up.
Statistical analysis: Descriptive analysis was carried out to compare the main characteristics of the overall population. Patient and treatment characteristics were presented as numbers and percentages for categorical variables, and as means ± standard deviation for continuous variables. The significance of differences was determined using the chi-square test or Fisher’s exact test for categorical variables and using the t-test or Mann–Whitney test for continuous variables, as appropriate. To determine OS, BMFS, and ECMFS, we used the Kaplan–Meier method to estimate median survival with a 95 percent confidence interval and the Cox model to determine hazard ratios (HR) for comparisons between groups (PCI vs. no-PCI) with a 95% confidence interval. Univariate and multivariate Cox analyses were used to identify prognostic factors for OS and BMFS. The multivariate analysis included all univariate significant clinical variables (p < 0.2) according to the performance or not of initial PCI. p < 0.05 was considered significant. The 95% confidence interval was reported. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 21.0 (IBM SPSS Inc., Chicago, IL, USA). As an exploratory analysis, a recursive partitioning analysis (RPA) using a survival tree for OS was generated based upon the most statistically significant variables in the univariate models and additional clinically meaningful variables. RPA divided patients at each step into two groups based on the covariate that provided maximum separation with respect to prognosis and accounted for interactions between factors. The RPA algorithm is based on the optimized binary partition of these subgroups, which enables the classification of patients into successively more homogeneous prognostic groups based on multiple input variables. RPA performs a ten-fold cross-classification by default to help assess the reliability of the tree model. The full sample is randomly divided into 10 sub-samples. Internally, the full RPA tree is carried out with 90% of the full sample, and the remaining 10% of the sample is used as a validation dataset to calculate a cross-classification error rate. This procedure is repeated 10 times, each time with 9 subsets as the modeling dataset and the remaining 1 subset as the validation dataset. Additional technical details can be found in this document [23].
3. Results
A total of 287 patients were treated for SCLC between 2014 and 2021 at APHM. Among them, 79 initially had BM. One hundred one either developed BM or extra-cranial metastases before the date for PCI or died during chemotherapy. A total of 107 patients were eligible to receive PCI, of whom 56 were initially diagnosed with ES-SCLC and were included in our study. Among these patients, 25 received PCI, 31 had no PCI, and 18 received immunotherapy. The initial characteristics of the population are described in Table 1.
Table 1.
Population characteristics.
| No PCI (N = 31) | PCI (N = 25) | p | |
|---|---|---|---|
| Age mean (min–max) | 64 (47–81) | 65 (47–87) | 0.656 |
| Smoking status | 0.580 | ||
| - Non-smoker | 1 (3.2%) | 0 | |
| - Former smoker | 23 (74.2%) | 15 (69.1%) | |
| - Current smoker | 7 (22.6%) | 9 (37.5%) | |
| Sex | 0.580 | ||
| - Men | 18 (58.1%) | 17 (68%) | |
| - Women | 13 (41.9%) | 8 (32%) | |
| Clinical tumor status | 0.450 | ||
| - T1 | 3(9.7%) | 0 | |
| - T2 | 6 (19.4%) | 4 (17.4%) | |
| - T3 | 13 (41.9%) | 9 (39.1%) | |
| - T4 | 9 (29%) | 10 (43.5%) | |
| Clinical node status | 0.112 | ||
| - N0 | 3 (9.7%) | 0 | |
| - N1 | 3 (9.7%) | 2 (8.3%) | |
| - N2 | 18 (58.1%) | 10 (41.7%) | |
| - N3 | 7 (22.6%) | 12 (50%) | |
| Clinical metastasis status | 0.688 | ||
| - M0 | 3 (9.7%) | 4 (16%) | |
| - M1 | 28 (90.3%) | 21 (84%) | |
| Stage | 0.606 | ||
| - Stage III | 3 (9.7%) | 4 (16%) | |
| - Stage IV | 28 (90.3%) | 21 (84%) | |
| Initial PS | 0.622 | ||
| - 0/1 | 25 (80.6%) | 21 (84%) | |
| - 2 | 6 (19.3%) | 4 (16%) | |
| Neurological history | 6 (19.4%) | 3 (33.3%) | 0.716 |
| Response befor PCI | 0.155 | ||
| - Stable | 0 | 2 (8%) | |
| - Partial response | 27 (87.1%) | 22 (88%) | |
| - Complete response | 4 (12.9%) | 1 (4%) | |
| Immunotherapy | 14 (45.2%) | 4 (16%) | 0.024 |
| Initial MRI | 15 (48.4%) | 12 (48%) | 0.977 |
| Diagnostic year (mean) | 2018 | 2015 | 0.000 |
| Monocyte to lymphocyte ratio MLR (mean) | 0.87 | 0.35 | 0.178 |
| Neutrophil to lymphocyte ratio NLR (mean) | 4.9 | 4.8 | 0.803 |
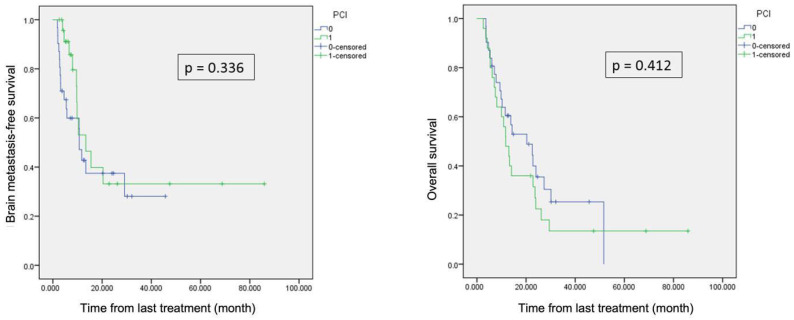
Median follow-up was 16 months for the whole population. There was no significant difference in terms of BMFS (p = 0.336) and OS (p = 0.412) between the two groups Figure 1. Median OS and BMFS were, respectively, 11.7 and 13.4 months in patients with PCI, and 20.3 and 10.7 months in patients without PCI. Median ECMFS was also comparable in the two groups, with 4.6 and 4.2 months, respectively, in the no-PCI and PCI groups (p = 0.446).
Figure 1.
(left) Brain metastasis-free survival; (right) overall survival. Patients with PCI in green, and patients without PCI in blue.
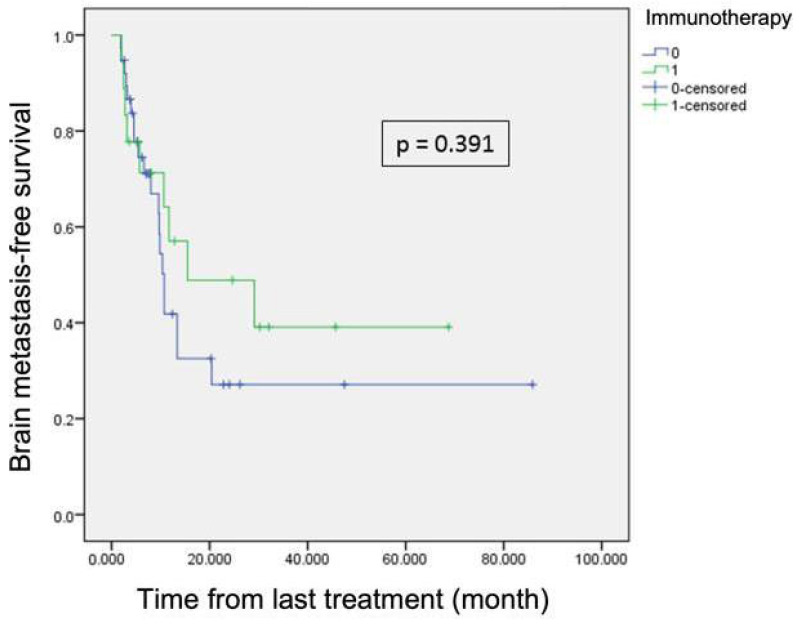
At the first cerebral event, the PS, size, and number of brain metastases were not statistically different between the two groups. PCI had no significant impact on the occurrence of a second cerebral event either from the inclusion date (p = 0.77) or from the first event (p = 0.669). Because the two groups were not balanced regarding immunotherapy, we also compared patients receiving immunotherapy or not in terms of survival. There was a trend in favor of immunotherapy but it was still not significant, in terms of OS (11.6 vs. 23.6 months p = 0.054) and ECMFS (4 vs. 5.7 months, p = 0.052). We observed a trend towards longer BMFS in immunotherapy-treated patients Figure 2, which, however, did not reach statistical significance.
Figure 2.
Brain metastasis-free survival for patients with immunotherapy in green and without in blue.
We performed univariate and multivariate analysis to determine the prognostic factors for OS and BMFS, including the PCI and adjuvant immunotherapy status. Univariate and multivariate analysis are summarized in Table 2 and Table 3. In multivariate analysis, only the ratio MLR was found to be associated with BMFS (p = 0.034), and PS ≥ 2 was found to be associated with OS (p = 0.014).
Table 2.
Univariate and multivariate analysis of BMFS.
| Univariate Analysis | ||
| HR (IC95%) | p-value | |
| Age | 0.99 (0.96–1.04) | 0.820 |
| Sex | 0.99 (0.47–2.10) | 0.998 |
| Initial PS (0/1 vs. 2) | 1.74 (0.64–4.72) | 0.275 |
| Neurological history | 1.17 (0.44–3.07) | 0.754 |
| Response before PCI: | ||
| - Partial response vs. stable | 1.54 (0.21–11.44) | 0.672 |
| - Complete response vs. stable | 1.24 (0.11–13.79) | 0.858 |
| Adjuvant immunotherapy | 0.71 (0.32–1.57) | 0.395 |
| PCI | 0.69 (0.33–1.47) | 0.341 |
| Hemoglobin | 1.05 (0.86–1.29) | 620 |
| MLR < 0.12 | 1.17 (0.99–1.39) | 0.069 |
| Multivariate Analysis | ||
| Adjuvant immunotherapy | 0.41 (0.15–1.10) | 0.077 |
| PCI | 0.52 (0.22–1.24) | 0.140 |
| MLR < 0.12 | 1.21 (1.01–1.45) | 0.034 |
Table 3.
Univariate and multivariate analysis of OS.
| Univariate Analysis | ||
| HR (IC95%) | p-value | |
| Age | 1.03 (0.99–1.7) | 0.057 |
| Sex | 1.7 (0.89–3.25) | 0.106 |
| Initial PS (0/1 vs. 2) | 3.97 (1.59–9.89) | 0.003 |
| Neurological history | 1.93 (0.92–4.06) | 0.082 |
| Response before PCI: | ||
| - Partial response vs. stable | 2.47 (0.34–18.07) | 0.373 |
| - Complete response vs. stable | 1.37 (0.14–13.27) | 0.785 |
| Adjuvant immunotherapy | 0.51 (0.25–1.03) | 0.060 |
| PCI | 1.28 (0.70–2.33) | 0.417 |
| Hemoglobin | 1.11 (0.92–1.09) | 0.267 |
| MLR < 0.12 | 1.05 (0.90–1.23) | 0.515 |
| Multivariate Analysis | ||
| Age | 1.01 (0.96–1.05) | 0.780 |
| Sex | 1.18 (0.78–4.04) | 0.675 |
| Initial PS (0/1 vs. 2) | 2.74 (1.23–6.13) | 0.014 |
| Neurological history | 1.77 (0.79–4.05) | 0.173 |
| Adjuvant immunotherapy | 0.59 (0.28–1.26) | 0.177 |
| PCI | 1.20 (0.60–2.41) | 0.601 |
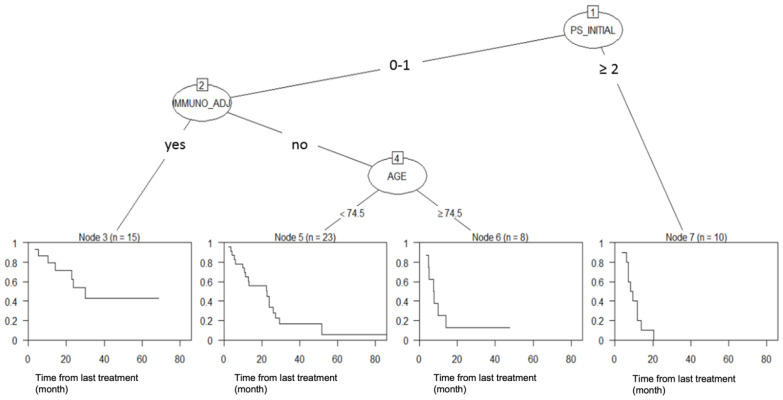
We performed a RPA to identify predictors of survival. The RPA divided patients into four risk groups according to PS, immunotherapy treatment, and age at treatment but not PCI (Figure 3).
Figure 3.
Recursive partitioning analysis of overall survival.
4. Discussion
ESMO 2021 recommendations are still in favor of performing PCI on patients with no progressive disease [3] after chemotherapy and remain unclear about PCI for patients treated with immunotherapy. In this context, the current study is one of the rare real-life studies reporting the results of omitting PCI for ES-SCLC integrating immunotherapy in the first-line strategy. We did not find that PCI had a positive impact in terms of OS and BMFS in our study population. Our data were in contradiction with the 2007 EORTC trial [6], which reported a benefit with PCI in terms of OS and BMFS. Moreover, OS was twice as long in both of our groups compared to this trial. In our study, median OS was 11.7 months for patients who received PCI and 20.3 months for those who did not, compared to only 6.7 months and 5.4 months, respectively, in the 2007 trial. This difference can be partly explained by poorer patient selection, especially in the absence of pre-therapeutic MRI, but mainly by the absence of immunotherapy at that time, which contributed to improving the OS of our patients.
However, our results in terms of OS remained consistent with more recent literature data. In a Japanese study [8] of ES-SCLC, median survival was comparable, with 11.6 months in the PCI group and 13.7 months in the MRI group, vs. 11.7 months and 20.3 months, respectively, in our study. Nevertheless, in their trial, PCI had an impact on reducing the cumulative incidence of BM, which was not observed in our study. It is important to note that the primary endpoint was OS for their trial, but it was stopped early at a planned interim analysis due to the demonstration of the futility of PCI.
The introduction of immunotherapy during this study period can explain the lack of impact of PCI on BMFS. The introduction of immunotherapy for patients after positive phase III trials has changed medical practices, and for these patients, PCI was more frequently omitted, as allowed in the trials guidelines [18,19]. In summary, our results could be interpreted either by the introduction of immunotherapy or by a lack of efficiency of PCI, supporting the idea that immunotherapy has modified the natural course of brain disease. Furthermore, in our RPA results, immunotherapy stands out as the second most important factor affecting overall survival, following PS, while PCI was not established as a relevant factor. These results support the impact of immunotherapy and underline the lack of PCI efficiency.
The PACIFIC trial showed that adjuvant therapy with the human monoclonal antibody durvalumab reduced the incidence of BM from 11.8% to 6.3% [24] in NSCLC, as was observed in the IM-POWER trial for SCLC and other trials [25]. Thus, the use of immunotherapy may potentially negate the marginal OS benefit of adding PCI.
The relationship between immunotherapy and brain control is still debated in the literature. No available randomized trials can help make the decision to defer brain irradiation in patients receiving immunotherapy. In this context, ESMO 2021 recommendations [3] still support PCI in progression-free patients without BM after chemotherapy who are under 75 years old and have a PS ≤ 2. However, they agree on the lack of appropriate data in patients undergoing immunotherapy and propose to share decision making for these patients. Meanwhile, NCCN currently recommends shared decision making regarding PCI for patients with ES-SCLC with a response to initial treatment with or without immunotherapy [26].
In our study, we observed that the MLR ratio had a potential impact on BMFS. A lower MLR (<0.12) was associated with an increased risk of cerebral progression with an HR of 1.21 (1.01–1.45). These data are comparable with the existing literature, particularly with the use of this ratio in nomograms attempting to predict the occurrence of BM in limited stage SCLC [27,28]. In contrast, other studies find a protective effect of a low MLR ratio in NSCLC [29,30] and in all other histologies [31]. Further studies are needed to validate the real role of the MLR ratio. However, the use of a biomarker such as MLR could help to predict the risk of cerebral progression and then to select high-risk patients who could benefit from PCI.
The limitations of our study primarily concern its retrospective nature and the small size of our series. The difference between the two groups regarding immunotherapy is also a limitation that prevents us from drawing definitive conclusions about the absence of the benefit of PCI on BMFS or the possible benefit of immunotherapy. Furthermore, due to ESMO 2021 recommendations, exhaustive monitoring via brain MRI was not systematically performed [3]. However, this study represents one of the rare clinical investigations reporting data on PCI in the context of immunotherapy for ES-SCLC and reports a strong signal in favor of omitting PCI. The results of Primalung (NCT0479025) and Swog-1827 are eagerly awaited.
5. Conclusions
In conclusion, the clinical results of our study do not show any benefit of PCI in terms of OS and BMFS for patients with ES-SCLC. Considering the current systemic strategies, a therapeutic de-escalation should be assessed in randomized trials that incorporate immunotherapy.
Acknowledgments
We thank Justine Buand for English language revision.
Abbreviations
The following abbreviations are used in this manuscript:
| SCLC | Small-cell lung cancer |
| ES-SCLC | Extensive-stage small-cell lung cancer |
| PCI | Prophylactic cerebral irradiation |
| BM | Brain metastases |
| APHM | Assistance Publique des Hôpitaux de Marseille |
| OS | Overall survival |
| BMFS | Brain metastasis-free survival |
| PS | Performans status |
| MLR | Monocyte lymphocyte ratio |
| HR | Hazard ratio |
| RPA | Recursive partitioning analysis |
| MRI | Magnetic resonance imaging |
| NSCLC | Non-small-cell lung cancer |
| WBRT | Whole-brain radiotherapy |
| ECMFS | Extra cerebral metastasis-free survival |
Author Contributions
Conceptualization, L.P.; methodology, C.B.; validation, L.P. and L.G.; formal analysis, M.B.; investigation, A.D.; resources, A.Z., B.K., A.M., E.G., J.P. and P.T.; writing—original draft preparation, A.D.; and supervision, L.P., S.B. and X.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Institutional Review Board of the French learned society for respiratory medicine, Société de Pneumologie de Langue Française (protocol code CEPRO 2023-051 and 2 January 2024).
Informed Consent Statement
Informed consent was obtained from all of the subjects involved in the study.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
S.B. was supported by a grant from the French government, managed by the National Research Agency (ANR), under the France 2030 program, reference ANR-22-PESN-0017.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Govindan R., Page N., Morgensztern D., Read W., Tierney R., Vlahiotis A., Spitznagel E.L., Piccirillo J. Changing epidemiology of small cell lung cancer in the United States over the last 30 years: Analysis of the Surveillance, Epidemiologic, and End Results database. J. Clin. Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Nugent J.L., Bunn P.A., Jr., Matthews M.J., Ihde D.C., Cohen M.H., Gazdar A., Minna J.D. CNS metastases in small cell bronchogenic carcinoma: Increasing frequency and changing pattern with lengthening survival. Cancer. 1979;44:1885–1893. doi: 10.1002/1097-0142(197911)44:5<1885::AID-CNCR2820440550>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Dingemans A., Früh M., Ardizzoni A., Besse B., Faivre-Finn C., Hendriks L.E., Lantuejoul S., Peters S., Reguart N., Rudin C.M., et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021;32:839–853. doi: 10.1016/j.annonc.2021.03.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aupérin A., Arriagada R., Pignon J.-P., Le Péchoux C., Gregor A., Stephens R.J., Kristjansen P.E.G., Johnson B.E., Ueoka H., Wagner H., et al. Prophylactic Cranial Irradiation for Patients with Small-Cell Lung Cancer in Complete Remission. N. Engl. J. Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 5.Meert A.-P., Paesmans M., Berghmans T., Martin B., Mascaux C., Vallot F., Verdebout J.M., Lafitte J.J., Sculieret J.P. Prophylactic cranial irradiation in small cell lung cancer: A systematic review of the literature with meta-analysis. BMC Cancer. 2001;1:5. doi: 10.1186/1471-2407-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slotman B., Faivre-Finn C., Kramer G., Rankin E., Snee M., Hatton M., Postmus P., Collette L., Musat E., Senan S. Prophylactic Cranial Prophylactic cranial irradiation in extensive small-cell lung cancer. N. Engl. J. Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 7.Seute T., Leffers P., ten Velde G.P.M., Twijnstra A. Detection of brain metastases from small cell lung cancer: Consequences of changing imaging techniques (CT vs. MRI) Cancer. 2008;112:1827–1834. doi: 10.1002/cncr.23361. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T., Yamanaka T., Seto T., Harada H., Nokihara H., Saka H., Nishio M., Kaneda H., Takayama K., Ishimoto O., et al. Prophylactic cranial irradiation vs. observation in patients with extensive-disease small-cell lung cancer: A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:663–671. doi: 10.1016/S1470-2045(17)30230-9. [DOI] [PubMed] [Google Scholar]
- 9.Gaebe K., Erickson A.W., Li A.Y., Youssef A.N., Sharma B., Chan K.K.W., Lok B.H., Dasa S. Re-examining prophylactic cranial irradiation in small cell lung cancer: A systematic review and meta-analysis. eClinicalMedicine. 2024;67:102396. doi: 10.1016/j.eclinm.2023.102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfson A.H., Bae K., Komaki R., Meyers C., Movsas B., Le Pechoux C., Werner-Wasik M., Videtic G., Garces Y.I., Choyet H. Primary Analysis of a Phase II Randomized Trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of Different Total Doses and Schedules of Prophylactic Cranial Irradiation on Chronic Neurotoxicity and Quality of Life for Patients With Limited-Disease Small-Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:77–84. doi: 10.1016/j.ijrobp.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., Bentzen S.M., Renschler M., Mehta M.P. Regression After Whole-Brain Radiation Therapy for Brain Metastases Correlates With Survival and Improved Neurocognitive Function. J. Clin. Oncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 12.Simó M., Vaquero L., Ripollés P., Jové J., Fuentes R., Cardenal F., Rodríguez-Fornells A., Bruna J. Brain damage following prophylactic cranial irradiation in lung cancer survivors. Brain Imaging Behav. 2016;10:283–295. doi: 10.1007/s11682-015-9393-5. [DOI] [PubMed] [Google Scholar]
- 13.Gondi V., Bauman G., Bradfield L., Burri S.H., Cabrera A.R., Cunningham D.A., Eaton B.R., Hattangadi-Gluth J.A., Kim M.M., Kotecha R., et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022;12:265–282. doi: 10.1016/j.prro.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Kocher M., Soffietti R., Abacioglu U., Villà S., Fauchon F., Baumert B.G., Fariselli L., Tzuk-Shina T., Kortmann R.D., Carrie C., et al. Adjuvant Whole-Brain Radiotherapy Versus Observation After Radiosurgery or Surgical Resection of One to Three Cerebral Metastases: Results of the EORTC 22952-26001 Study. J. Clin. Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown P.D., Jaeckle K., Ballman K.V., Farace E., Cerhan J.H., Anderson S.K., Carrero X., Barker F., Deming R., Burri S., et al. Effect of Radiosurgery Alone vs. Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown P.D., Ballman K.V., Cerhan J.H., Anderson S.K., Carrero X.W., Whitton A.C., Greenspoon J., Parney I.F., Laack N.N.I., Ashman J.B., et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rusthoven C.G., Yamamoto M., Bernhardt D., Smith D.E., Gao D., Serizawa T., Yomo S., Aiyama H., Higuchi Y., Shuto T., et al. Evaluation of First-line Radiosurgery vs. Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA. 2020;6:1028–1037. doi: 10.1001/jamaoncol.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn L., Mansfield A.S., Szczęsna A., Havel L., Krzakowski M., Hochmair M.J., Huemer F., Losonczy G., Johnson M.L., Nishio M., et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 19.Paz-Ares L., Dvorkin M., Chen Y., Reinmuth N., Hotta K., Trukhin D., Statsenko G., Hochmair M.J., Özgüroğlu M., Ji J.H., et al. Durvalumab plus platinum–etoposide vs. platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 20.Loiola de Alencar V.T., Camandaroba M., Pirolli R., Fogassa C., Cordeiro de Lima V.C. Immunotherapy as Single Treatment for Patients With NSCLC With Brain Metastases: A Systematic Review and Meta-Analysis-the META-L-BRAIN Study. J. Thorac. Oncol. 2021;16:1379–1391. doi: 10.1016/j.jtho.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S., Xie J., Huang Z., Deng L., Wu L., Yu J., Meng X. Anti-PD-(L)1 immunotherapy for brain metastases in non-small cell lung cancer: Mechanisms, advances, and challenges. Cancer Lett. 2021;502:166–179. doi: 10.1016/j.canlet.2020.12.043. [DOI] [PubMed] [Google Scholar]
- 22.Pathak R., Amini A., Hill A., Massarelli E., Salgia R. Immunotherapy in Non-Small Cell Lung Cancer Patients with Brain Metastases: Clinical Challenges and Future Directions. Cancers. 2021;13:3407. doi: 10.3390/cancers13143407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therneau T.M., Atkinson E.J. An Introduction to Recursive Partitioning Using the RPART Routine. Mayo Clinic Fundation; Rochester, NY, USA: 1997. [(accessed on 20 November 2024)]. Available online: https://www.mayo.edu/research/documents/rpartminipdf/doc-10027257. [Google Scholar]
- 24.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., Kurata T., Chiappori A., Lee K.H., de Wit M., et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 25.Long G.V., Atkinson V., Lo S., Sandhu S., Guminski A.D., Brown M.P., Wilmott J.S., Edwards J., Gonzalez M., Scolyer R.A., et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018;9:672–681. doi: 10.1016/S1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 26.Ganti A.K.P., Loo B.W., Bassetti M., Blakely C., Chiang A., D’Amico T.A., D’Avella C., Dowlati A., Downey R.J., Edelman M., et al. Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021;19:1441–14464. doi: 10.6004/jnccn.2021.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J., Ke D., Yu Y., Lin H., Zheng Q., Li H., Zheng H., Liu L., Wang Z., Wuet Y., et al. A New Nomogram and Risk Stratification of Brain Metastasis by Clinical and Inflammatory Parameters in Stage III Small Cell Lung Cancer Without Prophylactic Cranial Irradiation. Front. Oncol. 2022;12:882744. doi: 10.3389/fonc.2022.882744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W., Ding C., Sheng W., Wan Q., Cui Z., Qi G., Liu Y. Development and validation of a nomogram for the prediction of brain metastases in small cell lung cancer. Clin. Respir. J. 2023;17:456–467. doi: 10.1111/crj.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li A., Mu X., He K., Wang P., Wang D., Liu C., Yu J. Prognostic value of lymphocyte-to-monocyte ratio and systemic immune-inflammation index in non-small-cell lung cancer patients with brain metastases. Future Oncol. 2020;16:2433–2444. doi: 10.2217/fon-2020-0423. [DOI] [PubMed] [Google Scholar]
- 30.Chen T., Tang M., Zhou Y., Wang Z., Li S., Wang H., Lu Y., Wang J., Shen W. Pretreatment lymphocyte-to-monocyte ratio as a prognostic factor and influence on dose-effect in fractionated stereotactic radiotherapy for oligometastatic brain metastases in non-small cell lung cancer patients. Front. Oncol. 2023;16:2433–2444. doi: 10.3389/fonc.2023.1216852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishijima T., Muss H., Shachar S.S., Tamura K., Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2015;41:971–978. doi: 10.1016/j.ctrv.2015.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.