Abstract
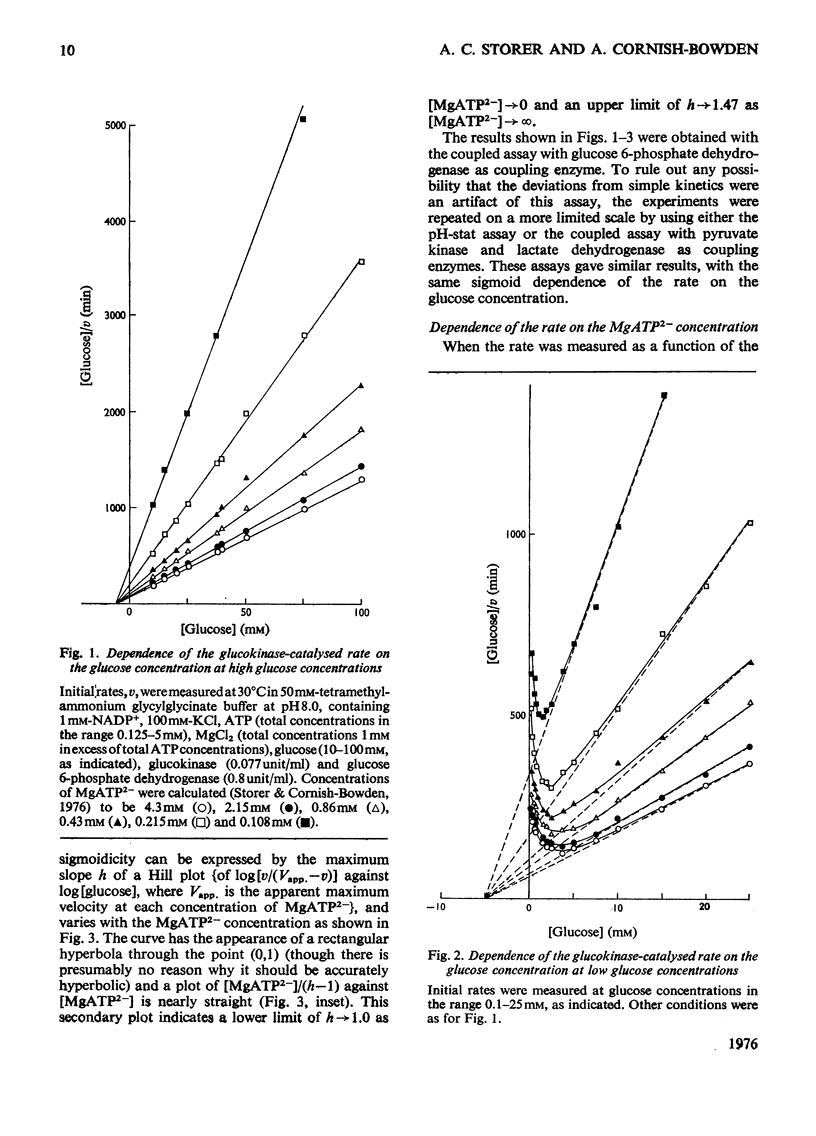
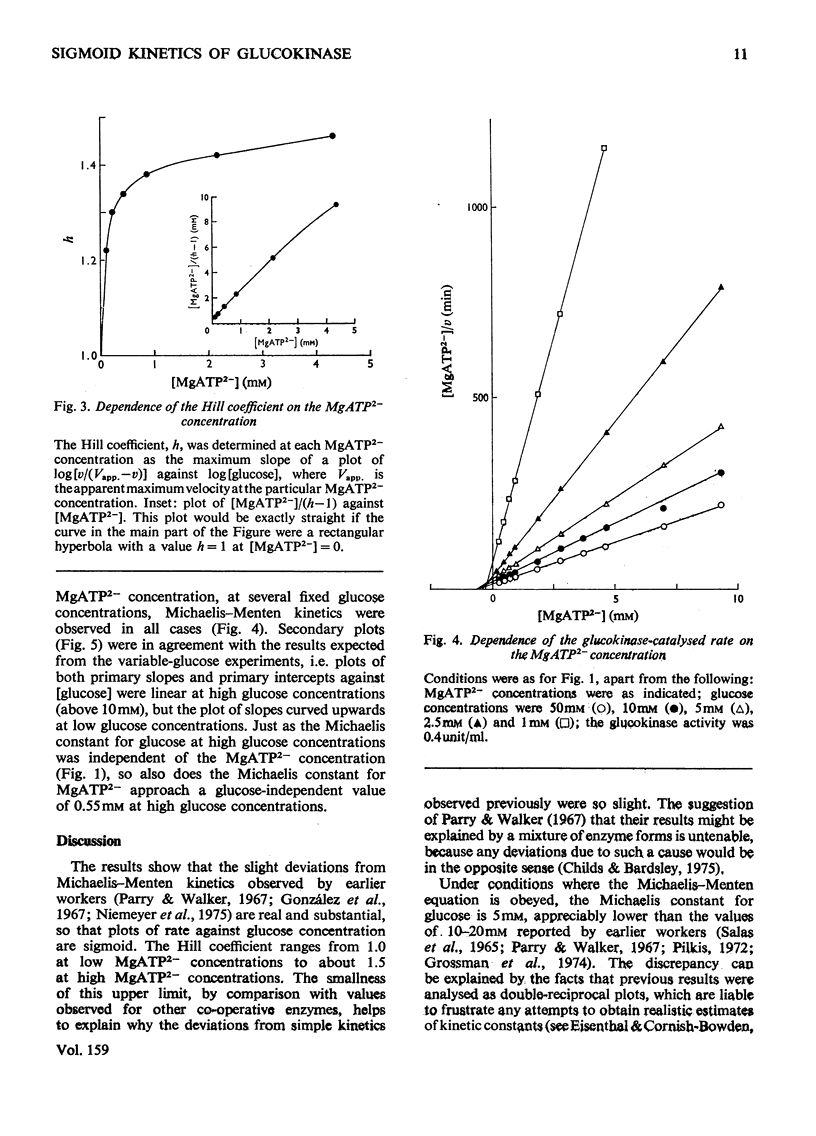
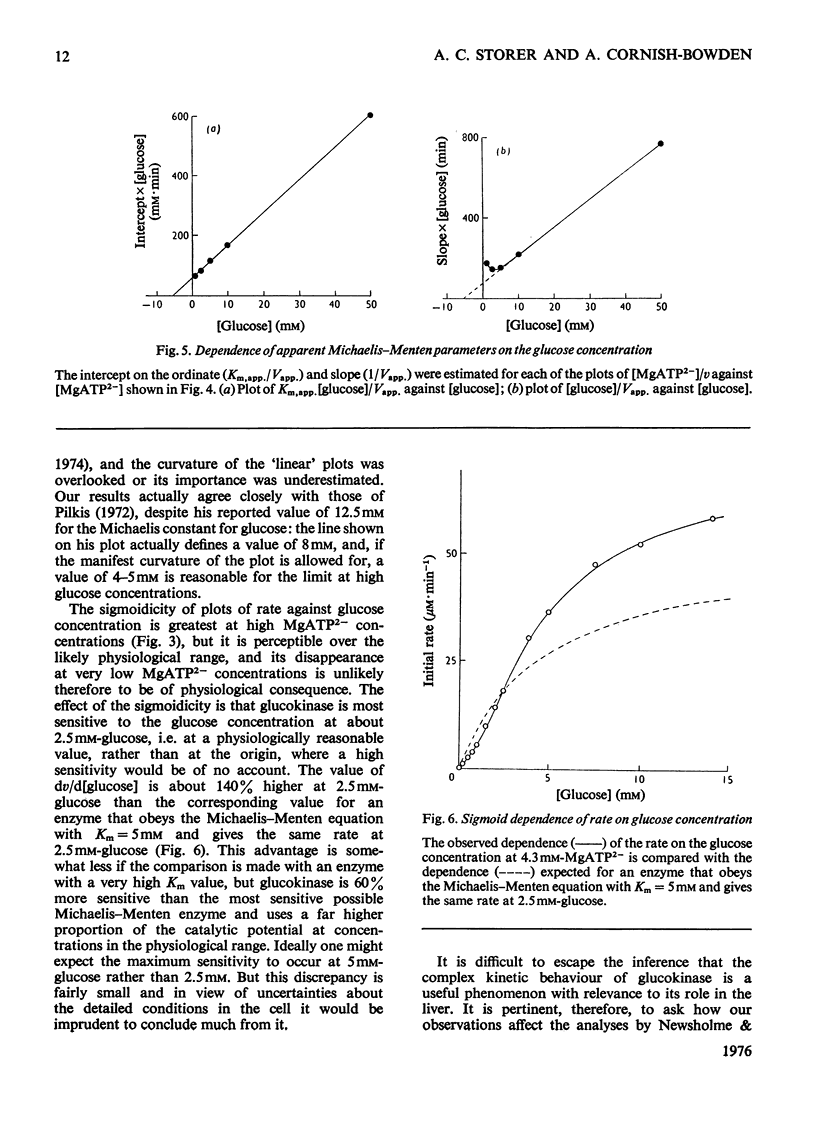
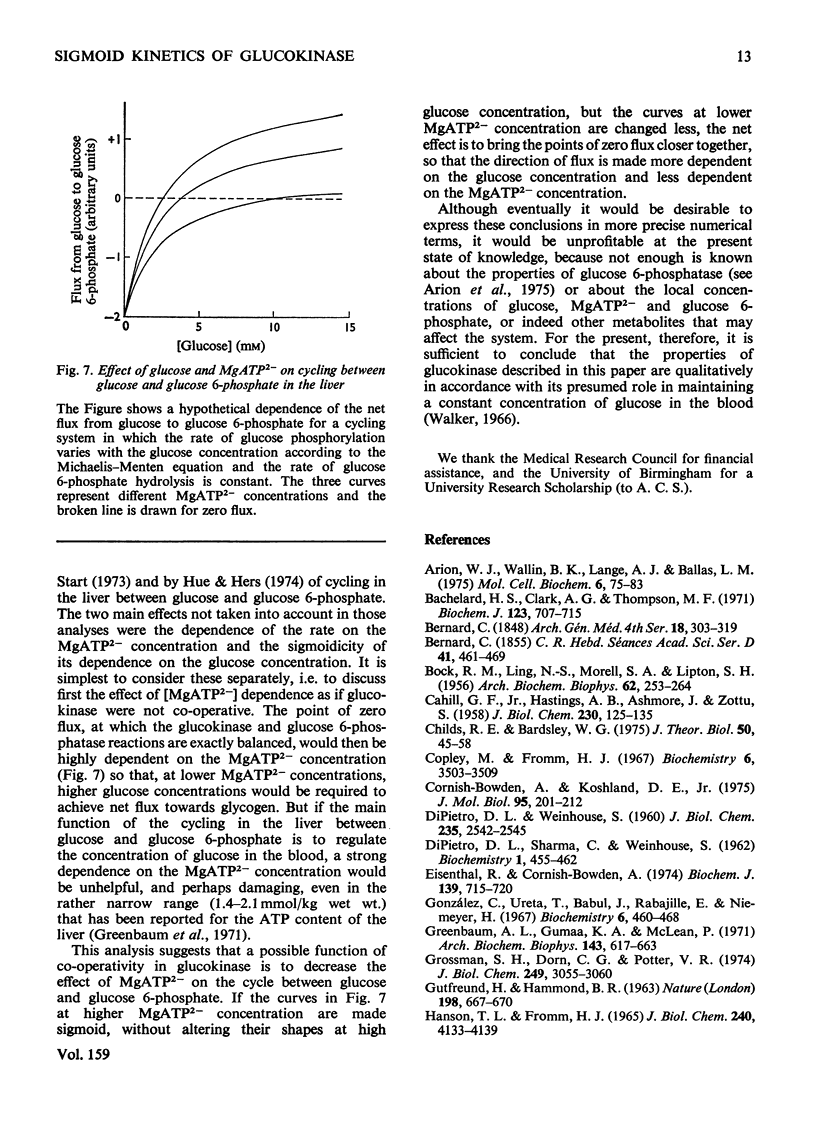
The kinetics of glucokinase from rat liver were studied over wide ranges of glucose and MgATP2- concentrations. The initial rate shows a co-operative dependence on the glucose concentration, with Hill coefficients in the range 1.2-1.5. The degree of glucose co-operativity increases with the MgATP2- concentration, but no co-operativity was detected for the dependence of the rate on the MgATP2- concentration. The effects observed occur at physiologically reasonable concentrations of glucose and MgATP2- and are consistent with the presumed function of glucokinase in maintaining a constant concentration of glucose in the blood.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion W. J., Wallin B. K., Lange A. J., Ballas L. M. On the involvement of a glucose 6-phosphate transport system in the function of microsomal glucose 6-phosphatase. Mol Cell Biochem. 1975 Feb 28;6(2):75–83. doi: 10.1007/BF01732001. [DOI] [PubMed] [Google Scholar]
- BOCK R. M., LING N. S., MORELL S. A., LIPTON S. H. Ultraviolet absorption spectra of adenosine-5'-triphosphate and related 5'-ribonucleotides. Arch Biochem Biophys. 1956 Jun;62(2):253–264. doi: 10.1016/0003-9861(56)90123-0. [DOI] [PubMed] [Google Scholar]
- Bachelard H. S., Clark A. G., Thompson M. F. Cerebral-cortex hexokinase. Elucidation of reaction mechanisms by substrate and dead-end inhibitor kinetic analysis. Biochem J. 1971 Aug;123(5):707–715. doi: 10.1042/bj1230707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAHILL G. F., Jr, HASTINGS A. B., ASHMORE J., ZOTTU S. Studies on carbohydrate metabolism in rat liver slices. X. Factors in the regulation of pathways of glucose metabolism. J Biol Chem. 1958 Jan;230(1):125–135. [PubMed] [Google Scholar]
- Childs R. E., Bardsley W. G. Allosteric and related phenomena: an analysis of sigmoid and non-hyperbolic functions. J Theor Biol. 1975 Mar;50(1):45–58. doi: 10.1016/0022-5193(75)90023-5. [DOI] [PubMed] [Google Scholar]
- Copley M., Fromm H. J. Kinetic studies of the brain hexokinase reaction. A reinvestigation with the solubilized bovine enzyme. Biochemistry. 1967 Nov;6(11):3503–3509. doi: 10.1021/bi00863a023. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Koshland D. E., Jr Diagnostic uses of the Hill (Logit and Nernst) plots. J Mol Biol. 1975 Jun 25;95(2):201–212. doi: 10.1016/0022-2836(75)90390-3. [DOI] [PubMed] [Google Scholar]
- DIPIETRO D. L., SHARMA C., WEINHOUSE S. Studies on glucose phosphorylation in rat liver. Biochemistry. 1962 May 25;1:455–462. doi: 10.1021/bi00909a014. [DOI] [PubMed] [Google Scholar]
- DIPIETRO D. L., WEINHOUSE S. Hepatic glucokinase in the fed, fasted, and alloxan-diabetic rat. J Biol Chem. 1960 Sep;235:2542–2545. [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTFREUND H., HAMMOND B. R. Records of pH changes during enzyme reactions and kinetic studies with yeast hexokinase. Nature. 1963 May 18;198:667–670. doi: 10.1038/198667a0. [DOI] [PubMed] [Google Scholar]
- González C., Ureta T., Babul J., Rabajille E., Niemeyer H. Characterization of isoenzymes of adenosine triphosphate: D-hexose 6-phosphotransferase from rat liver. Biochemistry. 1967 Feb;6(2):460–468. doi: 10.1021/bi00854a014. [DOI] [PubMed] [Google Scholar]
- Greenbaum A. L., Gumaa K. A., McLean P. The distribution of hepatic metabolites and the control of the pathways of carbohydrate metabolism in animals of different dietary and hormonal status. Arch Biochem Biophys. 1971 Apr;143(2):617–663. doi: 10.1016/0003-9861(71)90247-5. [DOI] [PubMed] [Google Scholar]
- Grossman S. H., Dorn C. G., Potter V. R. The preparation and characterization of pure rat liver glucokinase. J Biol Chem. 1974 May 25;249(10):3055–3060. [PubMed] [Google Scholar]
- Hanson T. L., Fromm H. J. Rat skeletal muscle hexokinase. I. Kinetics and reaction mechanism. J Biol Chem. 1965 Nov;240(11):4133–4139. [PubMed] [Google Scholar]
- Holroyde M. J., Allen M. B., Storer A. C., Warsy A. S., Chesher J. M., Trayer I. P., Cornish-Bowden A., Walker D. G. The purification in high yield and characterization of rat hepatic glucokinase. Biochem J. 1976 Feb 1;153(2):363–373. doi: 10.1042/bj1530363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Hers H. G. Utile and futile cycles in the liver. Biochem Biophys Res Commun. 1974 Jun 4;58(3):540–548. doi: 10.1016/s0006-291x(74)80454-7. [DOI] [PubMed] [Google Scholar]
- LUCAS N., KING H. K., BROWN S. J. Substrate attachment in enzymes. The interaction of pyridoxal phosphate with amino acids. Biochem J. 1962 Jul;84:118–124. doi: 10.1042/bj0840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer H., de la Luz Cárdenas M., Rabajille E., Ureta T., Clark-Turri L., Peñaranda J. Sigmoidal kinetics of glucokinase. Enzyme. 1975;20(6):321–333. doi: 10.1159/000458957. [DOI] [PubMed] [Google Scholar]
- Parry M. J., Walker D. G. Further properties and possibel mechanism of action of adenosine 5'-triphosphate-D-glucose 6-phosphotransferase from rat liver. Biochem J. 1967 Nov;105(2):473–482. doi: 10.1042/bj1050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. J., Walker D. G. Purification and properties of adenosine 5'-triphospae-D-glucose 6-phosphotransferase from rat liver. Biochem J. 1966 May;99(2):266–274. doi: 10.1042/bj0990266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic studies of the formation and ionization of the magnesium(II) complexes of ADP and ATP over the pH range 5 to 9. J Am Chem Soc. 1966 Jun 20;88(12):2631–2640. doi: 10.1021/ja00964a002. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J. Rat hepatic glucokinase: improved purification and some properties. Arch Biochem Biophys. 1972 Apr;149(2):349–360. doi: 10.1016/0003-9861(72)90333-5. [DOI] [PubMed] [Google Scholar]
- SALAS J., SALAS M., VINUELA E., SOLS A. GLUCOKINASE OF RABBIT LIVER. J Biol Chem. 1965 Mar;240:1014–1018. [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. The kinetics of coupled enzyme reactions. Applications to the assay of glucokinase, with glucose 6-phosphate dehydrogenase as coupling enzyme. Biochem J. 1974 Jul;141(1):205–209. doi: 10.1042/bj1410205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton C. W., Cornish-Bowden A., Brocklehurst K., Crook E. M. Kinetics of the hydrolysis of N-benzoyl-L-serine methyl ester catalysed by bromelain and by papain. Analysis of modifier mechanisms by lattice nomography, computational methods of parameter evaluation for substrate-activated catalyses and consequences of postulated non-productive binding in bromelain- and papain-catalysed hydrolyses. Biochem J. 1974 Aug;141(2):365–381. doi: 10.1042/bj1410365. [DOI] [PMC free article] [PubMed] [Google Scholar]


