Abstract
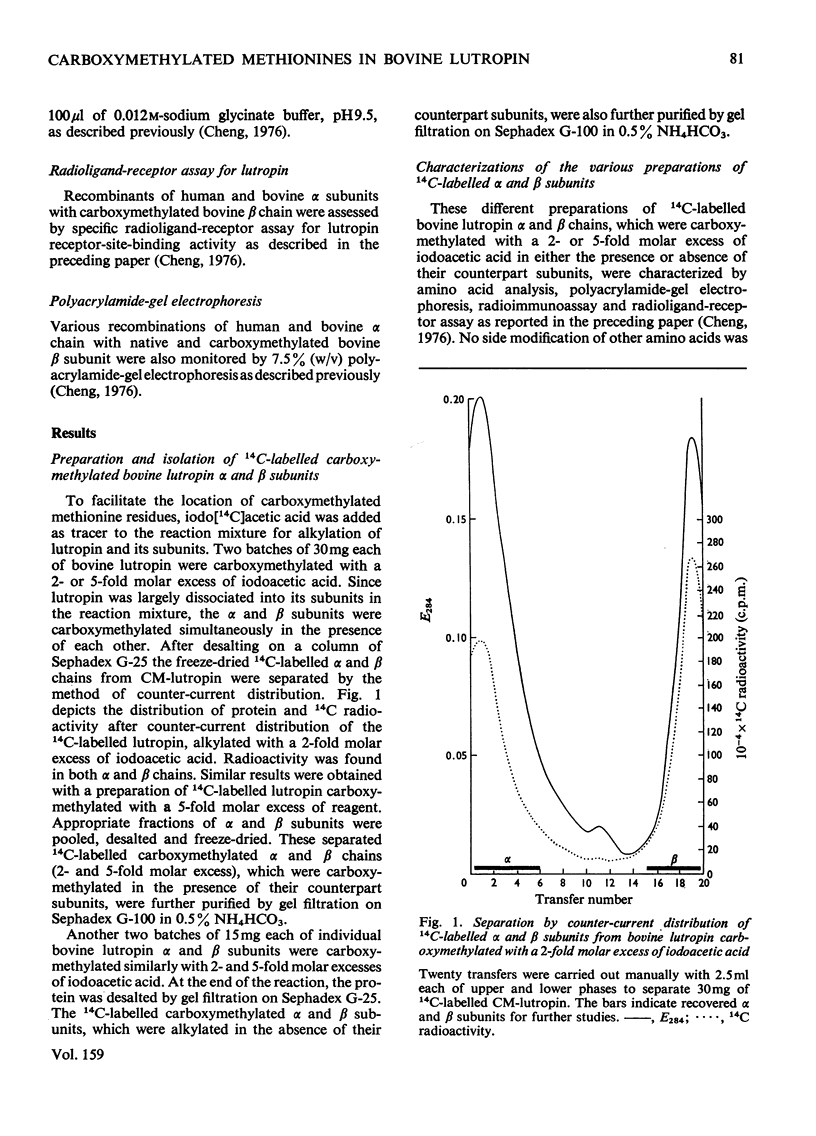
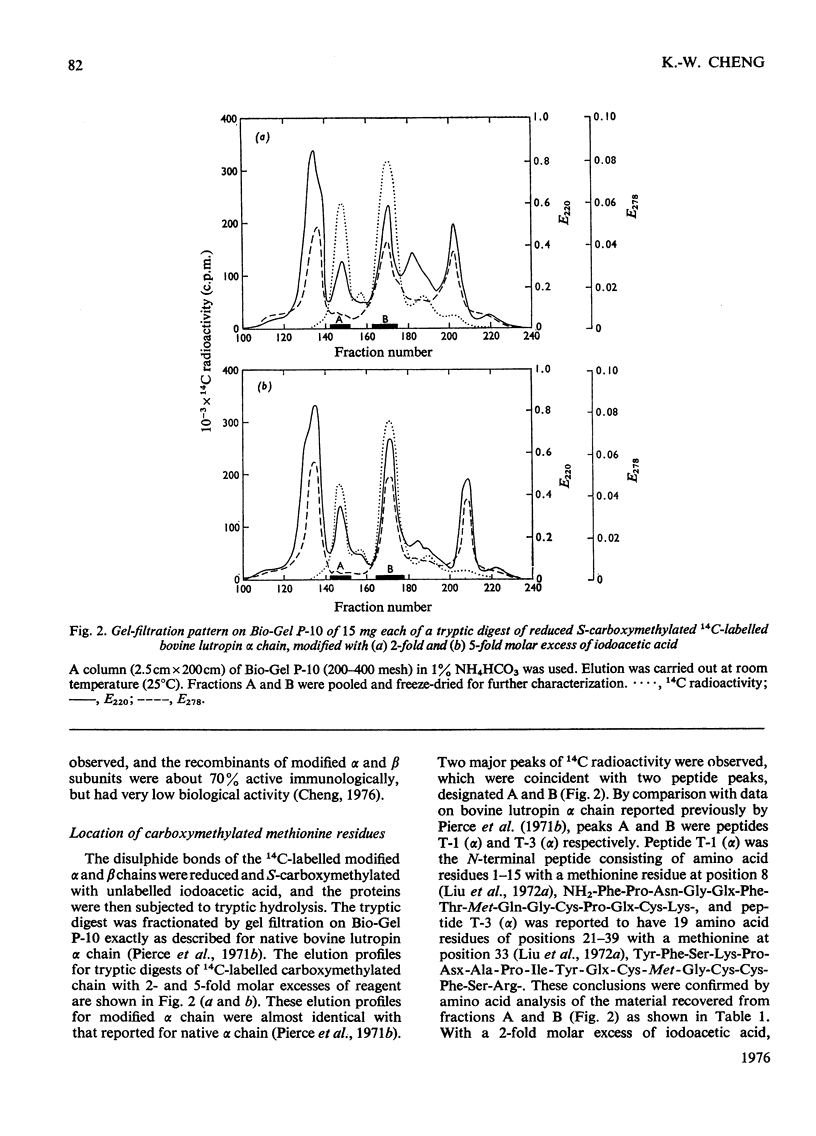
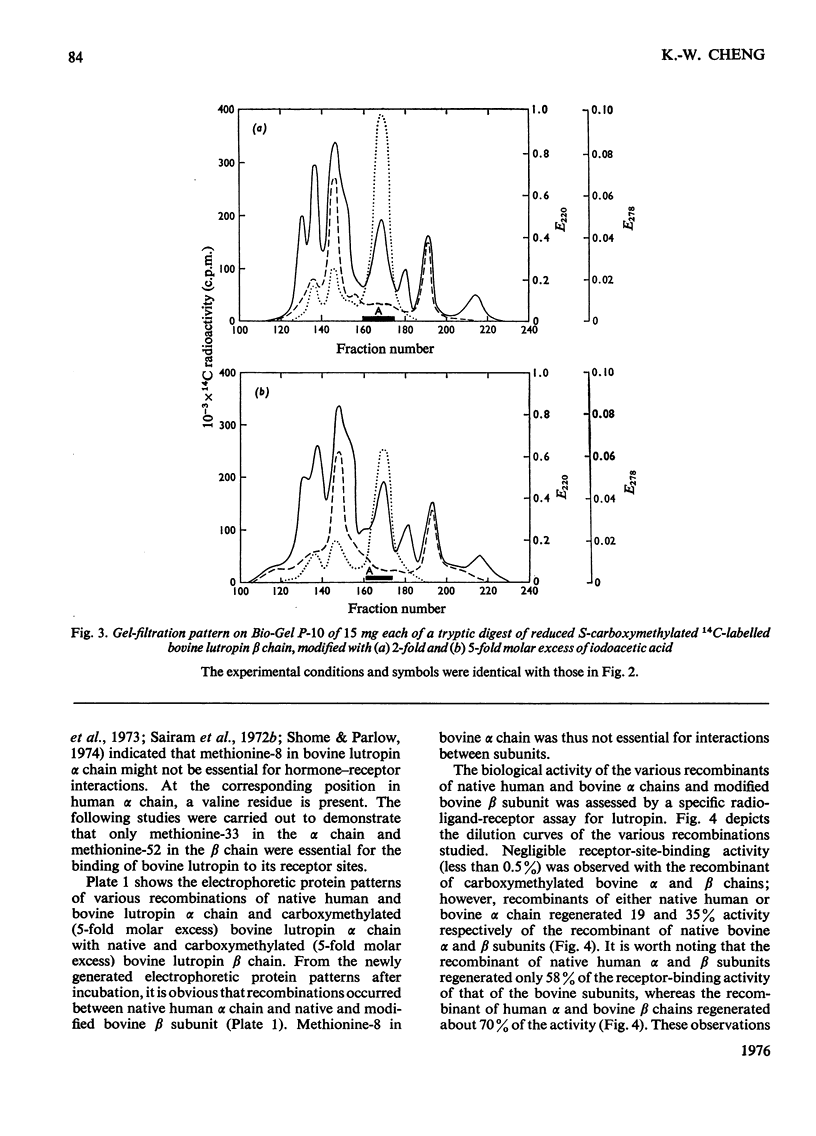
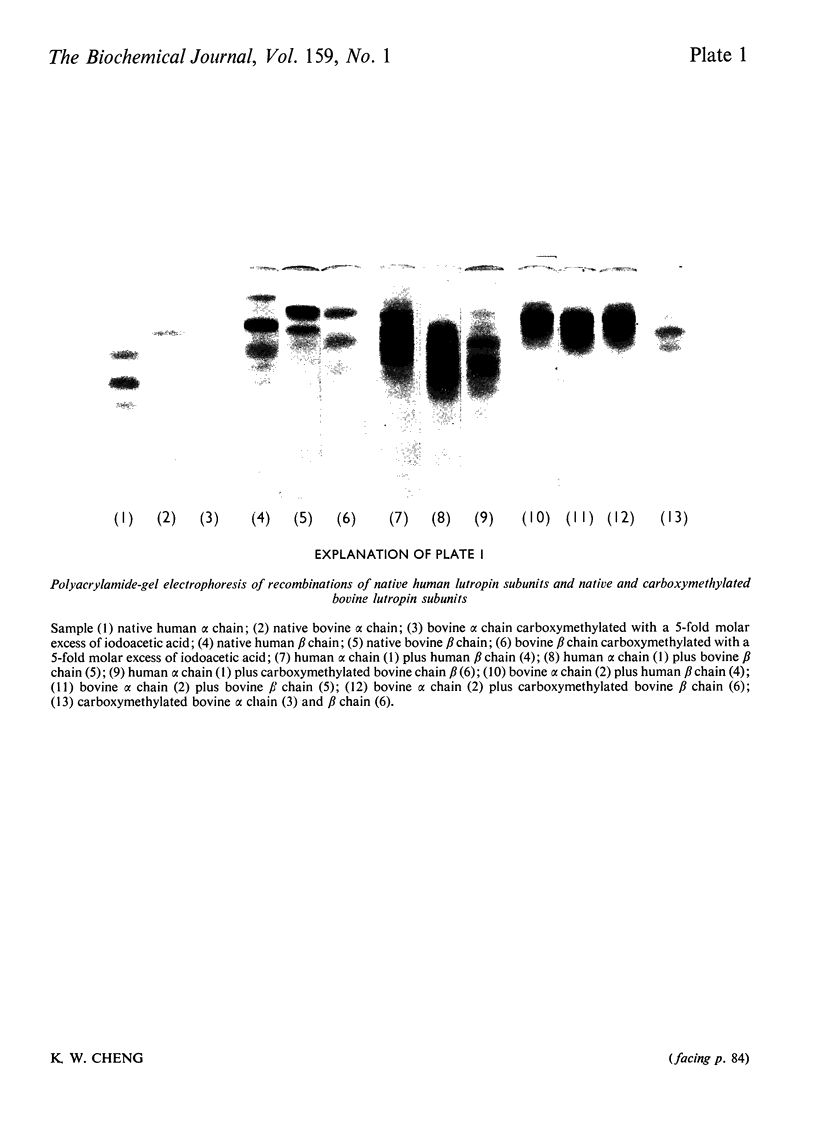
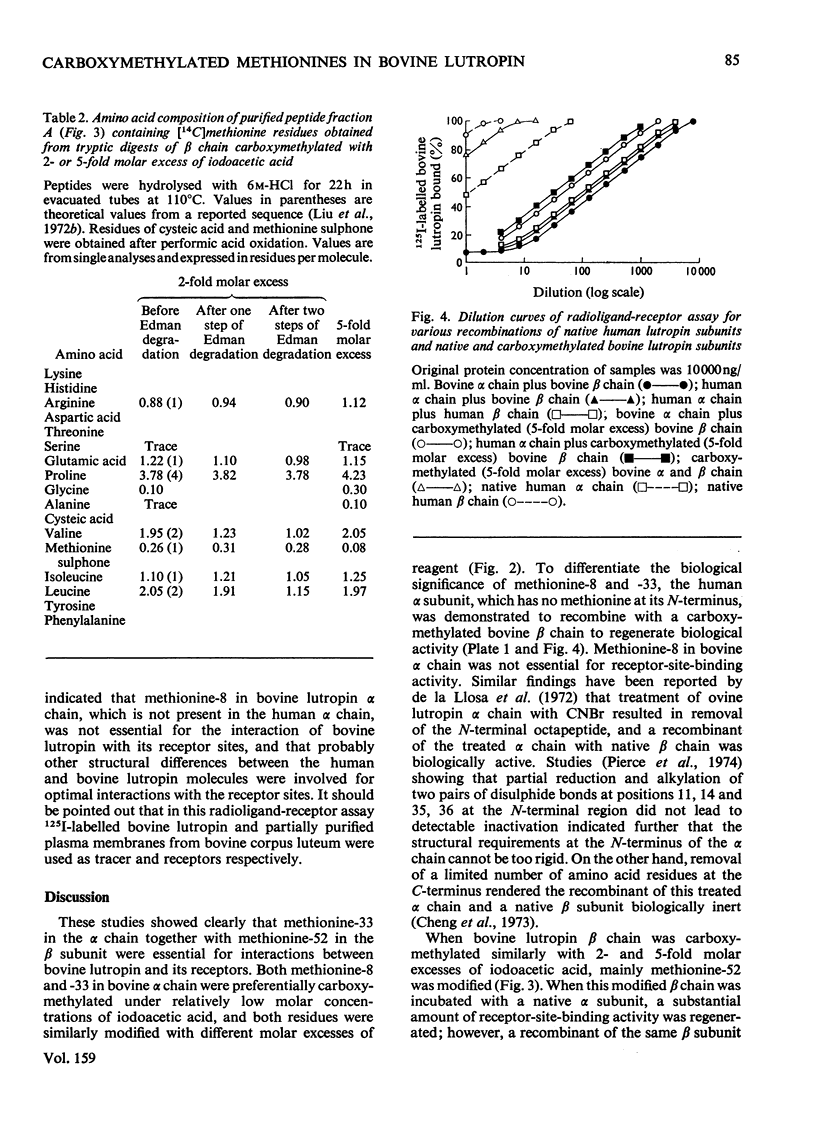
Bovine lutropin (luteinizing hormone) was carboxymethylated at pH3.0 for 12 h at 37 degrees C with iodoacetic acid for specific modification of methionine residues. To facilitate the location of preferentially modified methionine residues, iodoE114C]acetic acid was added as tracer. The alpha and beta subunits of bovine lutropin were carboxymethylated with a 2- or 5-fold molar excess of iodoacetic acid either in the presence or absence of their counterpart subunits. The modified subunits were separated and isolated by counter-current distribution followed by gel filtration on Sephadex G-100. To locate the modified methiones, the isolated alpha or beta chain was reduced. S-carboxymethylated and subjected to tryptic hydrolysis. The tryptic peptides were fractionated by gel filtration on Bio-Gel P-10. From analyses of the purified 14C-labelled tryptic peptides, it was observed that methionine-8 and -33 in bovine lutropin alpha chain and methionine-52 in the beta chain were preferentially modified. Similar results were obtained when isolated alpha and beta subunits were individually carboxymethylated in the absence of their counterpart subunit under identical conditions. The fact that a recombinant of native human lutropin alpha chain, in which a valine residue is present in the position corresponding to methionine-8 of bovine lutropin alpha chain, and carboxymethylated bovine lutropin beta chain regenerated a substantial amount of receptor-site-binding activity indicated that methionine-8 in bovine alpha chain was biologically not essential. These studies showed clearly that both methionine-33 in the alpha chain and methionine-52 in the beta subunit were involved for optimum binding between bovine lutropin and its receptors for expression of hormonal activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Cheng K. W. Carboxymethylation of methionine residues in bovine pituitary luteinizing hormone and its subunits. Effects on the binding activity with receptor sites and interactions between subunits. Biochem J. 1976 Oct 1;159(1):71–77. doi: 10.1042/bj1590071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. W., Glazer A. N., Pierce J. G. The effects of modification of the COOH-terminal regions of bovine thyrotropin and its subunits. J Biol Chem. 1973 Nov 25;248(22):7930–7937. [PubMed] [Google Scholar]
- Cheng K. W., Pierce J. G. The reaction of tetranitromethane with pituitary, luteinizing and thyroid-stimulating hormones. J Biol Chem. 1972 Nov 25;247(22):7163–7172. [PubMed] [Google Scholar]
- Closset J., Hennen G., Lequin R. M. Human luteinizing hormone. The amino acid sequence of the subunit. FEBS Lett. 1973 Jan 15;29(2):97–100. doi: 10.1016/0014-5793(73)80534-4. [DOI] [PubMed] [Google Scholar]
- Combarnous Y., Hennen G. Luteinizing hormone derivatives with covalently-linked subunits. FEBS Lett. 1974 Aug 25;44(2):224–228. doi: 10.1016/0014-5793(74)80730-1. [DOI] [PubMed] [Google Scholar]
- Combarnous Y., Maghuin-Rogister G. Luteinizing hormone. 2. Relative reactivities of tyrosyl residues of the porcine hormone towards iodination. Eur J Biochem. 1974 Feb 15;42(1):13–19. doi: 10.1111/j.1432-1033.1974.tb03308.x. [DOI] [PubMed] [Google Scholar]
- De la Llosa P., Durosay M., Tertrin-Clary C., Jutisz M. Chemical modification of lysine residues in ovine luteinizing hormone. Effect on biological activity. Biochim Biophys Acta. 1974 Mar 14;342(1):97–104. doi: 10.1016/0005-2795(74)90110-x. [DOI] [PubMed] [Google Scholar]
- Faith M. R., Pierce J. G. The carboxylic acid groups of bovine luteinizing hormone. The effects of their modification on receptor site binding and subunit-subunit interaction. J Biol Chem. 1975 Sep 10;250(17):6923–6929. [PubMed] [Google Scholar]
- Lamkin W. M., Fujino M., Mayfield J. D., Holcomb G. N., Ward D. N. Separation of the subunits of ovine luteinizing hormone by a chromatographic procedure and comparison with a countercurrent distribution procedure. Biochim Biophys Acta. 1970 Aug 21;214(2):290–298. doi: 10.1016/0005-2795(70)90006-1. [DOI] [PubMed] [Google Scholar]
- Liao T. H., Hennen G., Howard S. M., Shome B., Pierce J. G. Bovine thyrotropin. Countercurrent distribution and a comparison with the isolated subunits of luteinizing hormone. J Biol Chem. 1969 Dec 10;244(23):6458–6467. [PubMed] [Google Scholar]
- Liao T. H., Pierce J. G. The presence of a common type of subunit in bovine thyroid-stimulating and luteinizing hormones. J Biol Chem. 1970 Jul 10;245(13):3275–3281. [PubMed] [Google Scholar]
- Liao T. H., Pierce J. G. The primary structure of bovine thyrotropin. II. The amino acid sequences of the reduced, S-carboxymethyl alpha and beta chains. J Biol Chem. 1971 Feb 25;246(4):850–865. [PubMed] [Google Scholar]
- Liu W. K., Nahm H. S., Sweeney C. M., Holcomb G. N., Ward D. N. The primary structure of ovine luteinizing hormone. II. The amino acid sequence of the reduced, S-carboxymethylated A-subunit (LH- ). J Biol Chem. 1972 Jul 10;247(13):4365–4381. [PubMed] [Google Scholar]
- Liu W. K., Yang K. P., Nakagawa Y., Ward D. N. The role of the amino group in subunit association and receptor site interaction for ovine luteinizing hormone as studied by acylation. J Biol Chem. 1974 Sep 10;249(17):5544–5550. [PubMed] [Google Scholar]
- Maghuin-Rogister G., Dockier A. The amino acid sequence of the bovine luteinizing hormone beta subunit. FEBS Lett. 1971 Dec 15;19(3):209–213. doi: 10.1016/0014-5793(71)80515-x. [DOI] [PubMed] [Google Scholar]
- NEUMANN N. P., MOORE S., STEIN W. H. Modification of the methionine residues in ribonuclease. Biochemistry. 1962 Jan;1:68–75. doi: 10.1021/bi00907a011. [DOI] [PubMed] [Google Scholar]
- Papkoff H., Samy T. S. Isolation and partial characterization of the polypeptide chains of ovine interstitial cell-stimulating hormone. Biochim Biophys Acta. 1967 Sep 19;147(1):175–177. doi: 10.1016/0005-2795(67)90102-x. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Liao T. H., Carlsen R. B., Reimo T. Comparisons between the alpha chain of bovine thyrotropin and the CI chain of luteinizing hormone. Compositions of tryptic peptides, cyanogen bromide fragments, and carbohydrate moieties. J Biol Chem. 1971 Feb 25;246(4):866–872. [PubMed] [Google Scholar]
- Pierce J. G., Liao T., Howard S. M., Shome B., Cornell J. S. Studies on the structure of thyrotropin: its relationship to luteinizing hormone. Recent Prog Horm Res. 1971;27:165–212. doi: 10.1016/b978-0-12-571127-2.50029-8. [DOI] [PubMed] [Google Scholar]
- Sairam M. R., Papkoff H., Li C. H. Human pituitary interstitial cell stimulating hormone: primary structure of the subunit. Biochem Biophys Res Commun. 1972 Aug 7;48(3):530–537. doi: 10.1016/0006-291x(72)90380-4. [DOI] [PubMed] [Google Scholar]
- Sairam M. R., Papkoff H., Li C. H. The primary structure of ovine interstitial cell-stimulating hormone. I. The -subunit. Arch Biochem Biophys. 1972 Dec;153(2):554–571. doi: 10.1016/0003-9861(72)90374-8. [DOI] [PubMed] [Google Scholar]
- Sairam M. R., Samy T. S., Papkoff H., Li C. H. The primary structure of ovine interstitial cell-stimulating hormone. II. The -subunit. Arch Biochem Biophys. 1972 Dec;153(2):572–586. doi: 10.1016/0003-9861(72)90375-x. [DOI] [PubMed] [Google Scholar]
- Shome B., Parlow A. F. Human follicle stimulating hormone (hFSH): first proposal for the amino acid sequence of the alpha-subunit (hFSHa) and first demonstration of its identity with the alpha-subunit of human luteinizing hormone (hLHa). J Clin Endocrinol Metab. 1974 Jul;39(1):199–202. doi: 10.1210/jcem-39-1-199. [DOI] [PubMed] [Google Scholar]
- Wolff J., Winand R. J., Kohn L. D. The contribution of subunits of thyroid stimulating hormone to the binding and biological activity of thyrotropin. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3460–3464. doi: 10.1073/pnas.71.9.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]