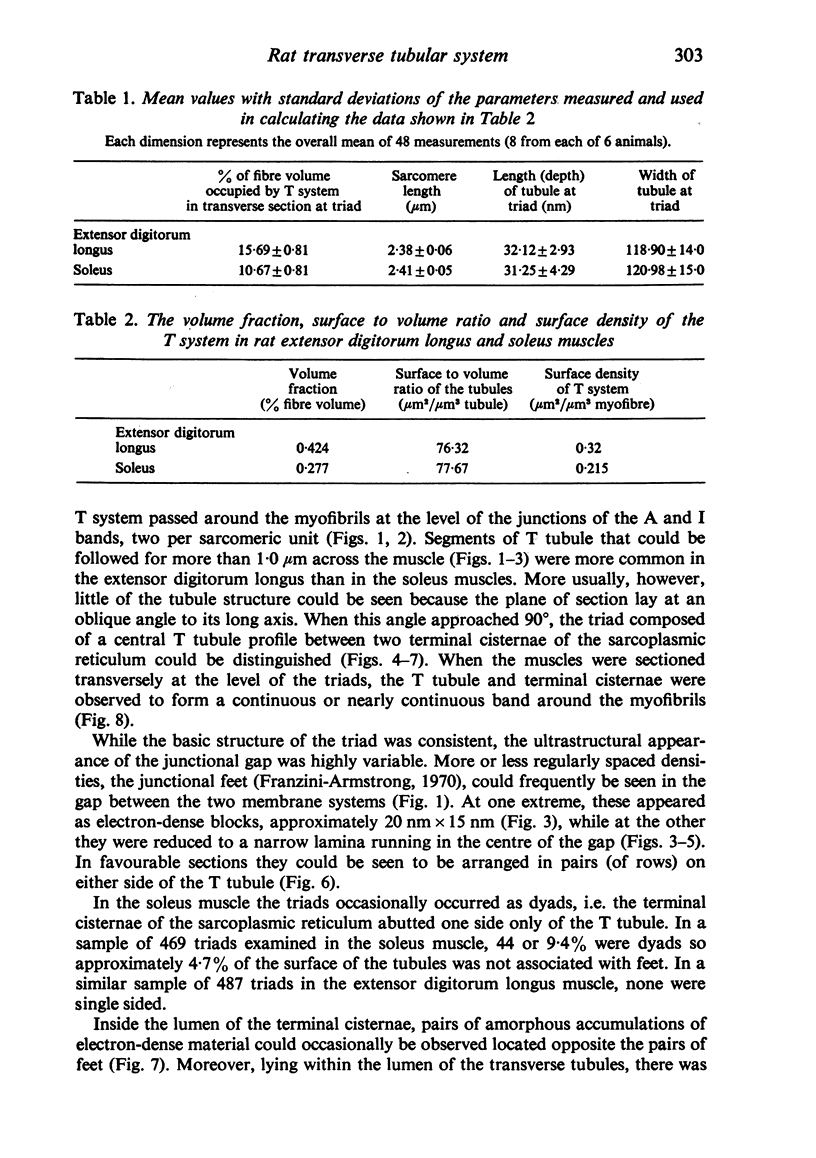
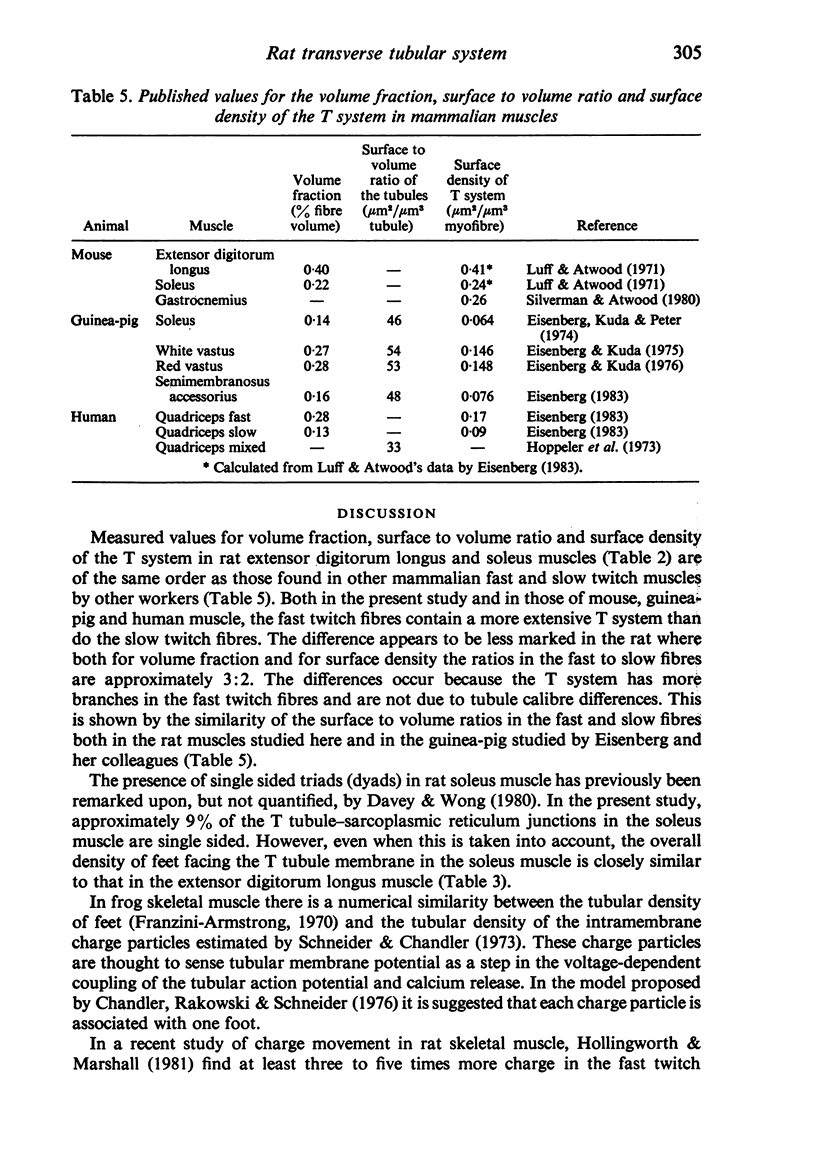
Abstract
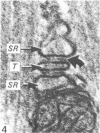
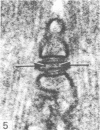
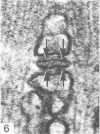
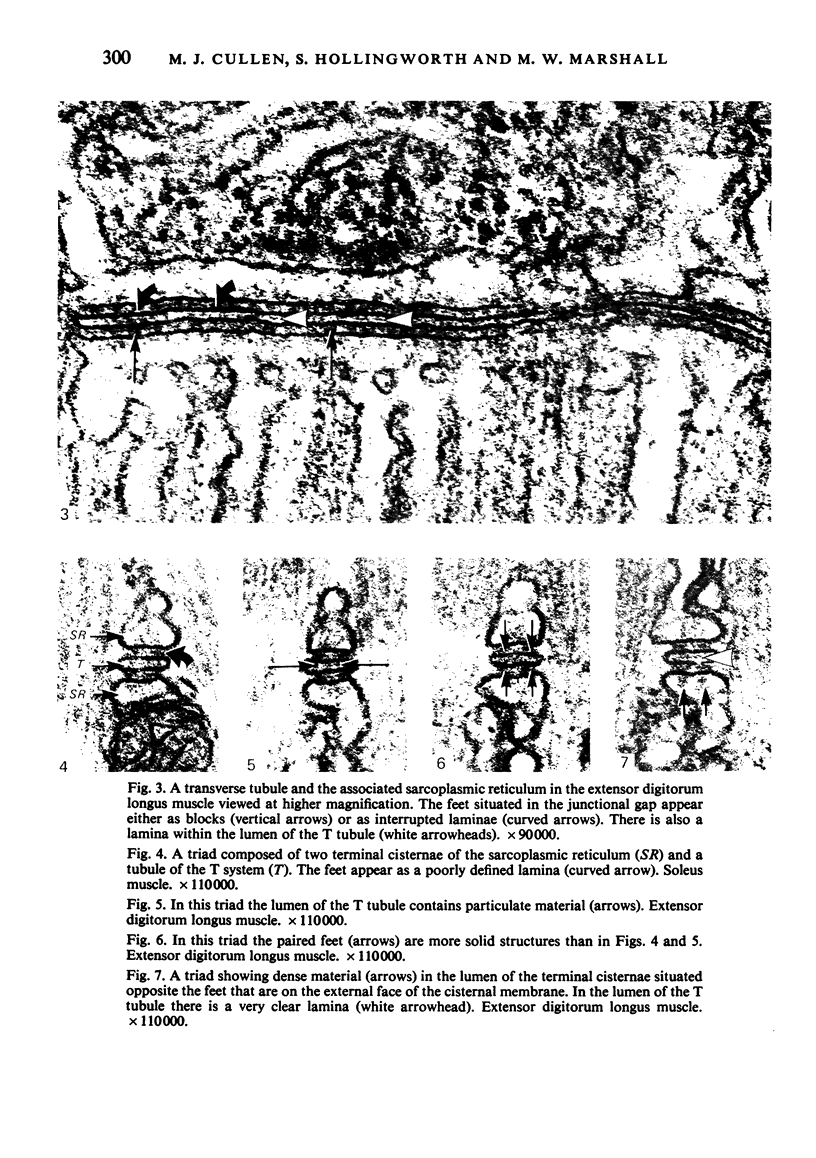
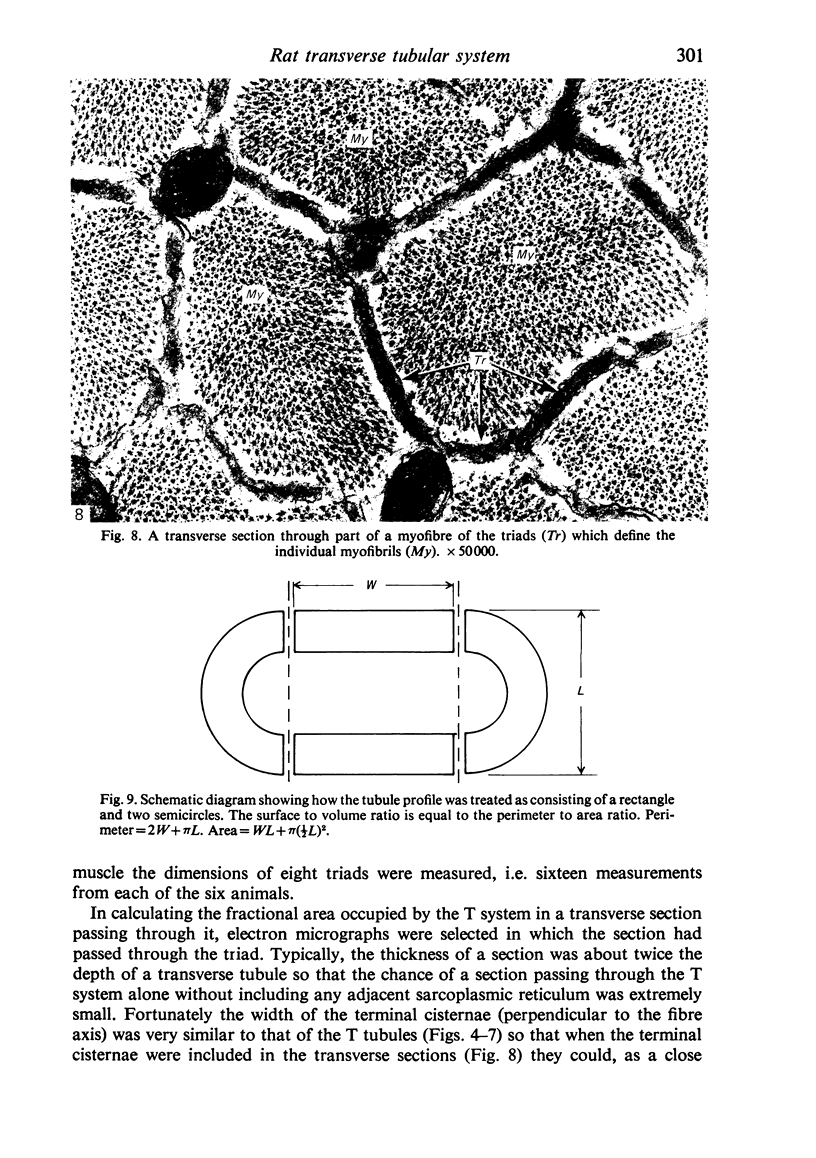
An ultrastructural comparison was made between the transverse tubular systems of the extensor digitorum longus (fast twitch) and the soleus (slow twitch) muscles in the rat. Quantitative measurements were made of the volume fraction, surface to volume ratio, surface density and foot density of the T system membrane. The values for the volume fraction and surface density were approximately 50% higher in the fast twitch (extensor digitorum longus) than the slow twitch (soleus) muscle. The dimensions of the tubules and hence the surface to volume ratio was the same in the two muscles. There was no difference in the spacing of the feet, so the reported three to fivefold difference in charge movement cannot be associated with a difference in foot density. Material lying within the lumen of the T tubules was observed in the majority of tubule profiles. This material, not hitherto described, was particulate or, more commonly, laminar in form.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M. J., Weightman D. The ultrastruct of normal human muscle in relation to fibre type. J Neurol Sci. 1975 May;25(1):43–56. doi: 10.1016/0022-510x(75)90185-9. [DOI] [PubMed] [Google Scholar]
- Davey D. F., Wong S. Y. Morphometric analysis of rat extensor digitorum longus and soleus muscles. Aust J Exp Biol Med Sci. 1980 Jun;58(3):213–230. doi: 10.1038/icb.1980.22. [DOI] [PubMed] [Google Scholar]
- Eisenberg B. R., Eisenberg R. S. The T-SR junction in contracting single skeletal muscle fibers. J Gen Physiol. 1982 Jan;79(1):1–19. doi: 10.1085/jgp.79.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B. R., Gilai A. Structural changes in single muscle fibers after stimulation at a low frequency. J Gen Physiol. 1979 Jul;74(1):1–16. doi: 10.1085/jgp.74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B. R., Kuda A. M. Discrimination between fiber populations in mammalian skeletal muscle by using ultrastructural parameters. J Ultrastruct Res. 1976 Jan;54(1):76–88. doi: 10.1016/s0022-5320(76)80010-x. [DOI] [PubMed] [Google Scholar]
- Eisenberg B. R., Kuda A. M., Peter J. B. Stereological analysis of mammalian skeletal muscle. I. Soleus muscle of the adult guinea pig. J Cell Biol. 1974 Mar;60(3):732–754. doi: 10.1083/jcb.60.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B. R., Kuda A. M. Stereological analysis of mammalian skeletal muscle. II. White vastus muscle of the adult guinea pig. J Ultrastruct Res. 1975 May;51(2):176–187. doi: 10.1016/s0022-5320(75)80146-8. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Membrane particles and transmission at the triad. Fed Proc. 1975 Apr;34(5):1382–1389. [PubMed] [Google Scholar]
- Franzini-Armstrong C. STUDIES OF THE TRIAD : I. Structure of the Junction in Frog Twitch Fibers. J Cell Biol. 1970 Nov 1;47(2):488–499. doi: 10.1083/jcb.47.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S., Marshall M. W. A comparative study of charge movement in rat and frog skeletal muscle fibres. J Physiol. 1981 Dec;321:583–602. doi: 10.1113/jphysiol.1981.sp014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppeler H., Lüthi P., Claassen H., Weibel E. R., Howald H. The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflugers Arch. 1973 Nov 28;344(3):217–232. doi: 10.1007/BF00588462. [DOI] [PubMed] [Google Scholar]
- Luff A. R., Atwood H. L. Changes in the sarcoplasmic reticulum and transverse tubular system of fast and slow skeletal muscles of the mouse during postnatal development. J Cell Biol. 1971 Nov;51(21):369–383. doi: 10.1083/jcb.51.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias R. T., Levis R. A., Eisenberg R. S. Electrical models of excitation-contraction coupling and charge movement in skeletal muscle. J Gen Physiol. 1980 Jul;76(1):1–31. doi: 10.1085/jgp.76.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., Conner G. E., Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973 Mar 16;298(2):246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- Mobley B. A., Eisenberg B. R. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975 Jul;66(1):31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE S. G., HUXLEY H. E. FILAMENT LENGTHS IN STRIATED MUSCLE. J Cell Biol. 1963 Nov;19:369–390. doi: 10.1083/jcb.19.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Salmons S., Gale D. R., Sréter F. A. Ultrastructural aspects of the transformation of muscle fibre type by long term stimulation: changes in Z discs and mitochondria. J Anat. 1978 Sep;127(Pt 1):17–31. [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Silverman H., Atwood H. L. Surface density of T tubules in normal and dystrophic mouse muscle. Exp Neurol. 1980 Oct;70(1):40–46. doi: 10.1016/0014-4886(80)90004-7. [DOI] [PubMed] [Google Scholar]
- Sjöström M., Squire J. M. Fine structure of the A-band in cryo-sections. The structure of the A-band of human skeletal muscle fibres from ultra-thin cryo-sections negatively stained. J Mol Biol. 1977 Jan 5;109(1):49–68. doi: 10.1016/s0022-2836(77)80045-4. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V. Bridging structures spanning the junctioning gap at the triad of skeletal muscle. J Cell Biol. 1979 Mar;80(3):743–750. doi: 10.1083/jcb.80.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]