Simple Summary
This study investigated the association between the number of synchronous ipsilateral T1 breast tumors and patient survival. A retrospective analysis of 45,881 patients with invasive breast cancer was conducted. Patients were categorized based on the number of tumors: one, two, or three or more. Overall survival (OS) and breast cancer-specific survival (BCSS) were compared across groups. Patients with three or more tumors had significantly lower OS and BCSS rates compared to those with one or two tumors. Multivariate analysis confirmed the number of tumors as an independent risk factor for poor prognosis. Our findings suggest that patients with three or more synchronous ipsilateral T1 breast tumors may benefit from escalated treatment strategies due to their increased risk of mortality.
Keywords: breast cancer, multiple tumors, overall survival, breast cancer-specific survival, T1 stage, escalated treatment
Abstract
Background/Objectives: The reported incidence of multiple breast cancers varies widely, ranging from 6 to 60%, depending on the definitions used and methods of detection. With advancements in preoperative imaging techniques, such as magnetic resonance imaging, the detection of multiple breast cancers has improved. However, the clinical significance of multiple breast cancers remains controversial, with conflicting results regarding their impact on prognosis. We investigated the association between the number of synchronous ipsilateral T1 breast tumors, overall survival (OS), and breast cancer-specific survival (BCSS). Methods: We retrospectively analyzed 45,881 patients diagnosed with invasive breast cancer who underwent surgery between 2004 and 2016. The patients were categorized based on the number of tumors: one (n = 43,234), two (n = 2241), and three or more (n = 406). The OS and BCSS scores were compared across the groups. Results: There were no significant differences between the one- and two-tumor groups (p = 0.490 and p = 0.650, respectively). However, patients with three or more tumors had significantly lower OS and BCSS rates than those with one or two tumors (p < 0.001 for both comparisons). Multivariate analysis confirmed that the number of tumors (three or more) was an independent risk factor for poor OS and BCSS. Conclusions: Our findings suggest that patients with synchronous ipsilateral T1 breast cancers and three or more tumors may benefit from escalated treatment strategies due to their potentially worse prognosis.
1. Introduction
Multiple breast cancers are classified into multifocal breast cancer, which refers to the presence of more than one tumor focus within the same quadrant of the breast, or multicentric breast cancer, which refers to the presence of more than one tumor focus in different quadrants of the breast [1]. However, owing to the lack of consistency in the criteria for classification and anatomical ambiguity, it is referred to as “multiple breast cancer” [2]. The incidence of multiple breast cancers varies widely in the literature, ranging from 6% to 60%, depending on the definitions and methods of detection used [3]. With advancements in preoperative imaging techniques, such as magnetic resonance imaging, the detection of multiple breast cancers has improved [4].
The clinical implications of multiple breast cancers are not well established. Some studies suggest that multiple breast cancer is associated with a higher tumor stage, a higher grade, lympho-vascular invasion, lymph node metastasis, and worse survival outcomes than unifocal breast cancer [1,3,5,6,7,8,9]. However, other studies report no significant differences in these parameters or prognostic factors between multiple and single breast cancers [10,11]. The existing TNM staging system does not consider the number of tumor foci or their location and considers only the size of the largest lesion to assign the T stage [12].
T1 breast cancer measures ≤ 2 cm in its greatest dimension [2]. It is considered an early-stage breast cancer with a favorable prognosis and a low risk of recurrence. However, T1 breast cancer can also be multifocal or multicentric, and the effect of multiple tumors on prognosis and management remains unclear [13].
The size of breast cancer is an important predictor of axillary metastasis and has a significant impact on prognosis. As the number of tumors increases, the tumor burden increases. However, according to the staging guidelines, the T stage is determined by the size of the largest mass in synchronous multiple breast cancer [2]. Therefore, it is highly likely that the tumor burden is underestimated in multiple breast cancers, especially T1 breast cancer, owing to small tumor size. Therefore, we evaluated the effect of tumor burden on prognosis according to the number of tumors in T1 breast cancer.
2. Materials and Methods
2.1. Patients
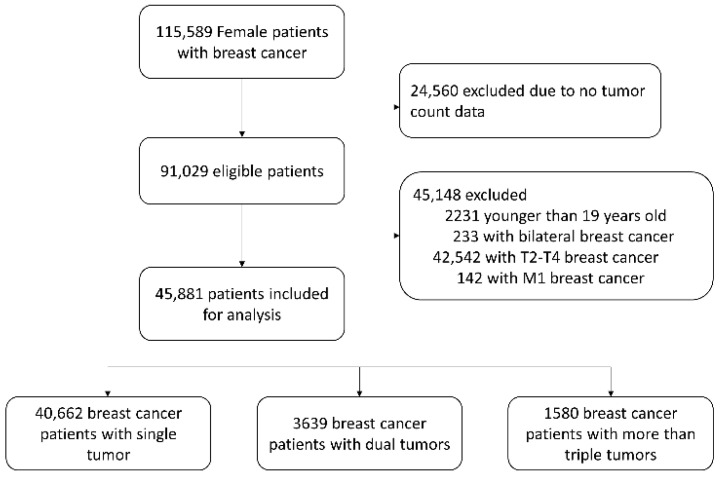
This study was conducted using nationwide multicenter data prospectively collected by the Korean Breast Cancer Society from 102 general hospitals in South Korea [14]. Treatment and follow-up of breast cancer patients adhered to the Korean breast cancer clinical practice guidelines, which were developed based on the NCCN guidelines [15]. We selected adult females with stage T1 cancer who had undergone surgery for invasive breast cancer between January 2004 and December 2016. Patients with bilateral breast cancer or distant metastases were excluded (Figure 1).
Figure 1.
Flow diagram of patient selection.
The data included patient age, TNM stage, number of tumors, size of the largest tumor, histological classification, surgical method, estrogen receptor (ER) status, human epidermal growth factor 2 (HER2) status, diagnostic date, and the date of death. HER2 positivity was defined as either 3+ overexpression in immunohistochemical staining or HER2 amplification in fluorescent in situ hybridization (HER2/CEP17 ratio ≥ 2.0). Hormone receptor positivity was defined as >1% staining for either ER or progesterone receptor or both. Human epidermal growth factor receptor 2 (HER2) positivity was defined as either 3+ overexpression or HER2 amplification observed via immunohistochemical staining or fluorescent in situ hybridization, respectively (HER2/chromosome enumeration probe 17 ratios: ≥2.0). Hormone receptor (HR) positivity was defined as >1% staining for either estrogen or progesterone receptors or both.
2.2. Statistical Analyses
Patients were classified into three groups based on the number of tumors: one, two, or three or more. Their clinical characteristics were compared using an analysis of variance and Student’s t-test for continuous and non-continuous variables, respectively. Survival was defined as the interval between the date of diagnosis and the date of death. Survival was estimated using the Kaplan–Meier method and compared using the restricted mean survival time (RMST) and log-rank test (two-sided, p < 0.05). Cox proportional regression models were used to estimate the hazard ratios (95% confidence intervals [CIs]) for breast cancer-specific survival (BCSS) and overall survival (OS). Propensity score matching (PSM) was performed using the MatchIt package. For improving study power, 1:4 nearest neighbor matching without replacement was used with propensity scores estimated through logistic regression [16]. Statistical analyses were performed using the R software (ver. 4.4.1, R Core Team, 2024, Vienna, Austria).
3. Results
3.1. Patient Characteristics
A total of 45,881 patients with invasive breast cancer were included in the analysis. Among them, 88.6% (n = 40,662) had a single tumor, 7.9% (n = 3639) had two tumors, and 3.4% had three or more tumors (n = 1580). The average size of the largest tumor was 1.24 cm, which did not differ among the three groups (p = 0.999; Table 1).
Table 1.
Clinicopathological characteristics.
| Number of Tumors (n = 45,881) | ||||
|---|---|---|---|---|
| One (n = 40,662) | Two (n = 3639) | Three or More (n = 1580) | p-Value | |
| Age, years | <0.001 | |||
| <30 | 378 (0.9) | 30 (0.8) | 19 (1.2) | |
| 30–50 | 21,861 (53.8) | 2245 (61.7) | 1041 (65.9) | |
| ≥50 | 18,423 (45.3) | 1364 (37.5) | 520 (32.9) | |
| Largest tumor size (cm) | 1.243 ± 1.048 | 1.244 ± 0.681 | 1.243 ± 0.766 | 0.999 |
| Surgery | <0.001 | |||
| Breast-conserving | 28,784 (70.8) | 1793 (49.3) | 554 (35.1) | |
| Mastectomy | 11,878 (29.2) | 1846 (50.7) | 1026 (64.9) | |
| N stage | <0.001 | |||
| N0 | 31136 (76.7) | 2635 (72.6) | 1052 (66.8) | |
| N1 | 7956 (19.6) | 833 (22.9) | 403 (25.6) | |
| N2 | 1079 (2.7) | 109 (3.0) | 76 (4.8) | |
| N3 | 429 (1.1) | 53 (1.5) | 44 (2.8) | |
| Unknown | 62 | 9 | 5 | |
| Hormonal receptor | <0.001 | |||
| Positive | 30,942 (76.2) | 2900 (79.8) | 1239 (78.6) | |
| Negative | 9667 (23.8) | 735 (20.2) | 337 (21.4) | |
| Unknown | 53 | 4 | 4 | |
| HER2 | <0.001 | |||
| Positive | 11,346 (27.9) | 1086 (29.9) | 554 (35.2) | |
| Negative | 29,263 (72.1) | 2549 (70.1) | 1022 (64.8) | |
| Unknown | 53 | 4 | 4 | |
| Chemotherapy | <0.001 | |||
| No | 18097 (44.6) | 1452 (40.0) | 549 (34.8) | |
| Yes | 22505 (55.4) | 2179 (60.0) | 1029 (65.2) | |
| Unknown | 60 | 8 | 2 | |
There was a significant positive correlation between the number of tumors and the proportion of patients aged 50 or younger (54.7% with one tumor, 62.5% with two, and 67.1% with three or more; p < 0.001). Additionally, a higher proportion of patients underwent mastectomy rather than breast-conserving surgery as the number of tumors increased (29.2% with one tumor, 50.7% with two, and 64.9% with three or more; p < 0.001).
3.2. Clinicopathological Characteristics
As the number of tumors increased, the proportion of patients with lobular carcinoma and HER2-positive breast cancer increased significantly. In patients with one, two, or three or more tumors, the incidence of axillary metastasis was 23.4%, 27.6%, and 33.5%, respectively (p < 0.001). Patients with a higher tumor count were significantly more likely to receive chemotherapy (55.4% with one tumor, 60.0% with two, and 65.2% with three or more; p < 0.001). Patient characteristics are summarized in Table 1.
3.3. Survival Analysis
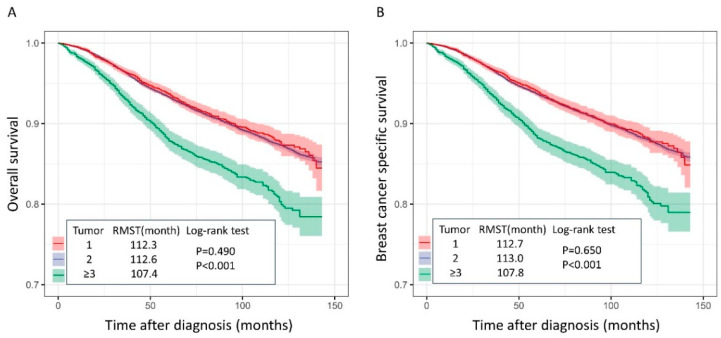
The median follow-up duration was 62.4 (±40.3) months. The log-rank test results showed no significant differences in OS and BCSS between the one- and two-tumor groups (log-rank test p = 0.490, p = 0.650). However, OS and BCSS were significantly lower in the group with three or more tumors than in the groups with one and two tumors (p < 0.001; Figure 2).
Figure 2.
Kaplan–Meier curves for (A) overall survival and (B) breast cancer-specific survival.
The RMST for OS was not significantly different between the one- (112.3 ± 0.1 months) and two-tumor groups (112.6 ± 0.3 months) (p = 0.299). However, the RMST for OS was significantly shorter in the group with three or more tumors (107.4 ± 0.6 months) than in the other groups (p < 0.001). Similarly, the RMST for BCSS did not differ significantly between the patients in the one- (112.7 ± 0.1 months) and two-tumor groups (113.0 ± 0.3 months) (p = 0.309). However, the RMST for BCSS was significantly shorter in the patients in the group with three or more tumors (107.8 ± 0.6 months) than in those in the other groups (p < 0.001; Figure 2).
Multivariate Cox proportional analysis showed that OS and BCSS were lower in patients with three or more tumors than in those in the other groups (OS 1.386 (1.096–1.754), p = 0.006; BCSS 1.349 (1.054–1.727), p = 0.017; Table 2 and Table 3).
Table 2.
Univariate and multivariate analyses of prognostic factors for overall survival.
| Univariate Analysis | p-Value | Multivariate Analysis | p-Value | |
|---|---|---|---|---|
| Age, years | ||||
| <30 | 1 | 1 | ||
| 30–50 | 0.620 (0.526–0.732) | <0.001 | 0.575 (0.382–0.865) | 0.008 |
| ≥50 | 0.909 (0.771–1.072) | 0.257 | 0.909 (0.606–1.365) | 0.647 |
| Surgery | ||||
| Breast-conserving | 1 | 1 | ||
| Mastectomy | 2.336 (2.231–2.445) | <0.001 | 1.946 (1.829–2.071) | <0.001 |
| Tumor type | ||||
| IDC | 1 | 1 | ||
| ILC | 0.848 (0.730–0.985) | 0.031 | 1.197 (0.832–1.723) | 0.332 |
| Others | 1.109 (0.967–1.271) | 0.139 | 1.264 (0.821–1.946) | 0.281 |
| Axillary metastasis | ||||
| No | 1 | 1 | ||
| Yes | 3.829 (3.656–4.010) | <0.001 | 2.635 (2.377–2.922) | <0.001 |
| Tumor count | ||||
| 1 | 1 | 1 | ||
| 2 | 0.959 (0.802–1.145) | 0.640 | 0.937 (0.768–1.144) | 0.525 |
| ≥3 | 1.489 (1.218–1.822) | <0.001 | 1.386 (1.096–1.754) | 0.006 |
| Molecular subtype | ||||
| Luminal A | 1 | 1 | ||
| Luminal B | 1.648 (0.455–0.550) | <0.001 | 1.355 (1.110–1.655) | 0.003 |
| Luminal HER2 | 2.312 (2.049–2.607) | <0.001 | 2.139 (1.707–2.681) | <0.001 |
| HER2 | 2.806 (2.490–3.162) | <0.001 | 2.492 (1.988–3.125) | <0.001 |
| TNBC | 3.651 (3.271–4.075) | <0.001 | 3.414 (2.774–4.202) | <0.001 |
Table 3.
Univariate and multivariate analyses of prognostic factors for breast cancer-specific survival.
| Univariate Analysis | p-Value | Multivariate Analysis | p-Value | |
|---|---|---|---|---|
| Age, years | ||||
| <30 | 1 | 1 | ||
| 30–50 | 0.610 (0.517–0.720) | <0.001 | 0.560 (0.372–0.843) | 0.005 |
| ≥50 | 0.843 (0.714–0.995) | 0.043 | 1.349 (0.603–0.924) | 0.017 |
| Surgery | ||||
| Breast-conserving | 1 | 1 | ||
| Mastectomy | 2.354 (2.246–2.467) | <0.001 | 1.947 (1.826–2.075) | <0.001 |
| Tumor type | ||||
| IDC | 1 | 1 | ||
| ILC | 0.850 (0.729–0.991) | 0.038 | 1.260 (0.865–1.837) | 0.229 |
| Others | 1.087 (0.944–1.252) | 0.248 | 1.258 (0.799–1.980) | 0.321 |
| Axillary metastasis | ||||
| No | 1 | 1 | ||
| Yes | 4.084 (3.893–4.285) | <0.001 | 2.818 (2.531–3.138) | <0.001 |
| Tumor count | ||||
| 1 | 1 | 1 | ||
| 2 | 0.992 (0.826–1.192) | 0.935 | 0.972 (0.792–1.192) | 0.785 |
| ≥3 | 1.473 (1.192–1.821) | <0.001 | 1.349 (1.054–1.727) | 0.017 |
| Molecular subtype | ||||
| Luminal A | 1 | 1 | ||
| Luminal B | 1.654 (1.478–1.849) | <0.001 | 1.343 (1.085–1.663) | 0.007 |
| Luminal HER2 | 2.389 (2.109–2.706) | <0.001 | 2.342 (1.847–2.970) | <0.001 |
| HER2 | 2.911 (2.573–3.293) | <0.001 | 2.719 (2.141–3.454) | <0.001 |
| TNBC | 3.789 (3.381–4.245) | <0.001 | 3.744 (3.005–4.664) | <0.001 |
To mitigate the confounding effects of intergroup differences, we employed 1:4 PSM, effectively eliminating all significant between-group disparities (Table 4).
Table 4.
Clinicopathological characteristics after propensity score matching.
| One or Two (n = 6292) | Three or More (n = 1573) | p-Value | |
|---|---|---|---|
| Age, years | 0.966 | ||
| <50 | 4226 (67.2) | 1055 (67.1) | |
| ≥50 | 18,423 (45.3) | 518 (32.9) | |
| Surgery | 1.000 | ||
| Breast-conserving | 2204 (35.0) | 551 (35.0) | |
| Mastectomy | 4088 (65.0) | 1022 (65.0) | |
| Nodal involvement | 1.000 | ||
| No | 4200 (66.8) | 1050 (66.8) | |
| Yes | 2092 (33.2) | 523 (33.2) | |
| Unknown | |||
| Hormonal receptor | 1.000 | ||
| Positive | 1342 (21.3) | 335 (21.3) | |
| Negative | 4950 (78.7) | 1238 (78.7) | |
| HER2 | 0.948 | ||
| Positive | 4072 (64.7) | 1020 (64.8) | |
| Negative | 2220 (35.3) | 553 (35.2) | |
| Tumor type | 0.980 | ||
| IDC | 5858 (93.1) | 1463 (93.0) | |
| ILC | 256 (4.1) | 64 (4.1) | |
| Others | 178 (2.8) | 46 (2.9) |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
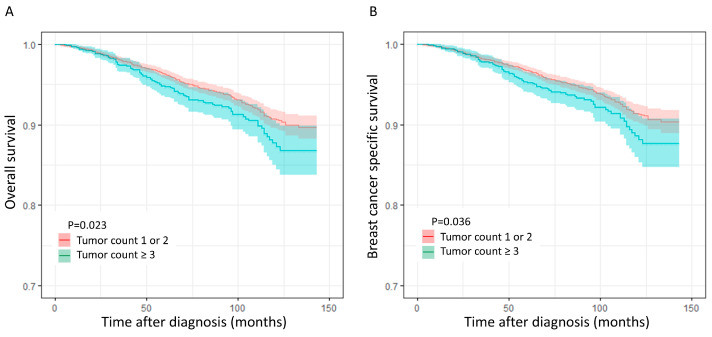
Figure 3 presents a Kaplan–Meier (KM) curve comparing the survival rates between patients with two or fewer tumors and those with three or more tumors after propensity score matching (PSM) was applied to adjust for potential biases. Subsequent log-rank tests following PSM revealed that the patients in the group with three or more tumors exhibited significantly inferior OS and BCSS compared with those in the groups with one or two tumors (OS; p = 0.023, BCSS; p = 0.036; Figure 3).
Figure 3.
Kaplan–Meier curves after propensity score matching, (A) overall survival, and (B) breast cancer-specific survival.
4. Discussion
Breast cancer is a heterogeneous disease with various histological and molecular features and is divided into three major subtypes depending on the presence or absence of molecular markers: ER or progesterone receptor and HER2 [17]. Because the response to and prognosis of chemotherapy for breast cancer vary depending on the subtype, the revised TNM prognostic staging reflects histological and molecular characteristics [2,12,14].
We focused exclusively on patients with T1 breast cancer. Notably, 11.3% of patients presented with multifocal disease, which aligns with the findings of a broader meta-analysis encompassing various tumor stages [4]. Interestingly, no significant difference in the largest tumor size was observed across the groups categorized according to the number of tumors. These findings suggest that the number of tumors may not directly correlate with the size of the largest tumor. Therefore, clinicians should maintain a high index of suspicion of multifocal breast cancer, irrespective of the size of the dominant lesion.
Multiple breast cancer is associated with a poorer prognosis compared with single breast cancer [1,5,6,18,19]. However, some studies have reported no significant difference in prognosis between multiple breast cancer and single breast cancer [10,11]. The effect of lymph node metastasis on the prognosis of multiple breast cancer remains controversial [20]. These studies often employed a binary classification (single vs. multiple tumors), which may be overly simplistic in capturing the true extent of the tumor burden. Although the sum of tumor diameters has been used to quantify tumor burden in multiple breast cancers, this approach may lack intuitiveness and ease of calculation [7,8]. To address these limitations, a straightforward and easily implemented method is required. We aimed to address this limitation by analyzing the relationship between prognosis and the number of tumors, a readily obtainable metric in clinical practice.
In the TNM staging system, the T stage relies solely on the size of the largest tumor for disease classification and treatment planning. However, this approach may not adequately capture the total tumor burden in patients with multifocal breast cancer, potentially underestimating disease aggressiveness. Our results align with the previous findings of Nathan et al. [8]. We observed a higher prevalence of axillary lymph node metastasis in patients with multiple breast cancers than in those with a single tumor. The rate of metastasis correlated positively with the number of tumors.
Interestingly, our study revealed no significant differences in OS or BCSS between patients with single and dual tumors. However, a statistically significant difference in survival rates emerged in patients with three or more tumors. These findings suggest a potential threshold effect in which tumor burden alone is a significant prognostic factor for three or more lesions. This may partially explain the heterogeneous results reported in other studies investigating the prognosis of multiple breast cancers.
The heterogeneous nature of tumors in patients with multiple breast cancer is another factor that contributes to the inconsistent prognostic outcomes observed in this patient population. Each tumor in a patient with multiple breast cancer may exhibit a distinct histological subtype, potentially influencing treatment response and OS. Buggi et al. reported that ER status was discordant in 4.4%, PR status was discordant in 15.9%, and HER2 status was discordant in 9.7% of patients with multiple breast cancers, resulting in a total of 12.4% of patients not receiving the correct adjuvant treatment due to heterogeneity [21]. Pekar et al. reported that 10% to 12.7% of women with ipsilateral multiple synchronous breast cancers had a heterogeneous subtype, and patients with a heterogeneous subtype had significantly worse survival [22].
Although our findings provide valuable insights into the prognostic implications of tumor burden in multiple breast cancers, it is important to acknowledge the limitations. First, the absence of molecular pathological data on individual tumors impedes a more comprehensive evaluation of tumor heterogeneity and its potential impact on prognosis. Future research incorporating the molecular characterization of each tumor could refine our understanding of this complex relationship. Second, the retrospective nature of our study limited our ability to draw definitive causal inferences. Prospective studies with rigorously controlled designs would be better suited to establish causal relationships between tumor burden and prognosis. Thirdly, the absence of information regarding BRCA germline mutations is noteworthy. While BRCA mutations are well-established risk factors for breast cancer, particularly bilateral disease, their association with ipsilateral multiple breast cancer remains unclear [23]. The prognostic impact of BRCA mutations on ipsilateral multiple breast cancer remains controversial, despite the majority of studies suggesting a worse outcome for BRCA-mutated breast cancer [24,25,26,27,28,29,30,31].
Despite these limitations, our study has several strengths. This is the first study to investigate the prognostic impact of tumor count in patients with T1 breast cancer utilizing a national database that encompasses a large and representative sample of patients with multiple breast cancers. We included ER and HER2 statuses, providing a more comprehensive assessment of prognostic factors than assessments limited to ER analysis [4].
5. Conclusions
Our findings suggest that tumor burden may be a significant prognostic factor for multiple breast cancers, particularly in patients with three or more tumors. These findings may inform treatment decisions, potentially favoring escalated therapeutic approaches for patients with a higher tumor burden. However, further research is needed to address these limitations, particularly regarding tumor heterogeneity. Future studies incorporating molecular analyses and rigorous classification criteria could definitively establish the role of these factors in predicting prognoses and guiding personalized treatment strategies in patients with multiple breast cancers.
Acknowledgments
This research was supported by the Korean Breast Cancer Society.
Author Contributions
Conceptualization, H.G. and S.H.K.; methodology, H.G.; formal analysis, H.G.; investigation, H.G.; resources, H.G.; data curation, H.G.; writing—original draft preparation, H.G.; writing—review and editing, H.G. and S.H.K.; visualization, H.G.; supervision, H.G.; project administration, H.G.; funding acquisition, H.G. Data curation, S.H.J., Y.J.S., S.J.N., J.H.H., S.J.O. and E.H.P.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to using a publicly available dataset.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Hallym University Research Fund 2023 (HURF-2023-49).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lynch S.P., Lei X., Chavez-MacGregor M., Hsu L., Meric-Bernstam F., Buchholz T.A., Zhang A., Hortobagyi G.N., Valero V., Gonzalez-Angulo A.M. Multifocality and multicentricity in breast cancer and survival outcomes. Ann. Oncol. 2012;23:3063–3069. doi: 10.1093/annonc/mds136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano A.E., Edge S.B., Hortobagyi G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018;25:1783–1785. doi: 10.1245/s10434-018-6486-6. [DOI] [PubMed] [Google Scholar]
- 3.Kapoor N.S., Shamonki J., Sim M.S., Chung C.T., Giuliano A.E. Impact of multifocality and lymph node metastasis on the prognosis and management of microinvasive breast cancer. Ann. Surg. Oncol. 2013;20:2576–2581. doi: 10.1245/s10434-013-2924-7. [DOI] [PubMed] [Google Scholar]
- 4.Vera-Badillo F.E., Napoleone M., Ocana A., Templeton A.J., Seruga B., Al-Mubarak M., AlHashem H., Tannock I.F., Amir E. Effect of multifocality and multicentricity on outcome in early stage breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2014;146:235–244. doi: 10.1007/s10549-014-3018-3. [DOI] [PubMed] [Google Scholar]
- 5.Boyages J., Jayasinghe U.W., Coombs N. Multifocal breast cancer and survival: Each focus does matter particularly for larger tumors. Eur. J. Cancer. 2010;46:1990–1996. doi: 10.1016/j.ejca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Chu J., Bae H., Seo Y., Cho S.Y., Kim S.H., Cho E.Y. The prognostic impact of synchronous ipsilateral multiple breast cancer: Survival outcomes according to the eighth American Joint Committee on Cancer staging and molecular subtype. J. Pathol. Transl. Med. 2018;52:396–403. doi: 10.4132/jptm.2018.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fushimi A., Yoshida A., Yagata H., Takahashi O., Hayashi N., Suzuki K., Tsunoda H., Nakamura S., Yamauchi H. Prognostic impact of multifocal and multicentric breast cancer versus unifocal breast cancer. Surg. Today. 2019;49:224–230. doi: 10.1007/s00595-018-1725-9. [DOI] [PubMed] [Google Scholar]
- 8.Coombs N.J., Boyages J. Multifocal and multicentric breast cancer: Does each focus matter? J. Clin. Oncol. 2005;23:7497–7502. doi: 10.1200/JCO.2005.02.1147. [DOI] [PubMed] [Google Scholar]
- 9.Chung A.P., Huynh K., Kidner T., Mirzadehgan P., Sim M.S., Giuliano A.E. Comparison of outcomes of breast conserving therapy in multifocal and unifocal invasive breast cancer. J. Am. Coll. Surg. 2012;215:137–146. doi: 10.1016/j.jamcollsurg.2012.05.006. discussion 137–146. [DOI] [PubMed] [Google Scholar]
- 10.Vlastos G., Rubio I.T., Mirza N.Q., Newman L.A., Aurora R., Alderfer J., Buzdar A.U., Singletary S.E. Impact of multicentricity on clinical outcomes in patients with T1-2, N0-1, M0 breast cancer. Ann. Surg. Oncol. 2000;7:581–587. doi: 10.1007/BF02725337. [DOI] [PubMed] [Google Scholar]
- 11.Joergensen L.E., Gunnarsdottir K.A., Lanng C., Moeller S., Rasmussen B.B. Multifocality as a prognostic factor in breast cancer patients registered in Danish Breast Cancer Cooperative Group (DBCG) 1996–2001. Breast. 2008;17:587–591. doi: 10.1016/j.breast.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Amin M.B., Greene F.L., Edge S., Compton C.C., Gershenwald J.E., Brookland R.K., Meyer L., Gress D.M., Byrd D.R., Winchester D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer. J. Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 13.Uthamalingam P., Rangarajan B., Sekar P., Mehta S. Impact of focality on prognostication of early and operable breast carcinomas of no special type. J. Cancer. Res. Ther. 2019;15:1024–1030. doi: 10.4103/jcrt.JCRT_804_17. [DOI] [PubMed] [Google Scholar]
- 14.Schneider J., Lee H.J., Nam S.J., Lee S.J., Jung J.H., Jung S.H., Lim S.T., Jeon Y.W., Gwak H. Relative survival benefit by hormonal receptor status of adding trastuzumab to neoadjuvant chemotherapy in breast cancer patients. J. Breast. Cancer. 2020;23:259–267. doi: 10.4048/jbc.2020.23.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwak G., Lee H.K., Kim H.J., Lee S.Y., Park Y.L., Lee J.W., Kim S.G., Huh H., Shin H., Kim J.R., et al. Korean Breast Cancer Society; Korea Breast Cancer Foundation. Survey of the application of the korean clinical practice recommendations on breast cancer treatment: The utility of the korean breast cancer society guidelines. J. Breast Cancer. 2012;15:239–243. doi: 10.4048/jbc.2012.15.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAfee A.T., Ming E.E., Seeger J.D., Quinn S.G., Ng E.W., Danielson J.D., Cutone J.A., Fox J.C., Walker A.M. The comparative safety of rosuvastatin: A retrospective matched cohort study in over 48,000 initiators of statin therapy. Pharmacoepidemiol. Drug Saf. 2006;15:444–453. doi: 10.1002/pds.1281. [DOI] [PubMed] [Google Scholar]
- 17.Waks A.G., Winer E.P. Breast Cancer Treatment: A Review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 18.Egan R.L. Multicentric breast carcinomas: Clinical-radiographic-pathologic whole organ studies and 10-year survival. Cancer. 1982;49:1123–1130. doi: 10.1002/1097-0142(19820315)49:6<1123::AID-CNCR2820490610>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Moon H.G., Han W., Kim J.Y., Kim S.J., Yoon J.H., Oh S.J., Yu J.H., Noh D.Y. Effect of multiple invasive foci on breast cancer outcomes according to the molecular subtypes: A report from the Korean Breast Cancer Society. Ann. Oncol. 2013;24:2298–2304. doi: 10.1093/annonc/mdt187. [DOI] [PubMed] [Google Scholar]
- 20.Ozturk A., Ilgun S., Ucuncu M., Gachayev F., Ordu C., Alco G., Elbuken F., Erdogan Z., Duymaz T., Aktepe F., et al. The effect of multifocal and multicentric tumours on local recurrence and survival outcomes in breast cancer. J. BUON. 2021;26:196–203. [PubMed] [Google Scholar]
- 21.Buggi F., Folli S., Curcio A., Casadei-Giunchi D., Rocca A., Pietri E., Medri L., Serra L. Multicentric/multifocal breast cancer with a single histotype: Is the biological characterization of all individual foci justified? Ann. Oncol. 2012;23:2042–2046. doi: 10.1093/annonc/mdr570. [DOI] [PubMed] [Google Scholar]
- 22.Pekar G., Gere M., Tarjan M., Hellberg D., Tot T. Molecular phenotype of the foci in multifocal invasive breast carcinomas: Intertumoral heterogeneity is related to shorter survival and may influence the choice of therapy. Cancer. 2014;120:26–34. doi: 10.1002/cncr.28375. [DOI] [PubMed] [Google Scholar]
- 23.Warner E., Zhu S., Plewes D.B., Hill K., Ramsay E.A., Causer P.A., Seely J., Jong R.A., Lenkov P., Elser C., et al. Breast Cancer Mortality among Women with a BRCA1 or BRCA2 Mutation in a Magnetic Resonance Imaging Plus Mammography Screening Program. Cancers. 2020;12:3479. doi: 10.3390/cancers12113479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antunes Meireles P., Fragoso S., Duarte T., Santos S., Bexiga C., Nejo P., Luís A., Mira B., Miguel I., Rodrigues P., et al. Comparing Prognosis for BRCA1, BRCA2, and Non-BRCA Breast Cancer. Cancers. 2023;15:5699. doi: 10.3390/cancers15235699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin T.Y., Park K.S., Nam S.E., Yoo Y.B., Park W.S., Yun I.J. BRCA1/2 Serves as a Biomarker for Poor Prognosis in Breast Carcinoma. Int. J. Mol. Sci. 2022;23:3754. doi: 10.3390/ijms23073754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conte L., Rizzo E., Civino E., Tarantino P., De Nunzio G., De Matteis E. Enhancing Breast Cancer Risk Prediction with Machine Learning: Integrating BMI, Smoking Habits, Hormonal Dynamics, and BRCA Gene Mutations—A Game-Changer Compared to Traditional Statistical Models? Appl. Sci. 2024;14:8474. doi: 10.3390/app14188474. [DOI] [Google Scholar]
- 27.Tinterri C., Di Maria Grimaldi S., Sagona A., Barbieri E., Darwish S., Bottini A., Canavese G., Gentile D. Comparison of Long-Term Oncological Results in Young Women with Breast Cancer between BRCA-Mutation Carriers Versus Non-Carriers: How Tumor and Genetic Risk Factors Influence the Clinical Prognosis. Cancers. 2023;15:4177. doi: 10.3390/cancers15164177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huszno J., Kołosza Z., Grzybowska E. BRCA1 mutation in breast cancer patients: Analysis of prognostic factors and survival. Oncol. Lett. 2019;17:1986–1995. doi: 10.3892/ol.2018.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Talhouet S., Peron J., Vuilleumier A., Friedlaender A., Viassolo V., Ayme A., Bodmer A., Treilleux I., Lang N., Christophe Tille J., et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci. Rep. 2020;10:7073. doi: 10.1038/s41598-020-63759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huzarski T., Byrski T., Gronwald J., Górski B., Domagala P., Cybulski C., Oszurek O., Szwiec M., Gugala K., Stawicka M., et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J. Clin. Oncol. 2013;31:3191–3196. doi: 10.1200/JCO.2012.45.3571. [DOI] [PubMed] [Google Scholar]
- 31.Park W.K., Chung S.Y., Jung Y.J., Ha C., Kim J.W., Nam S.J., Kim S.W., Yu J., Chae B.J., Lee J.E., et al. Long-term oncologic outcomes of unselected triple-negative breast cancer patients according to BRCA1/2 mutations. NPJ Precis. Oncol. 2024;30:96. doi: 10.1038/s41698-024-00559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.