Abstract
The reduction in fibre length of muscles immobilised in a shortened position is accompanied by reduced compliance of the muscle. Since the intramuscular connective tissue framework distributes the forces passively imposed on a muscle by stretching, it was decided to investigate the amount and distribution of connective tissue in immobilised muscles. Biochemical analysis of the hydroxyproline content of muscles immobilised in the shortened position for different periods of time showed an increase in the ratio of collagen to muscle fibre tissue. This occurred during the first few days of immobilisation, before there was any significant loss of sarcomeres. Thus the increase in connective tissue appeared to result directly from immobilisation rather than from redistribution of connective tissue, following shortening of the fibres. A detailed histological analysis of muscle sections stained for connective tissue with Sirius Red showed that the early increase in connective tissue in immobilised muscles occurred in the perimysium rather than the endomysium, although after a longer period of immobilisation there was also a thickening of the endomysium. Ultrastructural analysis of the perimysium in normal muscle showed that the angle the collagen fibres made with the muscle fibres changed with the state of stretch of the muscle; when the muscle was shortened, the angle was larger than when the muscle was lengthened. In immobilised muscle, collagen fibres were found to be arranged at a more acute angle to the axis of the muscle fibres than was found in normal muscle; this would be expected to affect the compliance of the muscle. The experiments described indicate that the increased stiffness of immobilised muscles could result from both quantitative and qualitative changes in the connective tissue.
Full text
PDF
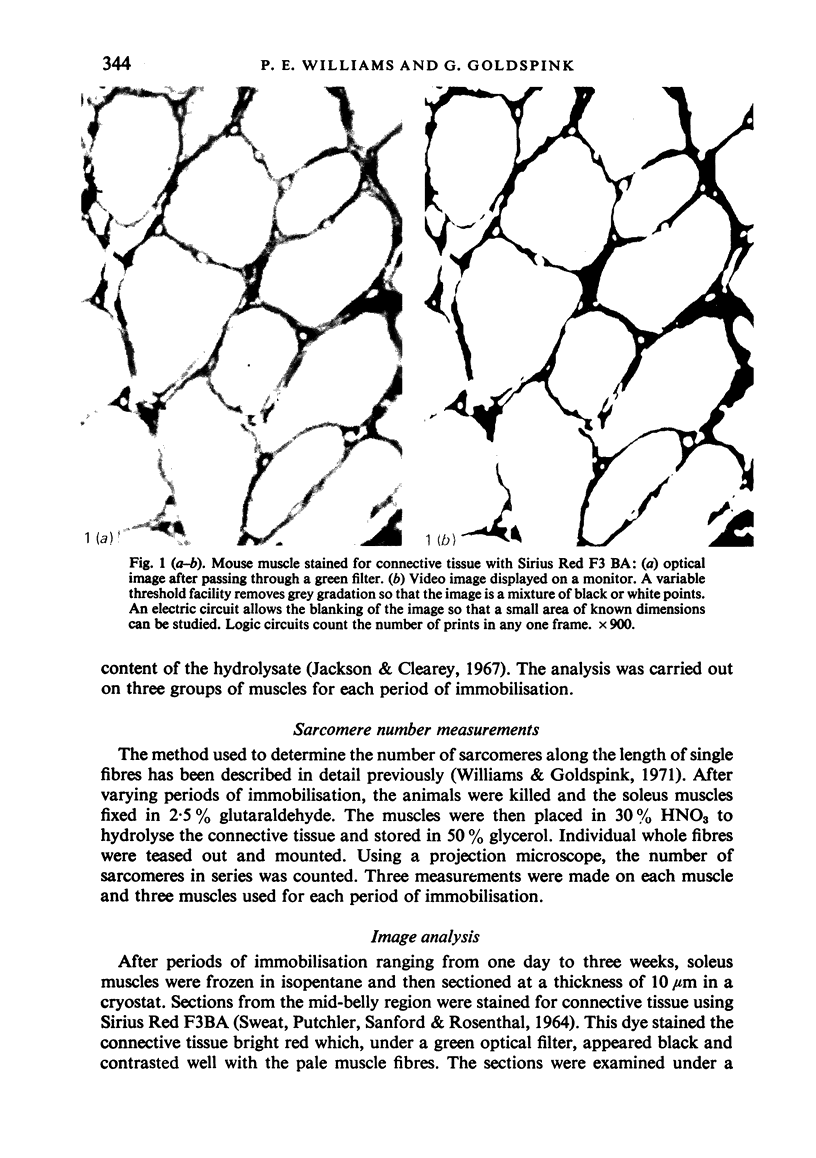

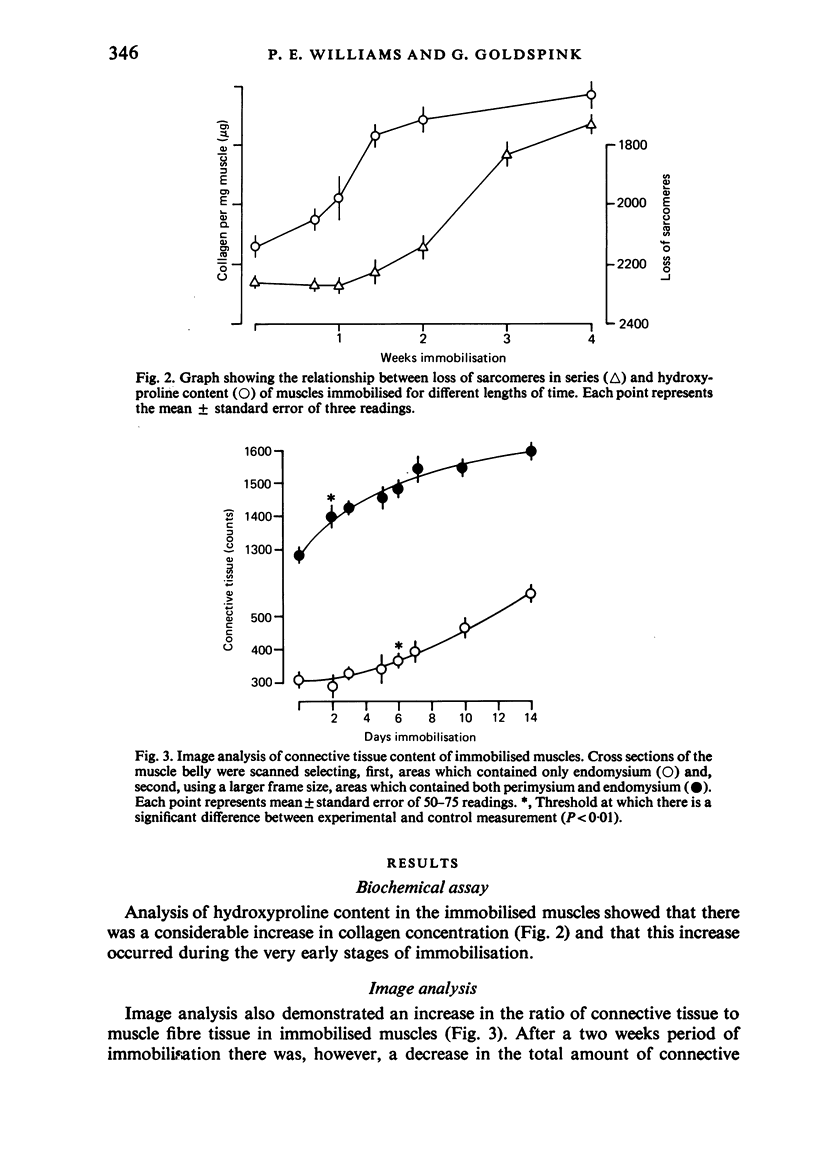
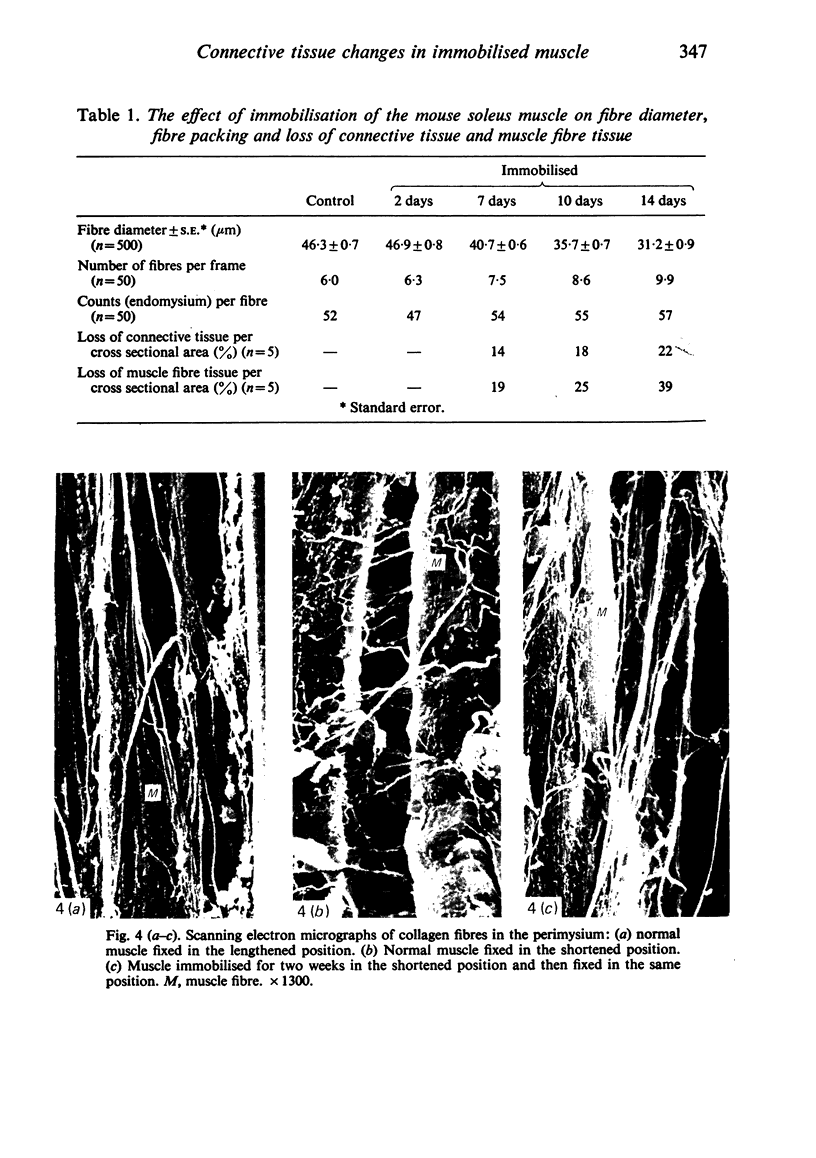



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borg T. K., Caulfield J. B. Morphology of connective tissue in skeletal muscle. Tissue Cell. 1980;12(1):197–207. doi: 10.1016/0040-8166(80)90061-0. [DOI] [PubMed] [Google Scholar]
- Brooks J. E. Disuse atrophy of muscle. Intracellular electromyography. Arch Neurol. 1970 Jan;22(1):27–30. doi: 10.1001/archneur.1970.00480190031005. [DOI] [PubMed] [Google Scholar]
- Caulfield J. B., Borg T. K. The collagen network of the heart. Lab Invest. 1979 Mar;40(3):364–372. [PubMed] [Google Scholar]
- GRANT R. A. ESTIMATION OF HYDROXYPROLINE BY THE AUTOANALYSER. J Clin Pathol. 1964 Nov;17:685–686. doi: 10.1136/jcp.17.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G., Williams P. E. The nature of the increased passive resistance in muscle following immobilization of the mouse soleus muscle [proceedings]. J Physiol. 1979 Apr;289:55P–55P. [PubMed] [Google Scholar]
- HILL A. V. The mechanics of active muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):104–117. doi: 10.1098/rspb.1953.0027. [DOI] [PubMed] [Google Scholar]
- HILL A. V. The series elastic component of muscle. Proc R Soc Lond B Biol Sci. 1950 Jul 24;137(887):273–280. doi: 10.1098/rspb.1950.0035. [DOI] [PubMed] [Google Scholar]
- Jackson D. S., Cleary E. G. The determination of collagen and elastin. Methods Biochem Anal. 1967;15:25–76. doi: 10.1002/9780470110331.ch2. [DOI] [PubMed] [Google Scholar]
- McLaughlin R. J., Sonnenblick E. H. Time behavior of series elasticity in cardiac muscle. Real-time measurement by controlled-length techniques. Circ Res. 1974 Jun;34(6):798–811. doi: 10.1161/01.res.34.6.798. [DOI] [PubMed] [Google Scholar]
- Rowe R. W. Morphology of perimysial and endomysial connective tissue in skeletal muscle. Tissue Cell. 1981;13(4):681–690. doi: 10.1016/s0040-8166(81)80005-5. [DOI] [PubMed] [Google Scholar]
- SWEAT F., PUCHTLER H., ROSENTHAL S. I. SIRIUS RED F3BA AS A STAIN FOR CONNECTIVE TISSUE. Arch Pathol. 1964 Jul;78:69–72. [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. Changes in sarcomere length and physiological properties in immobilized muscle. J Anat. 1978 Dec;127(Pt 3):459–468. [PMC free article] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. Longitudinal growth of striated muscle fibres. J Cell Sci. 1971 Nov;9(3):751–767. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973 Oct;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]
- Winegrad S., Robinson T. F. Force generation among cells in the relaxing heart. Eur J Cardiol. 1978 Jun;7 (Suppl):63–70. [PubMed] [Google Scholar]




