Abstract
Purpose:
To evaluate the use of hydrogen peroxide as an adjunct to ultrasonication (US) in biofilm removal and whether it can limit the spread of viable microorganisms in the aerosol.
Materials and Methods:
Multi-species biofilms were formed on dentin disks and titanium disks fixed on a plastic surface. After placing the specimens in a periodontal pocket model, an ultrasonic scaler was applied for 30 s, in part combined with 0.25% or 0.5% H2O2. After treatment, the remaining biofilm was analysed for bacterial counts (colony forming units [CFU]), biofilm quantity and metabolic activity. Further, the cytotoxic effect of hydrogen peroxide on periodontal ligament fibroblasts was assessed and the spread of bacteria in aerosol was quantified.
Results:
Ultrasonication reduced bacterial counts in biofilm, biofilm mass and metabolic activity on both dentin and titanium disks. Adjunctive use of 0.25% and 0.5% H2O2 more effectively reduced the viable bacteria in biofilm than ultrasonication alone; this was also found on both dentin and titanium. The different concentrations of H2O2 did not lead to corresponding differences in bacterial mass and metabolic activity. The spread of bacteria through aerosols was statistically significantly reduced when adjunctive H2O2 was used. However, a certain cytotoxic effect on periodontal ligament fibroblasts by H2O2 could not be ruled out.
Conclusions:
Irrigating with H2O2 during periodontal instrumentation with an ultrasonic scaler increases the reduction of viable bacteria within biofilms. It might limit bacterial spreading via aerosols.
Key words: aerosol, biofilm, hydrogen peroxide, periodontal therapy
Periodontitis is a chronic inflammatory disease of the tooth supporting tissues associated with high counts of certain bacterial species interacting with the host immune system.4 The primary goal in cause-related periodontitis treatment is to remove hard and soft bacterial deposits, which should result in a smooth and biocompatible root surface to facilitate fibroblast attachment and minimise bacterial adhesion.3 Despite the fact that hand instruments (i.e. curettes) are still widely used, ultrasonic scalers have gained popularity in dental practice. Comparisons between hand instruments and sonic and ultrasonic scalers did not show a clear advantage for any procedure,26 and traumatisation immediately after instrumentation is similar.2
Attempts were made to further improve the removal of the biofilms by using an ultrasonic scaler. A recent systematic review evaluated the adjunctive use of antiseptics in clinical trials:27 chlorhexidine, essential oils and povidone iodine were used and in part showed beneficial effects.
Over the last 20 to 30 years, dental implants have been widely used to replace missing teeth. The need for prevention and treatment of peri-implant diseases is continually increasing. The prevalence of inflammatory peri-implant diseases is high. In a recent systematic review, the weighted mean prevalence of peri-implant mucositis and peri-implantitis among individuals with implants was 43% and 22%, respectively.5
In the 1990s, a low-concentration hydrogen peroxide solution (≤ 3%) was discussed as being beneficial in reducing plaque scores and gingival inflammation.18 Hydrogen peroxide (H2O2) shows oxidising and antiseptic properties. It is effective against a wide range of viruses and bacteria. It exerts its antimicrobial effects by increasing cellular oxidative stress, thereby upregulating the expression of TLR3 and NF-kB, both of which activate the innate local immune response. Hydroxyl radicals that result from H2O2 cause lethal oxidative damage to microbes, e.g. via DNA oxidation or lipid peroxidation.23 For instance, H2O2 disinfectant proved to inactivate porcine epidemic diarrhea virus in swine feces on metal surfaces,10 and at a concentration of 3% was effective against rabies virus.1
The outbreak of a novel coronavirus, SARS-CoV-2, in China in December 2019 and its rapid spread raised serious global health concerns. Airborne transmission via respiratory droplets and aerosols, that are abundantly formed during dental treatment, is meanwhile considered the main route of infection.28 SARS-CoV-2 infected patients usually carry high loads of the virus in the oropharynx and the oral cavity. Therefore, pretreatment mouthrinses have been proposed to reduce the risk of nosocomial infections by healthcare professionals with close contact to this area, such as dentists or otorhinolaryngologists. One agent that has been proposed in this context is hydrogen peroxide.
A recent review12 including 26 studies showed that human coronaviruses such as Severe Acute Respiratory Syndrome (SARS) virus and Middle East Respiratory Syndrome (MERS) virus can persist on inanimate surfaces, but can effectively be inactivated by 0.5% H2O2 within 1 min.
Therefore, the purpose of the study was to evaluate in an established in vitro model for testing non-surgical therapy: a) the removal of biofilms, b) the potential cytotoxic activity on periodontal ligament cells and c) the spread of viable microorganisms in the aerosol.
The hypothesis was that the adjunctive use of hydrogen peroxide with ultrasonication (US) more effectively removes biofilms and viable microorganisms in aerosol than US alone. The primary outcome is that 0.25% hydrogen peroxide applied for 30 s and left in place thereafter for another 30 s reduces bacterial counts in a 10-species biofilm by about 1 log10 CFU more than US alone.
Materials and Methods
Ultrasonic Scaler
The study employed an established in vitro model for testing non-surgical therapy,8 using two different surfaces as test specimens (i.e. hydroxyapatite and SLA [sandblasted, large grit, acid-etched] titanium).
In all groups except for the negative control, a piezo scaler (W&H; Bürmoos, Austria) with 2U tips (W&H) for hydroxyapatite surfaces and tip 1I Implant-clean (W&H) for titanium surfaces was used. The exact parameters of the device (angle, distance to the surface, water and power setting) were set according to the manufacturer’s recommendation.
Biofilm Removal from Dentin and Titanium Specimens
Specimens
From a pool of extracted teeth, dentin disks with a diameter of 5 mm were prepared. Before extraction, patients were informed about the use of their teeth for research purposes and their spoken consent was obtained. The present experiment was carried out in accordance with the approved guidelines and regulations of the local ethics committee (KEK) for irreversibly anonymised samples. These dentin disks (diameter 5 mm) and the titanium disks (diameter 5 mm, with SLA surface) were fixed on a plastic surface. In preliminary tests, stable bonding to the plastic surface as the carrier was established.
Biofilm formation
All disks were colonised with a biofilm. For biofilm formation, a multiple species mixture consisting of 10 bacterial strains (Streptococcus gordonii ATCC 10558, Actinomyces naeslundii ATCC 12104, Fusobacterium nucleatum ATCC 25586, Campylobacter rectus ATCC 33238, Eikenella corrodens ATCC 23834, Parvimonas micra ATCC 33270, Prevotella intermedia ATCC 25611, Porphyromonas gingivalis ATCC 33277, Tannerella forsythia ATCC 43037, Treponema denticola ATCC 35405) was prepared. Before an experiment, all strains (except for T. denticola ATCC 35405) were precultivated on Schaedler agar plates (Oxoid; Basingstoke, UK) with 5% sheep blood in an anaerobic atmosphere or with 5% CO2 (S. gordonii ATCC 10558). T. denticola ATCC 35405 was anaerobically cultured in modified mycoplasma broth (BD; Franklin Lakes, NJ, USA) to which 1 mg/ml glucose, 400 µg/ml niacinamide, 150 µg/ml spermine tetrahydrochloride, 20 µg/ml Na isobutyrate enriched with 1 g/ml cysteine and 5 µg/ml cocaboxylase had been added.
First, 1.5% bovine serum albumin (BSA) solution was added to the disks for 10 min before placing them in tubes. Then the bacterial suspension was added. The medium was first changed after 48 h. P. gingivalis ATCC 33277, T. forsythia ATCC 43037 and T. denticola ATCC 35405 were again added to the nutrient medium before application on the disks. The renewed addition of selected bacterial strains guaranteed a sufficient number of these species in the biofilms.
Treatment
Treatment of specimens started after 3.5 days of biofilm formation. The disks were placed in the periodontal pocket model.8
The treatment protocol included two different modalities (1 and 2) and each with one treated control (US only, 3.) and one untreated control (4).
Specimens were treated with US combined with 0.25% H2O2 for 30 s
Specimens were treated with US combined with 0.5% H2O2 for 30 s
Specimens were treated with US only for 30 s
Specimens were left untreated.
All treatments were performed by the same calibrated operator (CL). After treatment, the remaining biofilm was analysed.
Analysis of Biofilm
At least six independent results were achieved for each type of treatment, disk surface and experiment.
On colonised and treated disks, biofilm samples were scraped from the surface and suspended in 0.9% NaCl solution. After making a serial dilution of each, 25 µl were spread on Schaedler agar plates, incubated under anaerobic conditions, and the total CFU were counted and recorded. The biofilms were quantified according to recently published protocols.16 After rinsing and heat-fixing the biofilms at 60°C, biofilms were stained with 50 µl of 0.06% (w/v) crystal violet (Sigma-Aldrich; St Louis, MO, USA) per well for 10 min. The staining was quantified using a plate reader (ELx808, Biotek Instruments; Winooski, VT, USA) at 600 nm.
Biofilm Metabolic Activity
Biofilm metabolic activity was assessed using Alamar blue as a redox indicator.22 Five µl of Alamar blue (alamarBlue reagent, Thermo Fisher Scientific; Waltham, MA, USA) was mixed with 100 µl of the nutrient medium and added to the biofilm. After extensive mixing with the biofilm and an incubation for 1 h at 37°C, absorbance was measured at 570 nm against 600 nm by using a microplate reader (ELx808, Biotek).
Experiments were made each in triplicates in three independent series resulting in at least nine single values each.
Cytotoxic Effect of Treatment on Periodontal Ligament Fibroblasts
Human periodontal ligament fibroblasts were anonymously collected from patients during regular surgical treatment (wisdom tooth removal) following written informed consent. This did not require approval by the Ethics Committee of the University of Bern. Fibroblasts were placed in T-25 cell culture flasks containing DMEM (Life Technologies / Invitrogen; Paisley, UK) with 10% foetal calf serum (FCS; Life Technologies / Invitrogen) to grow to confluency. At the beginning of the experiments, the fibroblasts were always in the 4th–6th passage.
Monolayers of fibroblasts were exposed to hydrogen peroxide solutions in different concentrations (two-fold dilution series, starting from 0.5% down to 0.008%). After 30 s, the hydrogen peroxide solution was replaced by cell cultivation medium and cells were cultivated for an additional 6 h before cytotoxicity against cells was determined by MTT assay according to Mosmann.20 Experiments were made in independent sextuplicates.
Spreading of Aerosols
Biofilms were formed in 96-well-plates as described before. Nutrient broth was removed. Then the tip of the ultrasonic device was placed close to the bottom and the biofilm was treated for 30 s. Agar plates (diameter 9 cm) were placed at a distance of 5 cm. After the treatment, the plates were incubated anaerobically.
Statistical Analysis
ANOVA with a post-hoc Bonferroni test was used for statistical analysis. The level of significance was set at p = 0.05. SPSS 24.0 software (IBM; Armonk, NY, USA) was used.
Results
Biofilm Reduction
Ultrasonication with water as a cooling medium decreased bacterial counts in biofilm by 3 log10 CFU (dentin) and 2.60 log10 CFU (titanium), and it also reduced biofim mass and metabolic activity. Ultrasonication of dentin disks with both 0.25% and 0.5% H2O2 statistically significantly (p < 0.001) reduced the bacteria in the biofilm compared to US alone (Fig 1). Similar results were observed for titanium disks. Both concentrations statistically significantly reduced bacterial counts compared to US alone (Fig 2). With regard to bacterial mass and metabolic activity, no differences between H2O2 concentrations were detected (Figs 1 and 2).
Fig 1.
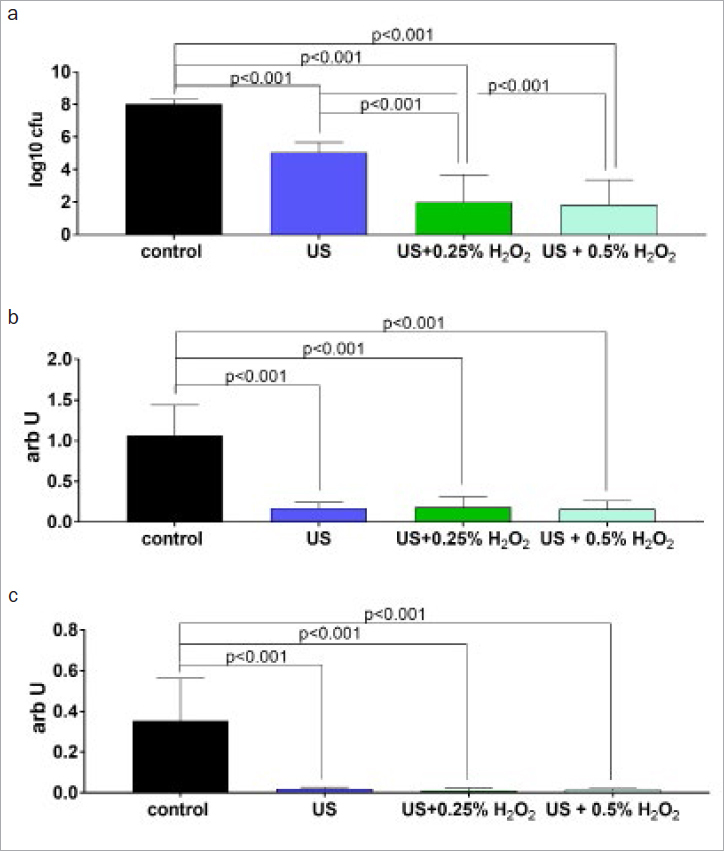
Mean (and SD) of total counts of colony forming units (CFU; a), mass (b) and metabolic activity in biofilm on dentin disks after 30 s of instrumentation with an ultrasonic scaler (US) without and with 0.25% and 0.5% H2O2.
Fig 2.
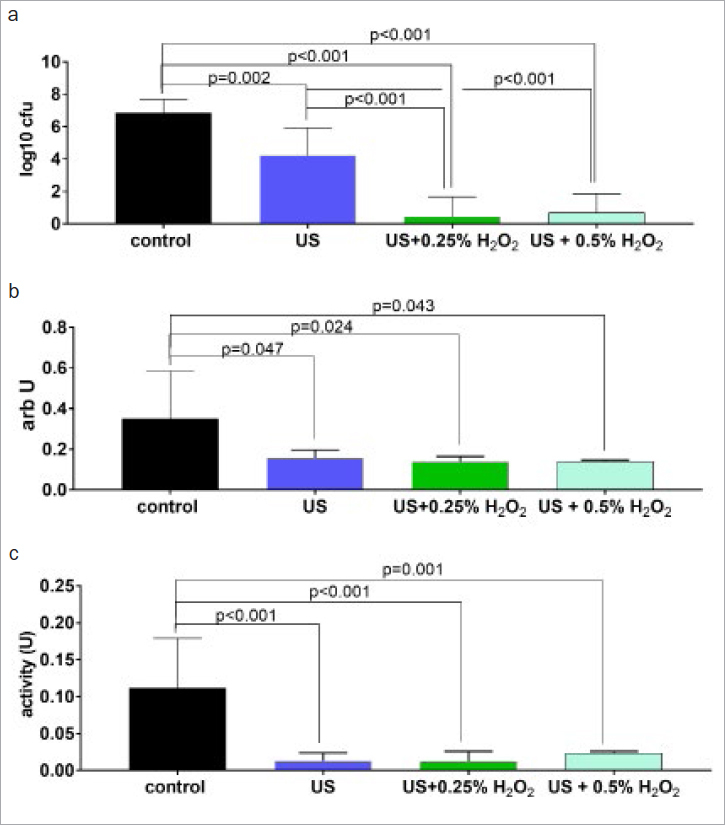
Mean (and SD) of total counts of colony forming units (CFU; A), mass and metabolic activity in biofilm on titanium disks after 30 s of instrumentation with an ultrasonic scaler (US) without and with 0.25% and 0.5% H2O2.
Spreading of Aerosols
Instrumentation in the periodontal pocket was always performed with an efficaceous evaporation system. The first-performed analyses in the surroundings resulted in only negative results. Subsequently, the protocol was repeated without evaporation, and the results (Table 1) showed a reduction of viable bacteria following the adjunctive use of H2O2 as indicated by the numbers of overall positive samples and the mean counts of positive samples.
Table 1.
Bacterial CFU per 63.5 cm2 in agar plates at a distance of 5 cm from the ultrasonic scaler application site
| Positive samples | Positive samples (%) | Mean count (CFU) of positive samples | |
|---|---|---|---|
| US | 4/4 | 100 | 17.75 |
| US + 0.25% H2O2 | 2/4 | 50 | 7.5 |
| US + 0.5% H2O2 | 1/4 | 25 | 0.5 |
Vitality of Periodontal Ligament Fibroblasts
In the assays, periodontal ligament fibroblasts of two donors were used. Whereas cells of donor 2 lost vitality only when exposed to 0.25% and to 0.5% hydrogen peroxide, the cells obtained from donor 1 showed less vitality after all concentrations of hydrogen peroxide tested (Fig 3).
Fig 3.
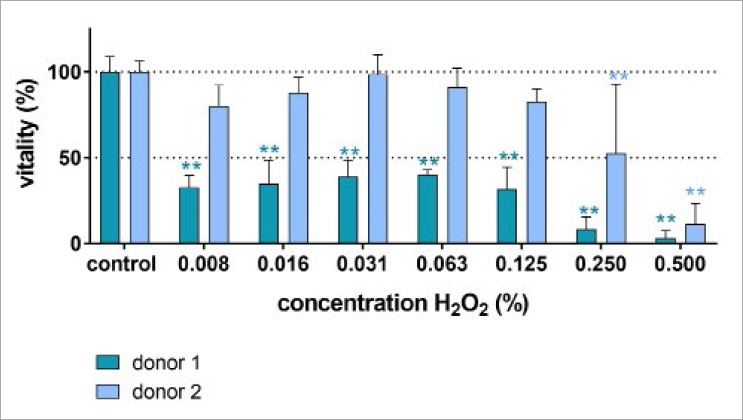
MTT ((3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide) tetrazolium) assay assessing the vitality of the periodontal ligament fibroblasts after exposure to different concentrations of hydrogen peroxide for 30 s and subsequent cultivation for 6 h. ** p<0.01 vs the unexposed control of the respective donor.
Discussion
This research was prompted not only by the intention to disinfect periodontal or peri-implant pockets concomitant with mechanical debridement, but also by the current global health concern about the SARS-CoV-2 pandemic and the ongoing discussion on aerosol contamination. The objective of the present in vitro study was to investigate whether the use of H2O2 solution adjunctive to an ultrasonic scaler augments the antibiofilm effect when used on dentin and titanium surfaces and if it was bactericidally active in the generated aerosols.
Our results demonstrated that H2O2 is able to reduce bacterial counts in biofilms on both dentin and titanium surfaces after instrumentation. However, H2O2 could not statistically significantly reduce the metabolic activity of bacteria within the remaining biofilm on both dentin and titanium surfaces. Furthermore, H2O2 showed the potential to reduce the spreading of bacteria via aerosols that are generated during mechanical debridement of inflamed periodontal or peri-implant pockets. Thus, this property might also be relevant for reducing viral transmission through aerosols.
H2O2 plays an important role in biofilm homeostasis. It is the major substance synthesised by commensal oral species to inhibit growth of pathogens.9 Oral streptococci represent the predominant species which are known to produce H2O2.14 Among them, Streptococcus sanguinis, Streptococcus gordonii, and Streptococcus oralis show inhibitory effects on the growth of A. actinomycetemcomitans, P. gingivalis and P. intermedia. Environmental alterations, however, can substantially influence or even neutralise this inhibitory effect of H2O2 and shift the equilibrium within the biofilm towards dysbiosis.
Previous research demonstrated that hydroxyl radicals generated from H2O2 by photocatalysis and applied after non-surgical therapy with US were more effective in reducing pocket probing depths and improving clinical attachment levels than US alone or US plus locally delivered minocycline gel.13 It can be speculated that these results might be a consequence of the bactericidal effect of H2O2 at the bottom of the periodontal pocket, creating an environment facilitating the healing process. The instability of hydrogen peroxide limits its use as a therapeutic agent; hydroxyl radicals generated by H2O2 have a very short half-life of approximately 10-9 s.11,25 Several attempts have been made to overcome this problem, e.g. silver was added to stabilise the molecule.19
In this study, we assessed bacterial counts in biofilms after treatment with US, US+0.25% H2O2 and US+0.5% H2O2. We found a statistically significantly stronger inhibitory effect when adjunctive H2O2 was used. Interestingly, no statistically significant difference between the two concentrations used was observed. These positive effects of H2O2 are in keeping with the findings of previous studies that reported similar effects when Staphylococcus aureus suspensions were exposed to H2O2 together with laser light and ultrasound, while ultrasonic irrigation alone had no effect.23 Moreover, in terms of oxidative stress, the combination of H2O2, laser light and US was most effective in inducing oxidative damage of bacterial DNA.23 A recent study treated titanium surfaces colonised by biofilms characteristic of peri-implantitis with an antimicrobial therapy employing H2O2 photolysis.11,21 Similar to our experimental setting, titanium disks colonised by a 3-species biofilm were subjected to ultrasonic scaling and application of 3% H2O2 photolysis. The results are in agreement with those of the present study, despite the differences in biofilm composition and H2O2 concentration (i.e. ultrasound scaling eliminated large portions of the biofilm by 3 log10, while additional treatment with H2O2 resulted in no detectable viable bacteria).21
Subsequently, we attempted to determine the biofilm mass and metabolic activity of the biofilm. All biofilms treated with US revealed a statistically significant reduction compared to the untreated control. However, no statistically significant differences among the groups were observed, pointing to the obvious effects exerted by US.
Next we looked at the spreading of bacteria via aerosols. A first round of experiments was performed using an effective evaporation system, while the next series was done without. While no spreading was observed when evaporation was used, ultrasonication alone without evaporation resulted in a conspicuous spreading of bacteria with 100% infected wells at a distance of 5 cm. Interestingly, H2O2 statistically significantly reduced the spreading effect. Clinically, these results indicate that 1. suction during mechanical therapy might be of importance to reduce bacterial loads of aerosols and 2. that H2O2 irrigation might further prevent bacterial spreading. In this context, a prospective clinical trial investigated the viral load of hospitalised SARS-CoV-2 patients before and after mouthrinsing with 1% H2O2 and found no statistically significant change in the oral viral load.6 However, the viral load was tested on RNA level, and these results indicate that H2O2 may not be able to inactivate RNA even though the viral cells might have been destroyed.
Another important aspect that needs to be discussed is the fact that bactericidal effects largely depend on whether bacteria are planktonic or in established biofilms. For instance, antibacterial photodynamic therapy (aPDT) shows substantially less activity on established biofilms than on planktonic periodontopathogenic bacteria.15 To increase the activity also against an established biofilm, an adjunctive irrigation with H2O2 might exert a synergistic effect. This question was investigated in a previous study by our group, also using the pocket model.15 While biofilms treated mechanically or with aPDT were reduced by less than 0.5 log10, the use of 3% H2O2 after mechanical therapy or aPDT completely eradicated the bacteria. Without mechanical therapy or aPDT and for biofilms cultivated in well plates, 3% H2O2 caused a reduction by 4 log10.15 The bactericidal potential of H2O2 is further bolstered by its effect on antibiotic-resistant bacteria, another emerging major threat to global health. H2O2 in combination with UV irradiation enhanced killing of methicillin-resistant Staphylococcus aureus (MRSA) and multi-drug resistant Pseudomonas aeruginosa.7
For the present study, we used the aforementioned periodontal pocket model. Further, in order to better mimic the periodontal pocket with its complex biofilm structure, all specimens were incubated with a multiple-species mixture consisting of 10 bacterial strains. Then, the specimens were inserted into the pocket model. Nevertheless, this model has some limitations, as the cellular interactions and cytokine secretion of epithelial cells, periodontal ligament cells, fibroblasts, macrophages and other cells stemming from the hematopoietic lineage are lacking. Neither does the oxygen content or pH reflect the in vivo situation. Thus, due to these missing manifold interactions, it is very difficult to extrapolate the results obtained in the present study to the in vivo situation. It cannot be ruled out that in a real clinical situation, it is more challenging to reach difficult anatomical areas/site of inflammation, such as furcations or deep pockets, with H2O2 irrigation.
Finally, we also attempted to assess the cytotoxicity of H2O2, which exhibited some cytotoxic activity on periodontal ligament fibroblasts. This cytotoxic activity was dependent on the concentration of H2O2, but there were clear differences in the reaction of the cells used. As those were anonymously collected, no further information on the donors is available. From other experiments in different studies, heretofore unpublished, it is of interest to note that cells showing less cytotoxic reaction produced much less MMP1 than cells with greater cytotoxic reaction, which responded with increased production. A cytotoxicity of 1% H2O2 against human gingival fibroblasts but also against human epithelial HSC-2 and murine osteoblastic MC3T3-E1 cells has been reported elsewhere.24 However, it should also be mentioned that in comparison to hydrogen peroxide, the widely used antiseptic chlorhexidine is already cytotoxic at much lower concentrations.17
Conclusion
The present results indicate that irrigating with H2O2 during periodontal instrumentation by means of ultrasonication increases the reduction of viable bacteria within biofilms and may limit bacterial spreading via aerosols, thus reducing the risk of bacterial transmission.
Acknowledgements
The laboratory work of Anna Magdon and Prashanthnj Sivapatham (Department of Periodontology, Laboratory of Oral Microbiology, University of Bern, Switzerland) is gratefully acknowledged. The authors thank W&H for providing an ultrasonic scaler for our in vitro experiments. The study was funded by the Department of Periodontology, School of Dental Medicine, University of Bern.
Funding Statement
The laboratory work of Anna Magdon and Prashanthnj Sivapatham (Department of Periodontology, Laboratory of Oral Microbiology, University of Bern, Switzerland) is gratefully acknowledged. The authors thank W&H for providing an ultrasonic scaler for our in vitro experiments. The study was funded by the Department of Periodontology, School of Dental Medicine, University of Bern.
References
- Abd-Elghaffar AA, Ali AE, Boseila AA, Amin MA. Inactivation of rabies virus by hydrogen peroxide. Vaccine. 2016;34:798–802. doi: 10.1016/j.vaccine.2015.12.041. [DOI] [PubMed] [Google Scholar]
- Alves RV, Machion L, Casati MZ, Nociti FH, Jr, Sallum EA, Sallum AW. Clinical attachment loss produced by curettes and ultrasonic scalers. J Clin Periodontol. 2005;32:691–694. doi: 10.1111/j.1600-051X.2005.00713.x. [DOI] [PubMed] [Google Scholar]
- Apatzidou DA, Kinane DF. Nonsurgical mechanical treatment strategies for periodontal disease. Dent Clin North Am. 2010;54:1–12. doi: 10.1016/j.cden.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(suppl 16):S158–S171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- Gottsauner MJ, Michaelides I, Schmidt B, Scholz KJ, Buchalla W, Widbiller M, Hitzenbichler F, et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin Oral Investig. 2020;24:3707–3713. doi: 10.1007/s00784-020-03549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Wang K, Hou S, Wan L, Lv J, Zhang Y, et al. H2O2 and/or TiO2 photocatalysis under UV irradiation for the removal of antibiotic resistant bacteria and their antibiotic resistance genes. J Hazard Mater. 2017;323(Pt B):710–718. doi: 10.1016/j.jhazmat.2016.10.041. [DOI] [PubMed] [Google Scholar]
- Hagi TT, Klemensberger S, Bereiter R, Nietzsche S, Cosgarea R, Flury S, et al. A biofilm pocket model to evaluate different non-surgical periodontal treatment modalities in terms of biofilm removal and reformation, surface alterations and attachment of periodontal ligament fibroblasts. PLoS One. 2015;10:e0131056. doi: 10.1371/journal.pone.0131056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero ER, Slomka V, Bernaerts K, Boon N, Hernandez-Sanabria E, Passoni BB, et al. Antimicrobial effects of commensal oral species are regulated by environmental factors. J Dent. 2016;47:23–33. doi: 10.1016/j.jdent.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Holtkamp DJ, Myers J, Thomas PR, Karriker LA, Ramirez A, Zhang J, et al. Efficacy of an accelerated hydrogen peroxide disinfectant to inactivate porcine epidemic diarrhea virus in swine feces on metal surfaces. Can J Vet Res. 2017;81:100–107. [PMC free article] [PubMed] [Google Scholar]
- Ikai H, Nakamura K, Shirato M, Kanno T, Iwasawa A, Sasaki K, Niwano Y, et al. Photolysis of hydrogen peroxide, an effective disinfection system via hydroxyl radical formation. Antimicrob Agents Chemother. 2010;54:5086–5091. doi: 10.1128/AAC.00751-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Nakamura K, Ishiyama K, Yamada Y, Shirato M, Niwano Y, et al. Adjunctive antimicrobial chemotherapy based on hydrogen peroxide photolysis for non-surgical treatment of moderate to severe periodontitis: a randomized controlled trial. Sci Rep. 2017;7:12247. doi: 10.1038/s41598-017-12514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Shi W, Qi F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol. 2005;187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz D, Wirth J, Sculean A, Eick S. In- vitro-activity of additive application of hydrogen peroxide in antimicrobial photodynamic therapy using LED in the blue spectrum against bacteria and biofilm associated with periodontal disease. Photodiagnosis Photodyn Ther. 2019;26:306–312. doi: 10.1016/j.pdpdt.2019.04.015. [DOI] [PubMed] [Google Scholar]
- Kwasny SM, Opperman TJ. Static biofilm cultures of Gram-positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Curr Protoc Pharmacol. 2010 doi: 10.1002/0471141755.ph13a08s50. Chapter 13: Unit 13A 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang O, Nagy KS, Lang J, Perczel-Kovach K, Herczegh A, Lohinai Z, et al. Comparative study of hyperpure chlorine dioxide with two other irrigants regarding the viability of periodontal ligament stem cells. Clin Oral Investig. 2021;25:2981–2992. doi: 10.1007/s00784-020-03618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MV, Cancro LP, Fischman SL. Hydrogen peroxide: a review of its use in dentistry. J Periodontol. 1995;66:786–796. doi: 10.1902/jop.1995.66.9.786. [DOI] [PubMed] [Google Scholar]
- Martin N, Bass L, Liss SN. Antibacterial properties and mechanism of activity of a novel silver-stabilized hydrogen peroxide. PLoS One. 2015;10:e0131345. doi: 10.1371/journal.pone.0131345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Shirato M, Tenkumo T, Kanno T, Westerlund A, Ortengren U, et al. Hydroxyl radicals generated by hydrogen peroxide photolysis recondition biofilm-contaminated titanium surfaces for subsequent osteoblastic cell proliferation. Sci Rep. 2019;9:4688. doi: 10.1038/s41598-019-41126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit RK, Weber CA, Kean MJ, Hoffmann H, Pettit GR, Tan R, et al. Microplate Alamar Blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob Agents Chemother. 2005;49:2612–2617. doi: 10.1128/AAC.49.7.2612-2617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Nakamura K, Kanno T, Sasaki K, Niwano Y. Bactericidal effect of photolysis of H2O2 in combination with sonolysis of water via hydroxyl radical generation. PLoS One. 2015;10:e0132445. doi: 10.1371/journal.pone.0132445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stähli A, Maheen CU, Strauss FJ, Eick S, Sculean A, Gruber R. Caffeic acid phenethyl ester protects against oxidative stress and dampens inflammation via heme oxygenase 1. Int J Oral Sci. 2019;11:6. doi: 10.1038/s41368-018-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki T, Nakamura K, Kurauchi M, Kanno T, Katsuda Y, Ikai H, Hayashi E, et al. Synergistic interaction between wavelength of light and concentration of H2O2 in bactericidal activity of photolysis of H2O2. J Biosci Bioeng. 2015;119:358–362. doi: 10.1016/j.jbiosc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Tunkel J, Heinecke A, Flemmig TF. A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol. 2002;29(suppl 3):72–81. doi: 10.1034/j.1600-051x.29.s3.4.x. discussion 90–91. [DOI] [PubMed] [Google Scholar]
- Van der Sluijs M, Van der Sluijs E, Van der Weijden F, Slot DE. The effect on clinical parameters of periodontal inflammation following non-surgical periodontal therapy with ultrasonics and chemotherapeutic cooling solutions: a systematic review. J Clin Periodontol. 2016;43:1074–1085. doi: 10.1111/jcpe.12613. [DOI] [PubMed] [Google Scholar]
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) as compared with SARS-CoV-1. N Engl J Med. 2020 Mar 17; doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]


