Abstract
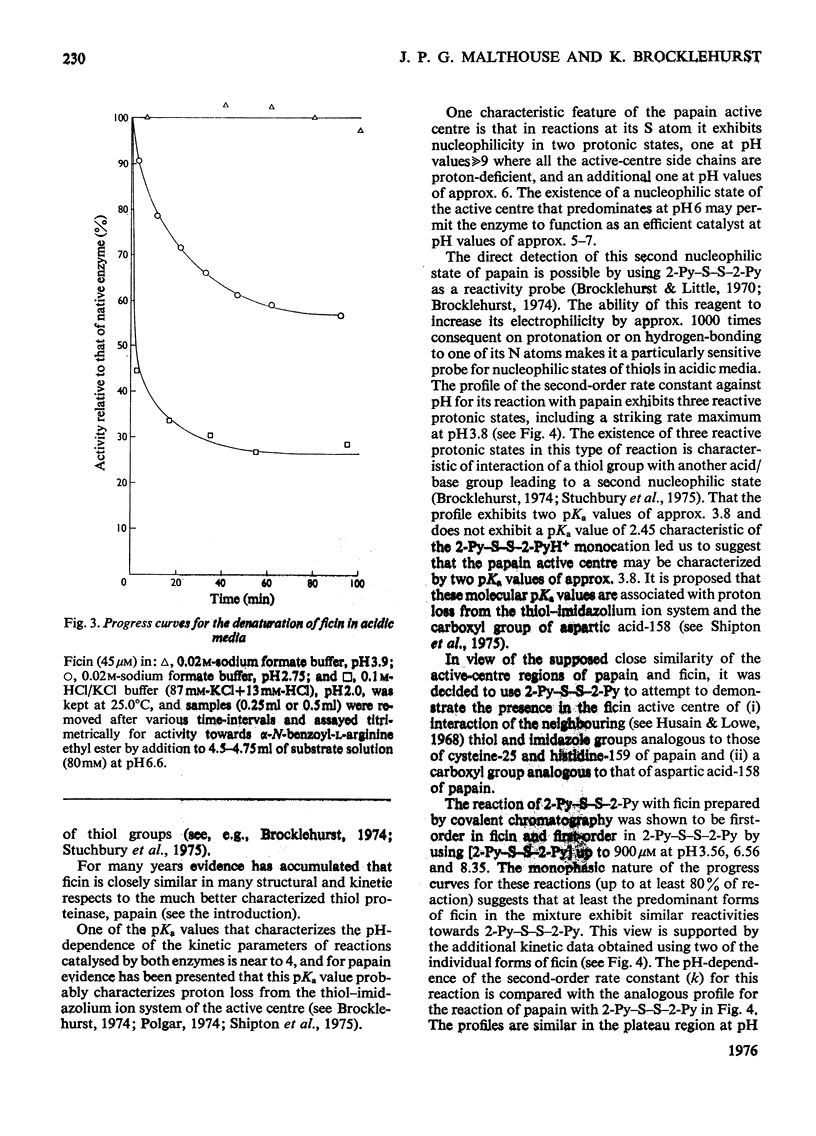
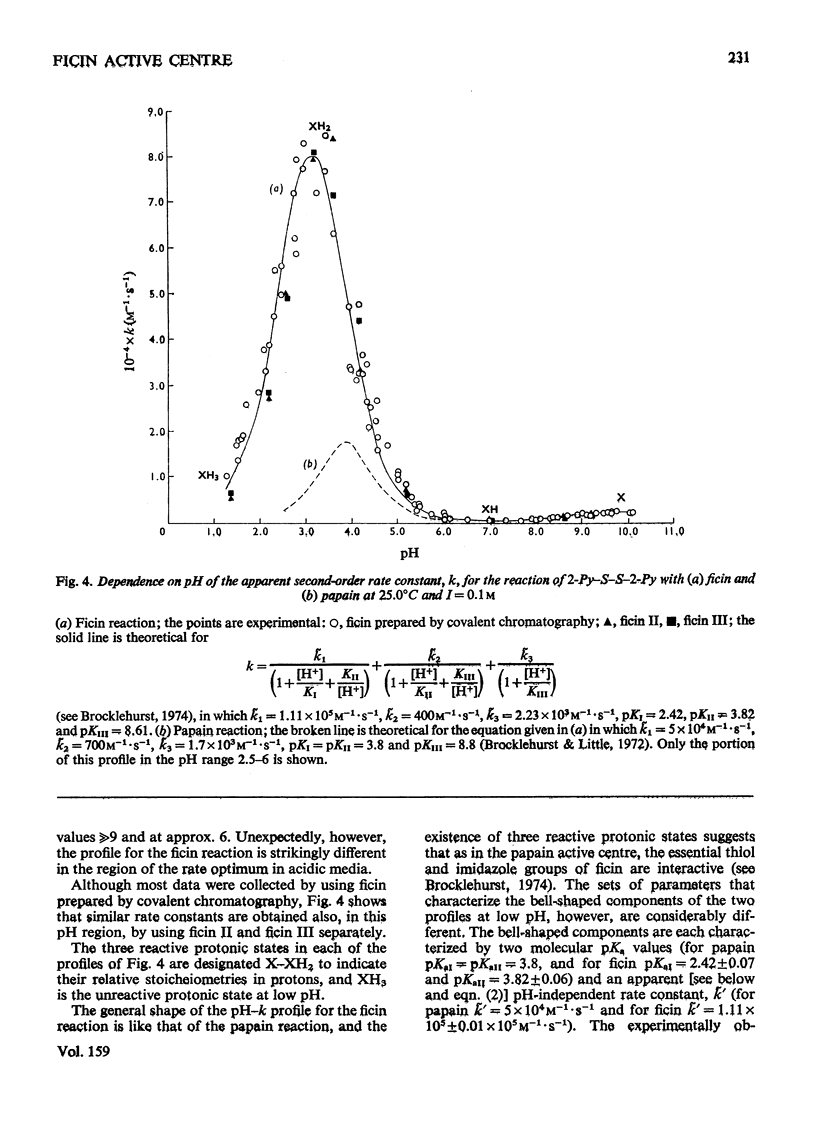
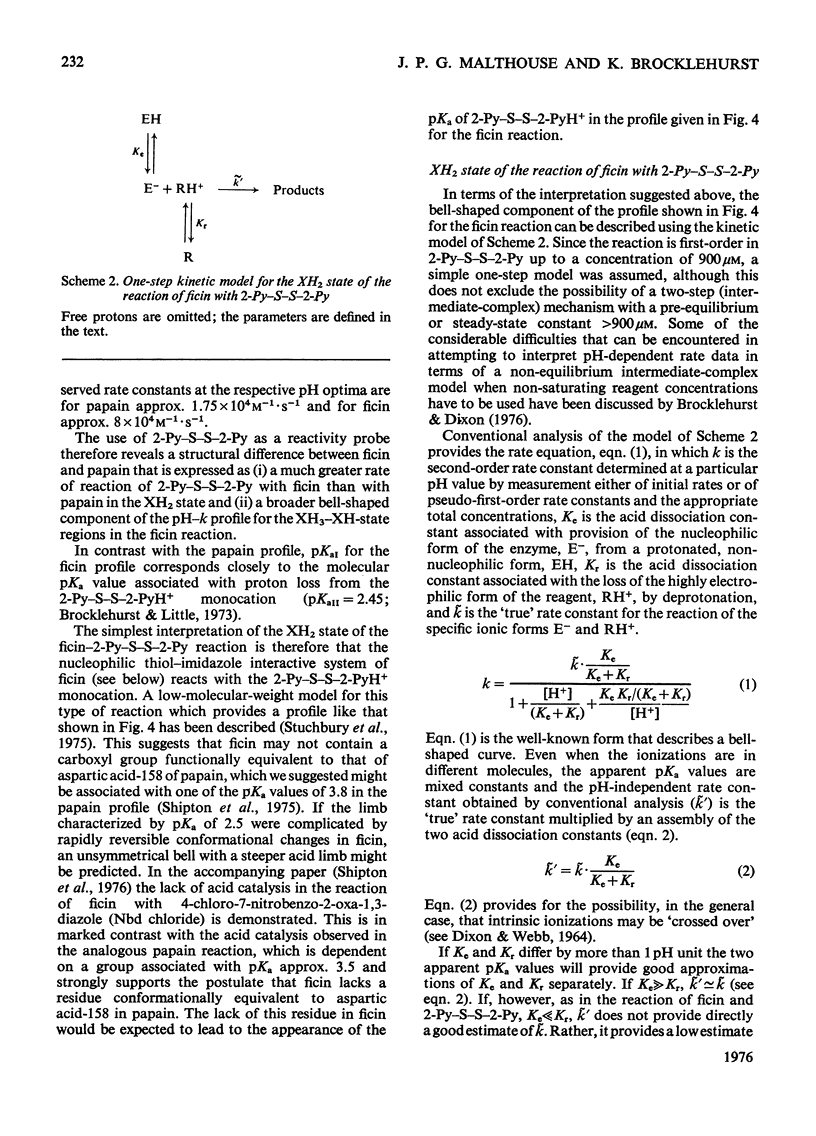
1. Fully active ficin (EC 3.4.22.3) containing 1 mol of thiol with high reactivity towards 2,2'-dipyridyl disulphide (2-Py-S-S-2-Py) at pH4.5 per mol of protein was prepared from the dried latex of Ficus glabrata by covalent chromatography on a Sepharose-glutathione-2-pyridyl disulphide gel. 2. Ficin thus prepared is a mixture of ficins I-IV and ficin G, in which ficins II and III predominate. The various ficins exhibit similar reactivity characteristics towards 2-Py-S-S-2-Py. 3. Use of 2-Py-S-S-2-Py as a reactivity probe demonstrates (a) that in ficin, as in papain (EC 3.4.22.2), the active-centre thiol and imidazole groups interact to provide a nucleophilic state at pH values of approx. 6 additional to the uncomplicated thiolate ion that predominates at pH values over 9, and (b) a structural difference between ficin and papain that leads to a much higher rate of reaction of 2-Py-S-S-2-Py with ficin than with papain at pH values 3-4. This difference is suggested to include a lack in ficin of a carboxyl group conformationally equivalent to that of aspartic acid-158 in papain. 4. The high electrophilicity of the 2-Py-S-S-2PyH+ monocation allows directly the detection of the exposure of the buried thiol group of ficin at pH values below 4.
Full text
PDF




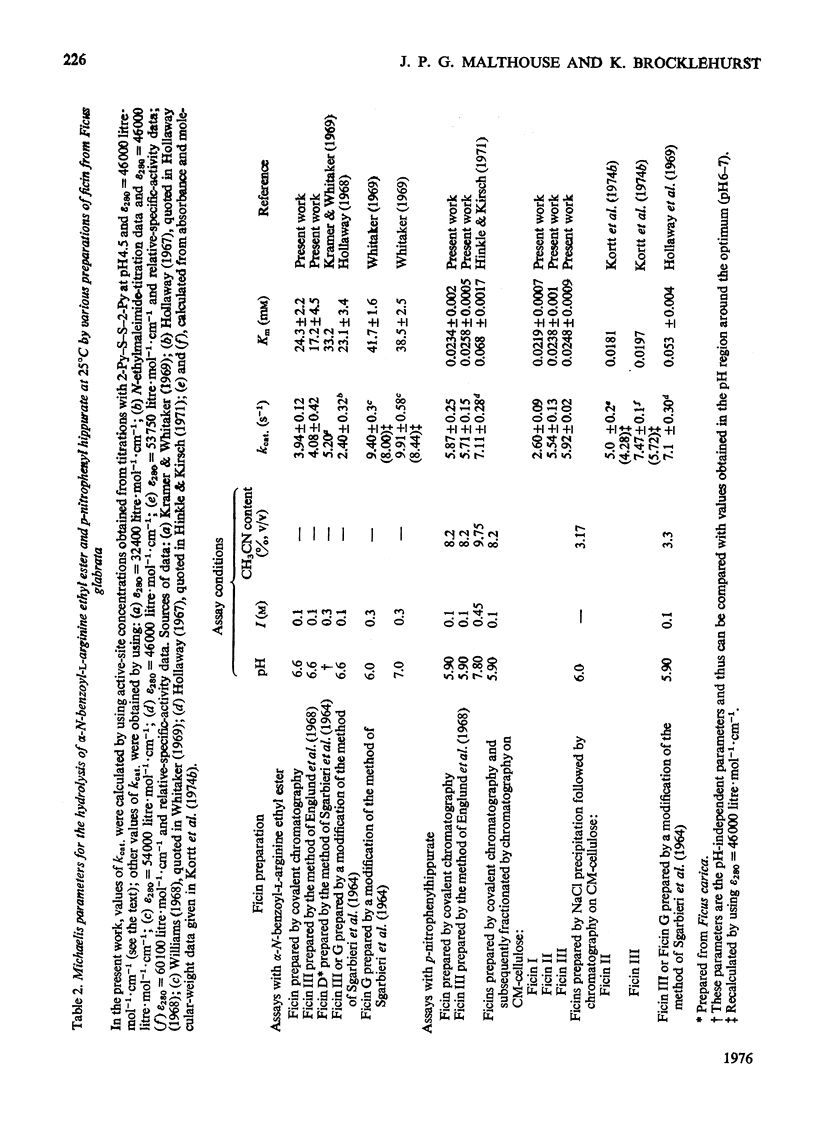
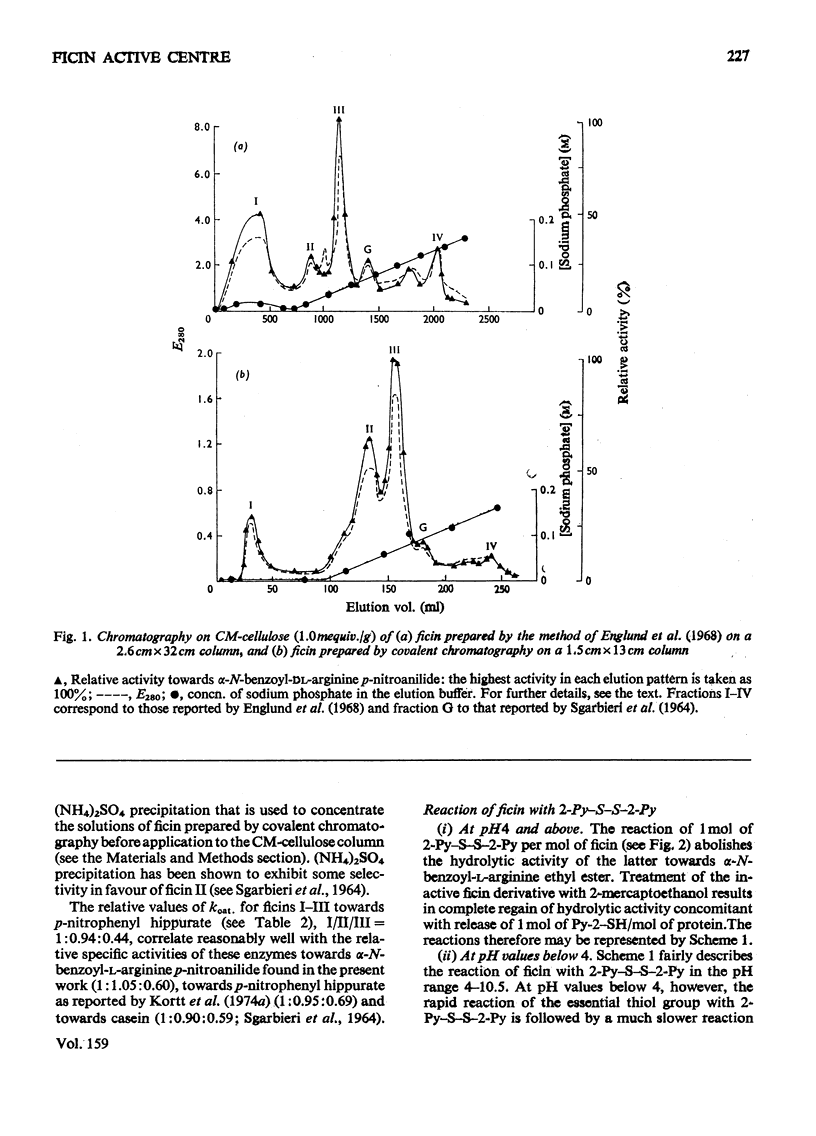

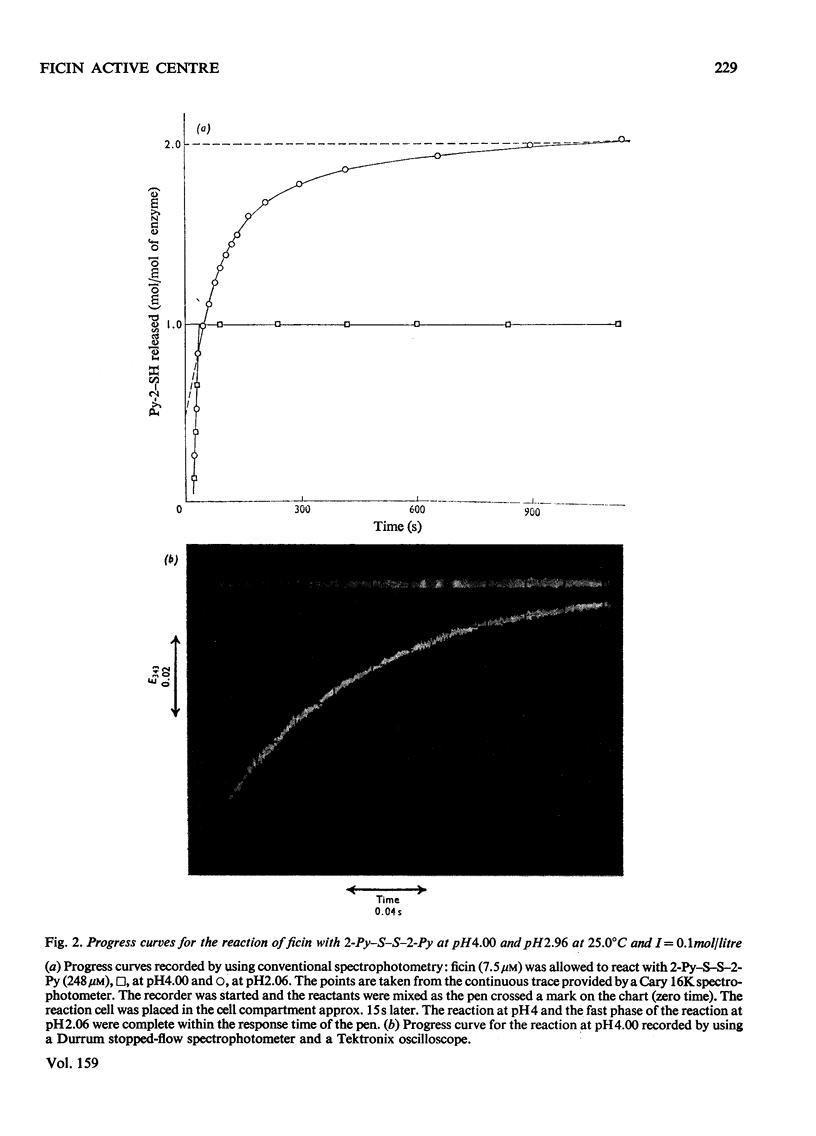


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. D., Hall P. L. Purification of ficin by affinity chromatography. Anal Biochem. 1974 Aug;60(2):417–423. doi: 10.1016/0003-2697(74)90250-4. [DOI] [PubMed] [Google Scholar]
- BERNHARD S. A., GUTFREUND H. Ficincatalysed reactions: the affinity of ficin for some arginine derivatives. Biochem J. 1956 May;63(1):61–64. doi: 10.1042/bj0630061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography by thiol-disulfide interchange. Methods Enzymol. 1974;34:531–544. doi: 10.1016/s0076-6879(74)34069-4. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography. Preparation of fully active papain from dried papaya latex. Biochem J. 1973 Jul;133(3):573–584. doi: 10.1042/bj1330573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Dixon H. B. PH-dependence of the steady-state rate of a two-step enzymic reaction. Biochem J. 1976 Apr 1;155(1):61–70. doi: 10.1042/bj1550061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Kierstan M. P. Propapain and its conversion to papain: a new type of zymogen activation mechanism involving intramolecular thiol-disulphide interchange. Nat New Biol. 1973 Apr 11;242(119):167–170. doi: 10.1038/newbio242167a0. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. A novel reactivity of papain and a convenient active site titration in the presence of other thiols. FEBS Lett. 1970 Jul 29;9(2):113–116. doi: 10.1016/0014-5793(70)80327-1. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactivities of the various protonic states in the reactions of papain and of L-cysteine with 2,2'- and with 4,4'- dipyridyl disulphide: evidence for nucleophilic reactivity in the un-ionized thiol group of the cysteine-25 residue of papain occasioned by its interaction with the histidine-159-asparagine-175 hydrogen-bonded system. Biochem J. 1972 Jun;128(2):471–474. doi: 10.1042/bj1280471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers L. D., Koshland D. E., Jr The specificity of induced conformational changes. The case of yeast glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1975 Aug 12;14(16):3661–3669. doi: 10.1021/bi00687a023. [DOI] [PubMed] [Google Scholar]
- Dixon H. B. The unreliability of estimates of group dissociation constants. Biochem J. 1976 Mar 1;153(3):627–629. doi: 10.1042/bj1530627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Englund P. T., King T. P., Craig L. C., Walti A. Studies on ficin. I. Its isolation and characterization. Biochemistry. 1968 Jan;7(1):163–175. doi: 10.1021/bi00841a021. [DOI] [PubMed] [Google Scholar]
- Goren H. J., Fridkin M. The hydrolysis of p-nitrophenylacetate in water. Mechanism and method of measurement. Eur J Biochem. 1974 Jan 16;41(2):263–272. doi: 10.1111/j.1432-1033.1974.tb03267.x. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- HAMMOND B. R., GUTFREUND H. The mechanism of ficin-catalysed reactions. Biochem J. 1959 Jun;72(2):349–357. doi: 10.1042/bj0720349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAWAY M. R., MATHIAS A. P., RABIN B. R. THE CHEMICAL REACTIVITY OF THE THIOL GROUP IN THE ACTIVE CENTRE OF FICIN. Biochim Biophys Acta. 1964 Oct 23;92:111–124. doi: 10.1016/0926-6569(64)90275-5. [DOI] [PubMed] [Google Scholar]
- Hollaway M. R., Antonini E., Brunori M. The pH-dependence of rates of individual steps in ficin catalysis. Eur J Biochem. 1971 Dec;24(2):332–341. doi: 10.1111/j.1432-1033.1971.tb19691.x. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. The amino acid sequence around the active-site cysteine and histidine residues, and the buried cysteine residue in ficin. Biochem J. 1970 Apr;117(2):333–340. doi: 10.1042/bj1170333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. The location of the active-site histidine residue in the primary sequence of papain. Biochem J. 1968 Aug;108(5):861–866. doi: 10.1042/bj1080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Kortt A. A., Hamilton S., Webb E. C., Zerner B. Ficins (EC 3.4.22.3). Purification and characterization of the enzymatic components of the latex of Ficus glabrata. Biochemistry. 1974 May 7;13(10):2023–2028. doi: 10.1021/bi00707a004. [DOI] [PubMed] [Google Scholar]
- Kortt A. A., Hinds J. A., Zerner B. On the specificity and pH dependence of ficin-catalyzed hydrolyses. Some comparisons with bromelain specificity. Biochemistry. 1974 May 7;13(10):2029–2037. doi: 10.1021/bi00707a005. [DOI] [PubMed] [Google Scholar]
- Kramer D. E., Whitaker J. R. Ficin-Catalyzed Reactions. Hydrolysis of alpha-N-Benzoyl-l-Arginine Ethyl Ester and alpha-N-Benzoyl-l-Argininamide. Plant Physiol. 1969 Apr;44(4):609–614. doi: 10.1104/pp.44.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIENER I. E. A study of the number and reactivity of the sulfhydryl groups of ficin. Biochim Biophys Acta. 1961 Oct 28;53:332–342. doi: 10.1016/0006-3002(61)90445-0. [DOI] [PubMed] [Google Scholar]
- LOWE G., WILLIAMS A. DIRECT EVIDENCE FOR AN ACYLATED THIOL AS AN INTERMEDIATE IN PAPAIN- AND FICIN-CATALYSED HYDROLYSES. Biochem J. 1965 Jul;96:189–193. doi: 10.1042/bj0960189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SGARBIERI V. C., GUPTE S. M., KRAMER D. E., WHITAKER J. R. FICUS ENZYMES. I. SEPARATION OF THE PROTEOLYTIC ENZYMES OF FICUS CARICA AND FICUS GLABRATA LATICES. J Biol Chem. 1964 Jul;239:2170–2177. [PubMed] [Google Scholar]
- Shipton M., Kierstan M. P., Malthouse J. P., Stuchbury T., Brocklehurst K. The case for assigning a value of approximately 4 to pKa-i of the essential histidine-cysteine interactive systems of papain, bromelain and ficin. FEBS Lett. 1975 Feb 15;50(3):365–368. doi: 10.1016/0014-5793(75)80529-1. [DOI] [PubMed] [Google Scholar]
- Shipton M., Stuchbury T., Brocklehurst K. 4-Chloro-7-nitrobenzo-2-oxa-1,3-diazole as a reactivity probe for the investigation of the thiol proteinases. evidence that ficin and bromelain may lack carboxyl groups conformationally equivalent to that of aspartic acid-158 of papain. Biochem J. 1976 Nov;159(2):235–244. doi: 10.1042/bj1590235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyterman L. A. The activation reaction of papain. Biochim Biophys Acta. 1967 Jul 11;139(2):430–438. doi: 10.1016/0005-2744(67)90046-0. [DOI] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J. R. Papain- and ficin-catalyzed reactions. Effect of pH on activity and conformation of ficin. Biochemistry. 1969 May;8(5):1896–1901. doi: 10.1021/bi00833a019. [DOI] [PubMed] [Google Scholar]