Abstract
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases. AD pathogenesis is associated with increased oxidative stress, impairment of the skin barrier, and activation of the immune response. Rosmarinic acid (RA), a caffeic acid ester, is known for its anti-inflammatory and antioxidant properties. However, the effects of RA on Dermatophagoides farinae extract (DfE)-induced AD-like skin inflammation, as well as its ability to regulate oxidative stress through the Nrf2/HO-1 pathway in TNF-α/IFN-γ-treated keratinocytes, remain unclear. We investigated RA activity in a DfE-induced AD-like skin inflammation mouse model and IFN-γ/TNF-α-stimulated keratinocytes. We found that RA attenuates DfE-induced inflammation by decreasing dermatitis scores and serum inflammatory marker levels and mast cell infiltration. Additionally, RA significantly suppressed IFN-γ/TNF-α-induced chemokine production in keratinocytes and reduced Th cytokine levels in concanavalin A-stimulated splenocytes. Importantly, RA also increased Nrf2/HO-1 expression in TNF-α/IFN-γ-treated keratinocytes. In conclusion, this study demonstrated that RA effectively alleviates DfE-induced AD-like skin lesions by reducing the levels of inflammatory cytokines and chemokines. Furthermore, RA promotes Nrf2/HO-1 signaling in keratinocytes, which may help mitigate DfE-induced oxidative stress, thereby alleviating AD-like skin inflammation. These findings highlight the potential of RA as a therapeutic agent for treating AD and other skin inflammation.
Keywords: rosmarinic acid, atopic dermatitis, Nrf2, HO-1, keratinocytes
1. Introduction
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases. AD pathogenesis involves allergic sensitization, oxidative stress induced by increased reactive oxygen species (ROS) generation, impaired skin barrier function, and increased Th immune reactions [1,2]. In the early response to external allergens, high amounts of ROS are rapidly generated in skin cells such as keratinocytes [3], causing the oxidization of cellular components and the degradation of extracellular matrix (ECM) components [4], which in turn stimulates immune reactions in skin lesions. Allergens activate immune cells and/or keratinocytes to produce proinflammatory cytokines and chemokines, which further evoke a systemic immune response with a predominant Th2 immune response exacerbated by mixed activation of Th1/2 cells at chronic stages of skin inflammatory diseases, such as AD [5,6]. Given the importance of oxidative stress in stimulating skin inflammation, the nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway has gained increasing attention [7,8]. Nrf2 is a ubiquitously expressed transcription factor that modulates redox balance to maintain skin homeostasis. Nrf2 binds to antioxidant response elements in ROS-protective genes, such as HO-1, glutathione S-transferase (GST), and superoxide dismutase (SOD) [9]. Additionally, Nrf2 promotes keratinocyte differentiation to maintain skin structure by regulating keratinocyte differentiation markers such as loricrin and keratin [10,11]. Nrf2 also protects keratinocytes from UV-induced oxidative damage [8] and reduces apoptosis in melanocytes by increasing the expression of SOD [12]. Inhibition of endogenous Nrf2 activity by a dominant-negative form of Nrf2 in loricrin-deficient epidermal cells causes severe loss of barrier function and cell death within 24 h [13]. However, excessive activation of Nrf2 in keratinocytes due to the deletion of its binding regulator, Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1 (Keap1), causes epithelial abnormalities, which can be rescued by concomitant deletion of the Nrf2 gene [14]. These findings underscore the critical role of the Nrf2-dependent redox balance system in maintaining skin homeostasis.
The long-term use of AD therapeutic agents using steroids or anti-histamines has been restricted due to severe side effects [15,16]. Therefore, the research to develop promising therapeutics derived from natural resources or their components is essential and in high demand. We previously reported the ethanol extract of Isodon inflexus containing rosmarinic acid (RA) has therapeutic effect against DfE-induced AD-like skin inflammation in NC/Nga mice [17]. Rosmarinic acid (RA), a naturally occurring caffeic acid ester identified in Rosmarinus officinalis L., is known to have various pharmaceutical activities, including antioxidative activity, anti-inflammatory activity, and antitumor activity [18]. In terms of skin inflammation, RA attenuates dibutyl squarate-induced pruritus or 2,4-dinitrofluorobenzene (DNFB)-induced AD in mice by inhibiting mast cell activation or T-cell-generated inflammatory cytokine levels [19], thereby contributing to improving the skin condition of patients with AD symptoms by reducing transepidermal water loss [20]. Additionally, RA suppresses Th2-related IL-4 and IL-5 levels, reducing intratracheal DfE-induced allergic inflammation in the lung [21]. However, its effects on DfE-induced AD-like skin inflammation remain unknown. Therefore, we investigated the potential effects of RA on DfE-induced AD-like skin inflammation in an NC/Nga mouse model. Previously, RA was shown to protect against UVB-induced oxidative damage in keratinocytes by activating Nrf2/HO-1 signaling or enhancing Akt/ERK/Nrf2-regulated glutathione synthesis [22]. RA also mitigates chlorpyrifos-induced kidney injury [23], vascular calcification [24], and ischemic stroke [25] by increasing Nrf2/HO-1 signaling to reduce oxidative stress. However, its effects on inflammatory cytokine-induced Nrf2/HO-1 signaling in keratinocytes have not been investigated.
Nishiki-nezumi Cinnamon/Nagoya (NC/Nga) mice spontaneously develop skin lesions similar to those in human AD [26,27]. Dermatophagoides farinae extract (DfE), the most prevalent house dust mite in daily life, is a potent allergen known to trigger AD symptoms [28]. DfE-treated NC/Nga mice display major clinical skin symptoms of human AD, including dryness, erythema, edema, and excoriation [29,30].
Keratinocytes are the primary cells responsible for expressing epidermal proteins to construct skin tissue and to protect against exogeneous allergens or stresses [31]. Environmental allergens stimulate keratinocytes to release proinflammatory factors, inducing the recruitment and activation of mast cells, dendritic cells, or T-cells [32]. Thus, an in vitro culture system using keratinocytes is a valuable approach for studying the cellular and molecular aspects of epidermal impairment in AD-like inflammatory diseases.
In this study, we explored the possible inhibitory effects of RA on DfE-induced AD-like skin inflammation in an NC/Nga murine model. To elucidate the underlying molecular mechanism by which RA influences skin inflammation, we examined its effect on IFN-γ/TNF-α-induced Nrf2/HO-1 expression in human epidermal keratinocytes (HEKs). We also evaluated the impact of RA on concanavalin A (Con A)-induced inflammatory cytokine levels in mouse splenocytes and on tumor necrosis factor (TNF)-α/ interferon (IFN)-γ-induced chemokine levels in HaCaT cells.
2. Results
2.1. Ra Attenuates Dfe-Induced Skin Inflammatory Lesions, Reduced Dermatitis Scores, and Decreased the Serum Levels of Inflammatory Factors in AD Model Mice
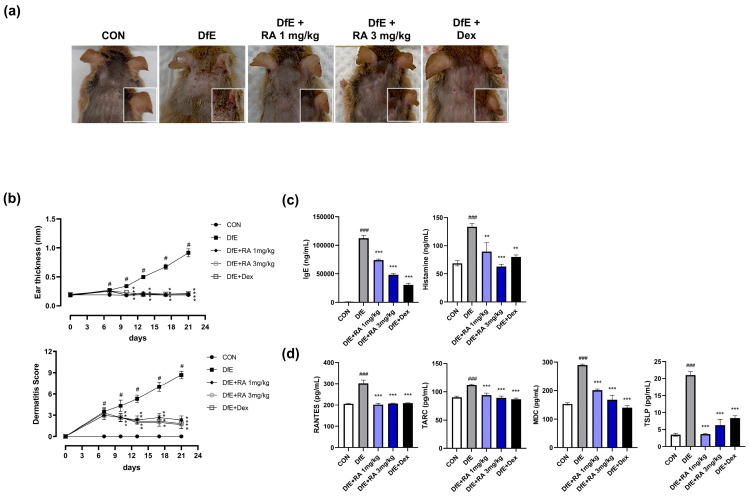
The topical application of DfE for two weeks significantly induced the development of dryness, excoriation, hemorrhage, and hematoma in both the ear and dorsal skin lesions of mice (Figure 1a). However, skin deterioration in these lesions was notably alleviated in the mice administered with RA (1 or 3 mg/kg) or dexamethasone. This mitigation of skin lesions was supported by a gradual and significant reduction in both the dermatitis score and ear thickness in all three groups (Figure 1b). The dermatitis score in the RA-treated groups decreased to less than 3, a score comparable to that of the dexamethasone group. We then analyzed the serum levels of IgE and histamine, which are standard markers of the early inflammatory response and involve the activation of mast cells, B cells, or monocytes. DfE application significantly elevated the levels of both markers compared with those in the control group, but these increases were significantly reduced by RA (1 or 3 mg/kg) or dexamethasone administration (Figure 1c). Additionally, the DfE-induced increase in the levels of inflammatory chemokines (RANTES, TACR, MDC, and TSLP) was also significantly decreased by RA (1 or 3 mg/kg) or dexamethasone treatment (Figure 1d). The inhibitory effects of 3 mg/kg RA on these inflammatory markers were similar to those of dexamethasone. These results suggest that RA may have an inhibitory effect on DfE-induced skin inflammation by reducing the levels of inflammatory factors.
Figure 1.
RA alleviated DfE-induced AD-like skin inflammation in NC/Nga mice. (a) Representative images of DfE-induced skin features in each mouse group are shown; (b) ear thickness and dermatitis score of dorsal lesions on each day for 21 days are presented; (c) serum levels of IgE, histamine, and (d) inflammatory chemokines (RANTES, TARC, MDC, and TSLP) in each group were examined via a bead-based immune assay. CON, normal control mice; DfE, DfE-induced mice; DfE + RA 1 mg/kg, DfE-induced RA (1 mg/kg)-administered mice; DfE + RA 3 mg/kg, DfE-induced RA (3 mg/kg)-administered mice; DfE + Dex, DfE-induced dexamethasone (1 mg/kg)-administered mice. The data are presented as the means ± standard errors of the means (SEMs) (n = 5). # p < 0.05 and ### p < 0.001 vs. CON; * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. DfE.
2.2. RA Alleviates Histological Damage in DfE-Induced Skin Lesions
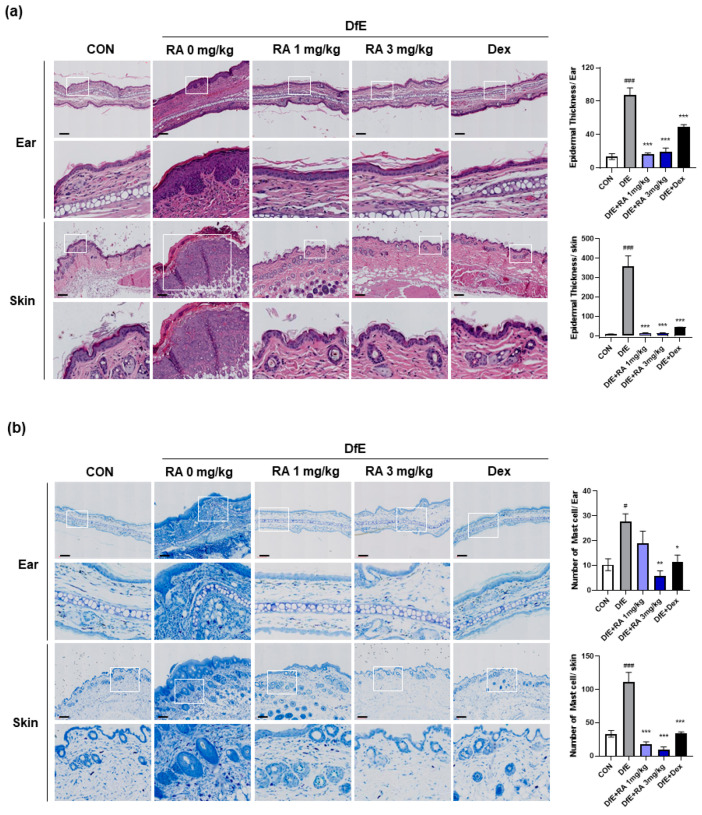
To confirm the physiological effect of RA-mediated suppression of DfE-induced skin inflammation, we assessed skin tissue histology via hematoxylin and eosin (H&E) staining and evaluated immune cell infiltration via toluidine blue (TB) staining. Mast cell infiltration induced by proinflammatory factors in resident skin cells is characteristic of the early phase of the systemic immune response. DfE application resulted in epidermal hyperplasia, as evidenced by increased epidermal thickness in ear and skin tissues (Figure 2a). However, RA (1 or 3 mg/kg) and dexamethasone significantly and markedly reduced the thickness of the skin lesions; the epidermal thickness of the RA treatment group was similar to that of the normal control group. RA also significantly inhibited mast cell infiltration in ear and skin lesions, reaching the level of the control group (Figure 2b). Notably, at a high dose (3 mg/kg), RA was even more effective than dexamethasone (1 mg/kg) in reducing mast cell infiltration in ear and skin lesions.
Figure 2.
RA mitigated DfE-induced pathological features and mast cell infiltration into the skin and ear lesions of the mice. Representative images of (a) H&E staining and (b) TB staining of skin and ear tissue sections. Epidermal thickness and mast cell numbers were analyzed in the mouse skin at 40× magnification (upper panel) and 400× magnification (lower panel) via microscopy, as shown in the accompanying bar graph on the left. The data are presented as the means ± SEMs (n = 5). # p < 0.05 and ### p < 0.001 vs. the normal control group (CON); * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. DfE. Scale bar = 100 μm.
2.3. RA Suppresses the IFN-γ/TNF-α-Induced Increase in Chemokine Levels in Keratinocytes
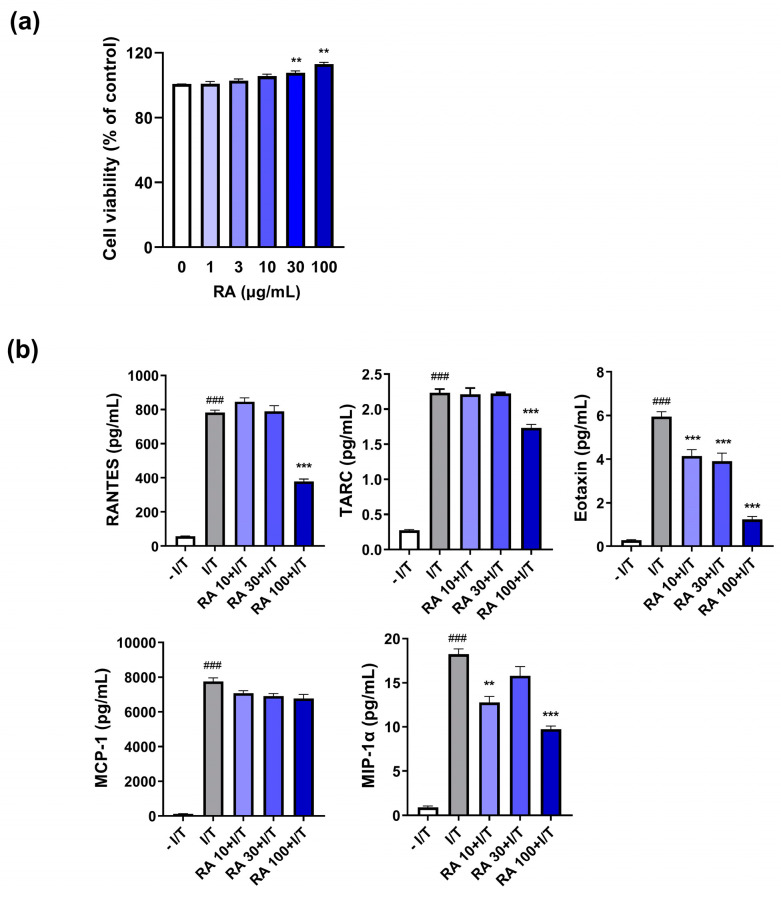
Inflammatory allergens stimulate keratinocytes to produce inflammatory chemokines that evoke a systemic immune response. Given that RA inhibits the serum levels of inflammatory chemokines, reflecting its inhibitory effect on the DfE-induced systemic immune response, we examined whether RA could also suppress the production of inflammatory chemokines from keratinocytes to inhibit the initiation of skin inflammation. RA at a high dose did not induce any cellular cytotoxicity in HaCaT cells (Figure 3a). IFN-γ/TNF-α increased the levels of five chemokines (RANTES, TARC, eotaxin, MCP-1, and MIP-1α) related to skin inflammation in HaCaT cells (Figure 3b). However, 100 µg/mL RA significantly decreased the levels of RANTES, TARC, eotaxin, and MIP-1α but not that of MCP-1 (Figure 3b).
Figure 3.
RA attenuated inflammatory-related chemokine levels in IFN-γ/TNF-α-stimulated HaCaT cells. (a) The cells were treated with RA (1, 3, 10, 30, or 100 µg/mL) for 24 h, and cell viability was measured by an CCK-8 assay. (b) The levels of five inflammatory chemokines (RANTES, TARC, eotaxin, MCP-1, and MIP-1α) in the media were measured via ELISA as described in the Section 4. ### p < 0.001 vs. the vehicle-treated cells without IFN-γ/TNF-α (– I/T) (10 ng/mL); ** p < 0.01 and *** p < 0.001 vs. the vehicle-treated cells with IFN-γ/TNF-α (I/T).
2.4. RA Attenuates Concanavalin A (Con A)-Induced Th Cytokine Levels in Splenocytes
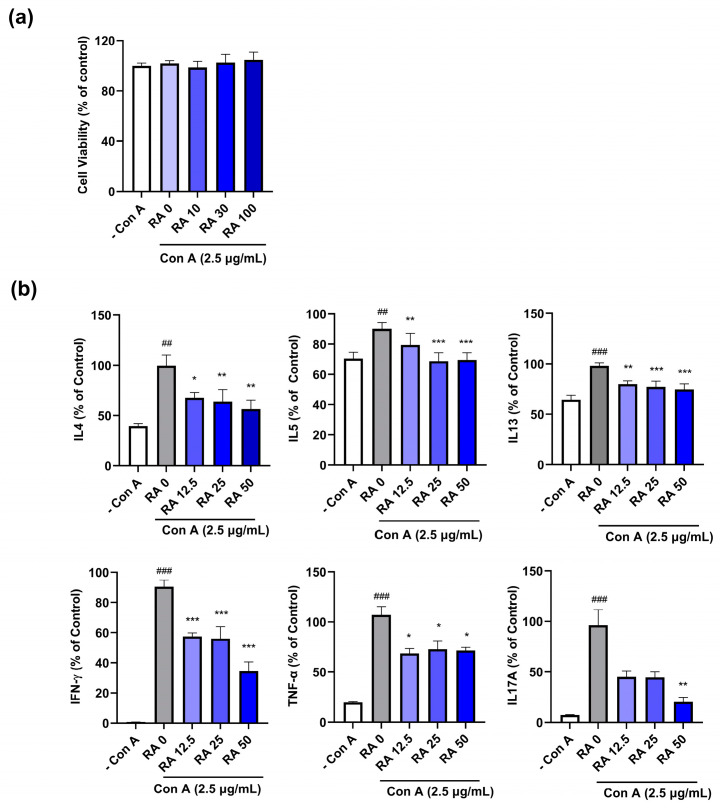
Skin inflammatory chemokines and activated immune cells, such as mast cells, induce a systemic immune response by activating Th1/2 cells. Th2 inflammation is dominant at the initial or acute stage of skin inflammation in diseases such as AD, followed by a Th1/2 mixed response at the chronic stage [5]. Th17 cell activation also increases with increasing severity of inflammation in AD patients [33]. Thus, we examined the inhibitory effect of RA on the Con A-induced increase in Th1/2/17 cytokine levels in primary mouse splenocytes. Con A (2.5 µg/mL) and RA (0, 10, 30, or 100 µg/mL) did not induce cell cytotoxicity (Figure 4a). We found that Con A significantly induced Th1 (IFN-γ and TNF-α), Th2 (IL-4, IL-5, and IL-13), and Th17A cytokines, which were inhibited by RA treatment in primary splenocytes (Figure 4b).
Figure 4.
RA suppressed the Con A-induced increase in inflammatory Th cytokine levels in primary mouse splenocytes. (a) The viability of the splenocytes was examined after 24 h of incubation with Con A (2.5 µg/mL) or RA (0, 10, 30, or 100 µg/mL); (b) The levels of nine cytokines (IL-4, IL-5, IL-13, IFN-γ, TNF-α, and IL-17A) in the culture media of splenocytes were analyzed via ELISA as described in the Section 4. ## p < 0.01 and ### p < 0.001 vs. the vehicle-treated cells without Con A (– Con A); * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. the vehicle-treated cells with Con A (RA 0).
2.5. RA Increases Nrf2/HO-1 Expression
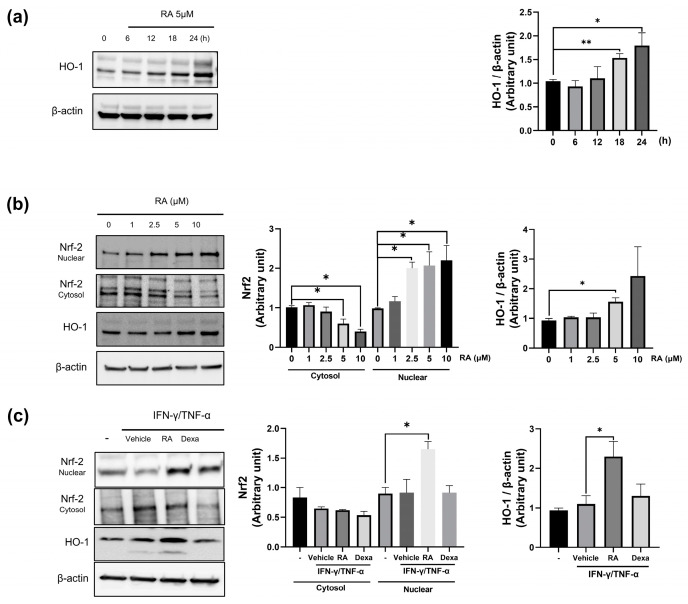
To explore the molecular mechanism by which RA protects against skin inflammation, we focused on the Nrf-2/HO-1 pathway. Our results revealed that RA induces HO-1 expression in a time-dependent manner, with HO-1 expression peaking after 24 h of incubation with RA (Figure 5a). While RA gradually decreased cytosolic Nrf-2 levels, it significantly increased nuclear Nrf-2 expression in a dose-dependent manner (Figure 5b). Correspondingly, high doses of RA (5 and 10 μM) increased HO-1 expression. The increase in nuclear expression after RA treatment, coupled with the reduction in cytosolic Nrf2 expression, may suggest that Nrf2 is translocated to the nucleus to upregulate HO-1 expression. Notably, 5 μM RA significantly increased nuclear Nrf-2 expression and further increased cytosolic HO-1 expression in IFN-γ/TNF-α-treated keratinocytes (Figure 5c). IFN-γ/TNF-α treatment appeared to reduce cytosolic Nrf-2 levels, although the difference was not statistically significant.
Figure 5.
RA-induced Nrf2 and HO-1. (a) RA increases HO-1 expression in keratinocytes in a time-dependent manner; (b) RA increases cytosolic HO-1 and nuclear Nrf2 levels but decreases cytosolic Nrf2 levels in a dose-dependent manner. HO-1 and Nrf2 levels were quantified by densitometric analysis. Nrf2 and HO-1 levels were examined in keratinocytes after RA (1, 2.5, 5, or 10 μM) treatment; (c) RA increases nuclear Nrf2 and cytosolic HO-1 levels in cells treated with IFN-γ/TNF-α (I/T) (10 ng/mL). Vehicle: DMSO-treated cells; RA: RA (5 μM)-treated cells; Dexa: dexamethasone (20 μM)-treated cells. The quantified Western blot data are shown as the means ± standard deviations (SDs) (n = 3). * p < 0.05, and ** p < 0.01 vs. the vehicle-treated cells as determined by two-tailed t tests.
3. Discussion
In this study, we investigated the inhibitory effect of RA on mice with AD-like skin inflammation induced by repeated topical application of DfE. RA was found to activate the Nrf-2/HO-1 pathway to alleviate ROS-induced oxidative stress in keratinocytes, which could contribute to the inhibition of DfE-induced local and systemic inflammatory reactions in the skin.
Chemical or biological allergens initially induce skin inflammation by recruiting mast cells or dendritic cells to activate the T-cell immune response. Repeated DfE application on NC/Nga mice spontaneously induces excessive oxidative stress to induce redox alterations in skin lesions [3]. It initiates mast cell accumulation and a T-cell-mediated immune response, increasing the levels of Th1/2 cytokines in skin lesions of NC/Nga mice [34], which mirrors the early phase immune response of skin inflammation observed in human AD patients. Previous studies have suggested that the pharmaceutical activity of RA against AD-like skin inflammation involves regulating the initial process of the immune response [35,36]. Subcutaneous injection of RA into the dorsal skin of mice alleviates dibutyl square acid (SADBE)-induced pruritus and skin inflammation by inhibiting the MRGPRX2-PLCγ1PKC-NF-κB axis, which is involved in mast cell activation [35]. Additionally, the intraperitoneal injection of RA inhibits DNFB-induced CD4+ and CD8+ T-cell infiltration in mouse skin lesions and decreases Th1/2 cytokine levels in CD4+ T-cells [19]. Consistent with these previous studies, we found that oral administration of RA inhibited DfE-induced increases in the levels of inflammatory chemokines (RANTES, TARC, MDC, and TSLP) in the serum and mast cell infiltration in skin lesions. These chemokines primarily function to induce the infiltration of eosinophils and mast cells in skin lesions during the early phase of inflammation, contributing to the activation of T-cells [5,37]. Repeated topical application of DfE increases the level of ROS in the dermis [3], resulting in the excessive accumulation of mast cells and a T-cell-mediated immune response, increasing the levels of Th1/2 cytokines in NC/Nga mice [34]. Thus, our results suggest that RA reduces DfE-induced inflammatory factor levels, including ROS, thereby decreasing mast cell infiltration in DfE-induced AD-like model mice at the early disease phase.
Keratinocytes produce chemokines and Th2 cytokines, including interleukin (IL)-4 and IL-13, to downregulate skin barrier protein expression in keratinocytes and stimulate the apoptosis of keratinocytes, contributing to cutaneous spongiosis in acute skin lesions [38,39]. Inflammatory cytokines or chemokines stimulate the initiation of systemic CD4+ T-cell activation in the lymph nodes or spleens of DfE-induced AD model mice [40,41]. Increased levels of Th2 cytokines reflect a predominant Th2 response at the acute stage of AD, while it becomes a Th1/Th2 mixed response at chronic stages, resembling the pathological phenotypes of human AD [5,42]. Additionally, keratinocyte-derived chemokines (e.g., thymic stromal lymphopoietin) enhance the itching sensation, which can lead to increased scratching and worsening of skin lesions [43]. In this study, we also reported that RA inhibited Con A-induced Th1/Th2/Th17 inflammatory cytokine levels in mouse splenocytes and inhibited the levels of IFN-γ/TNF-α-induced chemokines (RANTES, TARC, eotaxin, and MIP-1α) in keratinocytes. Several herbal components or medicinal herbs inhibit DfE-induced AD-like skin inflammation by attenuating immune cell infiltration, alleviating skin barrier dysfunction, or mitigating the Th1/2 imbalance [44,45]. Therefore, the inhibitory effects of RA on chemokine levels in keratinocytes and on Th cytokine generation in splenocytes could contribute to alleviating immune cell infiltration and systemic T-cell activation, subsequently reducing DfE-induced skin inflammation in mouse skin lesions.
The Keap1–Nrf2 pathway is a crucial cytoprotective mechanism that transcriptionally regulates antioxidant response elements (AREs) of antioxidant proteins, such as HO-1, in response to oxidative and electrophilic stress [46]. Various phytochemicals or small molecules indirectly modulate the Keap1–Nrf2 pathway by forming covalent adducts with the sulfhydryl groups of cysteine or through electrophilic reactions with cysteine in Keap1 [47]. This interaction has dual effects: it prevents the association of Keap1 with Cullin for ubiquitin-mediated degradation of Nrf2 and induces allosteric changes in Nrf2-interacting sites in Keap1, releasing Nrf2 for nuclear translocation [47,48]. RA modulates the Keap1–Nrf2 pathway via covalent modification of the Michael acceptor of RA with the cysteine 151 residue in the N-terminal BTB (Broad complex, Tramtrack, and Bric-a-Brac) domain of Keap1 in inflammation-stimulated RW264.7 cells [49]. When cysteine residues of the BTB and IVR domains in Keap1 are covalently modified under oxidative stress, homodimerization of Keap1 and Cullin for Nrf2 degradation is disturbed, resulting in the release of Nrf2 for translocation [48]. Additionally, RA can interact with both polar and hydrophobic residues in the Kelch 1-6 domains of Keap1, which consist of positively charged P1 and P2 subpockets and hydrophobic P4 and P5 subpockets for Nrf2 binding [50,51]. The electrostatic and hydrophobic interactions between RA and Keap1 could decrease the binding strength of Keap1 that results in the dissociation of Keap1 from Nrf2 [52]. In keratinocytes, RA exhibits excellent antioxidant properties by abrogating UVB-induced reduction in Nrf2 expression and increasing the expression of antioxidant proteins such as glutamate-cysteine ligase catalytic subunit (GCLC) and glutathione synthetase (GSS) to protect the skin from UVB-induced oxidative damage [22,53]. In this study, we found that RA increased the nuclear translocation of Nrf2 to induce HO-1 expression in keratinocytes in a dose- and time-dependent manner. RA profoundly increased Nrf2/HO-1 expression in IFN-γ/TNF-α-treated keratinocytes. These findings suggest that the interaction of RA with Nrf2 regulatory domains of Keap1 could induce allosteric changes in Keap1 to disassemble the Keap1–Nrf2 complex, leading to the release and nuclear translocation of Nrf2, which consequently increases HO-1 expression to protect against oxidative stress in keratinocytes.
RA inhibits skin inflammation by regulating various molecular pathways. RA inhibits imiquimod-induced psoriasis-like dermatitis in mice by reducing JAK2/Stat3-dependent Th17 cell differentiation and IL-17A production [54]. RA also significantly suppresses the poly(I:C)-induced expression of inflammatory cytokines, including Il-1β and TNF-α, in human keratinocytes by suppressing the NF-κB pathway [55]. We also found that RA inhibited DfE-induced skin inflammation by attenuating chemokine and Th1/2/17 cytokine generation and increasing Nrf2/HO-1 expression to decrease inflammatory factor-induced oxidative stress. The topical application of RA to improve epidermal conditions in AD patients by reducing transepidermal water loss and erythema on the antecubital fossa [20] could suggest the pharmaceutical potential of RA in treating skin inflammatory diseases such as AD or psoriasis through various molecular pathways. Further studies investigating these relevant action mechanisms of RA against skin inflammation, along with the therapeutic safety of RA in clinical conditions, could be beneficial for developing skin application remedies.
Skin also functions as a neuroendocrine organ, mediating the nervous, immune, and endocrine systems in response to skin stress [56]. Chronic itching inevitably triggers scratching behavior via the IgE–mast-cell–histamine-neuronal axis [57]. The persistence of this itching–scratching cycle connecting the immune and neuronal systems exacerbates atopic inflammation and skin barrier dysfunction. Further research is needed to investigate the effects of RA on neurohormonal mediators or cytokines involved in neuroendocrine signaling within the skin. It could elucidate how RA modulates various nervous and immune pathways for the pharmaceutical activity of RA.
4. Materials and Methods
4.1. Materials
House dust mite ointment was purchased from Biostir Inc. (Kobe, Japan). The LBIS Mouse IgE ELISA Kit was purchased from Fujifilm (Shibukawa, Japan). Histamine Research ELISATM was purchased from LDN (Nordhorn, Germany). The LEGEND-plex™ Mouse Proinflammatory Chemokine Panel was purchased from BioLegend (San Diego, CA, USA). The toluidine blue staining kit was purchased from VitroVivo Biotech (Rockville, MA, USA). H&E staining kit, Roswell Park Memorial Institute (RPMI) 1640 medium, fetal bovine serum (FBS), penicillin/streptomycin, NE-PER Nuclear and Cytoplasmic Extraction Reagents, BCA assay kit, TNF-α, IFN-γ, and Super Signal West Femto Chemiluminescent Substrate were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Concanavalin A was purchased from Sigma Aldrich (Burlington, MA, USA). Normal human epidermal keratinocytes (HEKs) (catalog number 00192627) and KBM-Gold Basal medium supplemented with a SingleQuots Supplement pack were purchased from LonzaBioscience (Basel, Switzerland). HaCaT human keratinocyte cells were purchased from American Type Culture Collection (Manassas, VA, USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies Inc. (Rockville, MD, USA). Antibodies against Nrf2 (12721), HO-1 (70081), and β-actin (4967) were purchased from Cell Signaling Technology (Boston, MA, USA).
4.2. Animals
The animal experimental protocol was designed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (2013). The protocol for the AD experiment was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the Korea Institute of Oriental Medicine (KIOM) (Approval No. 22-073; approval date, 1 August 2022). The animal experiment for the isolation of splenocytes to analyze Th cytokines was approved by the IACUC of KIOM (approval number: 23-021; Approval date, 30 March 2022). All methods are reported in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for the reporting of animal experiments. NC/Nga male mice (body weight 19–24 g, 8 weeks old) (Central Lab Animal Inc., Seoul, Republic of Korea) were housed in a specific-pathogen-free (SPF) facility at KIOM, where they were maintained under standard laboratory animal conditions including a controlled temperature (23 ± 2 °C) and humidity (55 ± 10%) level and a 12 h light–dark cycle. The mice were provided a standard laboratory diet, and water was provided ad libitum.
4.3. AD Induction and Dermatitis Scoring
The animals were randomly categorized into five groups (n = 5): the Nc/Nga normal control group, which was administered phosphate-buffered saline (PBS); the DfE-induced AD group administrated PBS; the DfE-induced AD group administrated 1 mg/kg RA; the DfE-induced AD group administrated 3 mg/kg RA; and the DfE-induced AD group administrated 1 mg/kg dexamethasone (positive control) [56]. All the treatments were administered orally once daily. AD-like skin lesions were induced by sensitization to 200 μL of 4% SDS sprayed on the dorsal skin and ear of the mice, which were shaven the previous day. House dust mite DfE ointment (100 mg, Biostir Inc., Kobe, Japan) was repeatedly applied on the dorsal skin and ears twice a week for 3 weeks. After 7 days of AD induction, RA (1 or 3 mg/kg) or dexamethasone (1 mg/kg) was orally administered by a syringe with plastic feeding tubes for the remaining 14 days. Ear thickness was measured via a digital caliper (CAS©, Seoul, Republic of Korea) twice a week after the health status of each mouse was assessed. Dermatitis scoring was based on the sum of individual scores for AD-related symptoms (scarring/dryness, erythema/hemorrhage, edema, and excoriation/erosion), ranging from 0 (no symptoms) to 1 (mild), 2 (moderate), or 3 (severe), as determined by the scorer [57].
4.4. Inflammatory Factor Analysis
After the final treatment of the sample and a 12 h fasting period, the mice were sacrificed using CO2 anesthesia, and the serum samples were collected. Following centrifugation at 3000 rpm for 15 min, the serum was collected and stored at −70 °C. Inflammatory factors in the serum or cell culture media were quantified via the LBIS Mouse IgE ELISA Kit (Fujifilm), Histamine Research ELISATM (LDN), or the LEGEND-plex™ Mouse Proinflammatory Chemokine Panel (BioLegend) according to the manufacturer’s instructions. A SpectraMax and BD LSRFortessa™ flow cytometer (BD Biosciences, San Jose, CA, USA) with BD CellQuest™ and LEGENDplex™ Software v8.0 (VigeneTech Inc., Carlisle, MA, USA) were used for the experiments.
4.5. Histological Analysis
The excised tissues were preserved in a 10% formaldehyde solution and then embedded in paraffin for cryosectioning. H&E staining was performed to evaluate hyperkeratosis and epidermal hyperplasia of the skin tissue. To identify mast cell infiltration, a toluidine blue staining kit (VitroVivo Biotech) was employed. A digital slide scanning system (Kfbio, Ningbo, China) in semiautomatic mode was used to scan and analyze the stained tissue samples.
4.6. Cell Culture and Viability Assay
The spleens of BALB/c mice (Central Lab Animal Inc., Seoul, Republic of Korea) were mashed with a medical spatula and filtered into a culture dish using a 70 μm cell strainer. After removing red blood cells (RBCs) via incubation with an RBC lysis buffer for 5 min, the cells were collected using centrifugation. The isolated primary splenocytes were incubated in RPMI 1640 medium with 10% FBS and 1% penicillin/streptomycin (Thermo Fisher Scientific) [58,59]. Normal human epidermal keratinocytes (HEKs) (catalog number 00192627, LonzaBioscience) were cultured in KBM-Gold Basal medium supplemented with a SingleQuots Supplement Pack (LonzaBioscience). HaCaT human keratinocyte cells (American Type Culture Collection) were cultured in Dulbecco’s modified Eagle’s medium supplemented with heat-inactivated 10% FBS and the above antibiotics in a HeracellTM incubator (Thermo Fisher Scientific) with 5% CO2 at 37 °C. TNF-α and IFN-γ (10 ng/mL) (Thermo Fisher Scientific) were used to activate the keratinocytes, and Con A (2.5 μg/mL) (Sigma Aldrich) was used to treat the splenocytes in the culture medium.
The cells were incubated in growth media supplemented with various concentrations of RA (0–100 µg/mL) for 24 h. After adding 10% CCK-8 (Dojindo Molecular Technologies Inc.) reagent to the media, the colorimetric results of the viability assay were measured at a wavelength of 450 nm using a SpectraMax 340 microplate reader (Molecular Devices, San Jose, CA, USA) according to the manufacturer’s instructions.
4.7. Western Blot Analysis
Detergent-based whole-cell lysis with RIPA buffer (Thermo Fisher Scientific) was performed to extract total cellular protein. NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific) were used for fractionation. A BCA assay was performed to quantify the protein concentration (Pierce™ BCA assay kit, Thermo Fisher Scientific). The extracted proteins were separated by their molecular weight under denaturing conditions with SDS on Mini-PROTEAN TGX precast protein gels (Bio-Rad, Hercules, CA, USA). The separated proteins were then electrically transferred to polyvinylidene fluoride membranes via a Trans-Blot Turbo Transfer system (Bio-Rad) under semidry conditions. The membranes were blocked with a commercial blocking solution (Atto, Tokyo, Japan) for 1 h and subsequently incubated with specific primary or HRP-conjugated secondary antibodies for an additional recommended time. Visualization of the immunoreactive bands was achieved via the Super Signal West Femto Chemiluminescent Substrate (Thermo Fisher Scientific) with a computerized densitometer (Bio-Rad).
4.8. Statistical Analysis
The data are presented as the means ± SEMs. A one-way ANOVA followed by Dunnett’s test was employed for multiple group comparisons to verify statistical significance. GraphPad Prism software (Version 8.0; GraphPad Software, Inc., San Diego, CA, USA) was used for the analysis. p < 0.05 was considered to indicate statistical significance.
5. Conclusions
In conclusion, this study demonstrated that oral administration of RA effectively alleviated DfE-induced AD-like skin lesions in a mouse model of AD by decreasing the levels of inflammatory cytokines and chemokines. Additionally, RA promotes HO-1 activation in keratinocytes by inducing Nrf2 expression, which may contribute to reducing the DfE-induced oxidative stress that triggers skin inflammation. These findings underscore the anti-inflammatory and antioxidant properties of RA against DfE-induced AD-like skin conditions, suggesting its potential as a pharmaceutical agent for preventing skin inflammation-related diseases such as AD.
Author Contributions
Conceptualization, T.K. and K.M.K.; methodology, K.-Y.J., D.H.J. and S.H.P.; investigation, H.J.K. and H.-K.S.; data curation, K.-S.S.; writing—original draft preparation, K.-S.S. and K.M.K.; writing—review and editing, T.K. and K.M.K.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Animal Care and Use Committee at the Korea Institute of Oriental Medicine (protocol code 22-073; date of approval, 1 August 2022) (approval number: 23-021; approval date, 30 March 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
All collected data are presented in this article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding Statement
This work was funded by the Korea Institute of Oriental Medicine, Ministry of Education, Science, and Technology, Republic of Korea [Grant no. KSN2023331].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Palmer C.N., Irvine A.D., Terron-Kwiatkowski A., Zhao Y., Liao H., Lee S.P., Goudie D.R., Sandilands A., Campbell L.E., Smith F.J., et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 2.Ji H., Li X.K. Oxidative Stress in Atopic Dermatitis. Oxid. Med. Cell. Longev. 2016;2016:2721469. doi: 10.1155/2016/2721469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eto H., Tsuji G., Chiba T., Furue M., Hyodo F. Non-invasive evaluation of atopic dermatitis based on redox status using in vivo dynamic nuclear polarization magnetic resonance imaging. Free Radic. Biol. Med. 2017;103:209–215. doi: 10.1016/j.freeradbiomed.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 4.Esser P.R., Wolfle U., Durr C., von Loewenich F.D., Schempp C.M., Freudenberg M.A., Jakob T., Martin S.F. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS ONE. 2012;7:e41340. doi: 10.1371/journal.pone.0041340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimada Y., Takehara K., Sato S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J. Dermatol. Sci. 2004;34:201–208. doi: 10.1016/j.jdermsci.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Gittler J.K., Shemer A., Suarez-Farinas M., Fuentes-Duculan J., Gulewicz K.J., Wang C.Q., Mitsui H., Cardinale I., de Guzman Strong C., Krueger J.G., et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishitsuka Y., Ogawa T., Roop D. The KEAP1/NRF2 Signaling Pathway in Keratinization. Antioxidants. 2020;9:751. doi: 10.3390/antiox9080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schafer M., Werner S. Nrf2—A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015;88:243–252. doi: 10.1016/j.freeradbiomed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Ngo V., Karunatilleke N.C., Brickenden A., Choy W.Y., Duennwald M.L. Oxidative Stress-Induced Misfolding and Inclusion Formation of Nrf2 and Keap1. Antioxidants. 2022;11:243. doi: 10.3390/antiox11020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L., Fan X., Cui T., Dang E., Wang G. Nrf2 Promotes Keratinocyte Proliferation in Psoriasis through Up-Regulation of Keratin 6, Keratin 16, and Keratin 17. J. Investig. Dermatol. 2017;137:2168–2176. doi: 10.1016/j.jid.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y., Shin J.M., Jang S., Choi D.K., Seo M.S., Kim H.R., Sohn K.C., Im M., Seo Y.J., Lee J.H., et al. Role of nuclear factor E2-related factor 2 (Nrf2) in epidermal differentiation. Arch. Dermatol. Res. 2014;306:677–682. doi: 10.1007/s00403-014-1470-x. [DOI] [PubMed] [Google Scholar]
- 12.Jeayeng S., Wongkajornsilp A., Slominski A.T., Jirawatnotai S., Sampattavanich S., Panich U. Nrf2 in keratinocytes modulates UVB-induced DNA damage and apoptosis in melanocytes through MAPK signaling. Free Radic. Biol. Med. 2017;108:918–928. doi: 10.1016/j.freeradbiomed.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huebner A.J., Dai D., Morasso M., Schmidt E.E., Schafer M., Werner S., Roop D.R. Amniotic fluid activates the nrf2/keap1 pathway to repair an epidermal barrier defect in utero. Dev. Cell. 2012;23:1238–1246. doi: 10.1016/j.devcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakabayashi N., Itoh K., Wakabayashi J., Motohashi H., Noda S., Takahashi S., Imakado S., Kotsuji T., Otsuka F., Roop D.R., et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 15.Buchman A.L. Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 2001;33:289–294. doi: 10.1097/00004836-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Del Rosso J., Friedlander S.F. Corticosteroids: Options in the era of steroid-sparing therapy. J. Am. Acad. Dermatol. 2005;53:S50–S58. doi: 10.1016/j.jaad.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Park S.H., Jang S., An J.E., Choo B.K., Kim H.K. I. inflexus (Thunb.) Kudo extract improves atopic dermatitis and depressive-like behavior in DfE-induced atopic dermatitis-like disease. Phytomedicine. 2020;67:153137. doi: 10.1016/j.phymed.2019.153137. [DOI] [PubMed] [Google Scholar]
- 18.Guan H., Luo W., Bao B., Cao Y., Cheng F., Yu S., Fan Q., Zhang L., Wu Q., Shan M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules. 2022;27:3292. doi: 10.3390/molecules27103292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang A.H., Kim T.H., Kim G.D., Kim J.E., Kim H.J., Kim S.S., Jin Y.H., Park Y.S., Park C.S. Rosmarinic acid attenuates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int. Immunopharmacol. 2011;11:1271–1277. doi: 10.1016/j.intimp.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Lee J., Jung E., Koh J., Kim Y.S., Park D. Effect of rosmarinic acid on atopic dermatitis. J. Dermatol. 2008;35:768–771. doi: 10.1111/j.1346-8138.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 21.Sanbongi C., Takano H., Osakabe N., Sasa N., Natsume M., Yanagisawa R., Inoue K.I., Sadakane K., Ichinose T., Yoshikawa T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin. Exp. Allergy. 2004;34:971–977. doi: 10.1111/j.1365-2222.2004.01979.x. [DOI] [PubMed] [Google Scholar]
- 22.Piao M.J., Fernando P., Kang K.A., Fernando P., Herath H., Kim Y.R., Hyun J.W. Rosmarinic Acid Inhibits Ultraviolet B-Mediated Oxidative Damage via the AKT/ERK-NRF2-GSH Pathway In Vitro and In Vivo. Biomol. Ther. 2024;32:84–93. doi: 10.4062/biomolther.2023.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abduh M.S., Alruhaimi R.S., Alqhtani H.A., Hussein O.E., Abukhalil M.H., Kamel E.M., Mahmoud A.M. Rosmarinic acid mitigates chlorpyrifos-induced oxidative stress, inflammation, and kidney injury in rats by modulating SIRT1 and Nrf2/HO-1 signaling. Life Sci. 2023;313:121281. doi: 10.1016/j.lfs.2022.121281. [DOI] [PubMed] [Google Scholar]
- 24.Ji R., Sun H., Peng J., Ma X., Bao L., Fu Y., Zhang X., Luo C., Gao C., Jin Y., et al. Rosmarinic acid exerts an antagonistic effect on vascular calcification by regulating the Nrf2 signalling pathway. Free Radic. Res. 2019;53:187–197. doi: 10.1080/10715762.2018.1558447. [DOI] [PubMed] [Google Scholar]
- 25.Cui H.Y., Zhang X.J., Yang Y., Zhang C., Zhu C.H., Miao J.Y., Chen R. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural Regen. Res. 2018;13:2119–2128. doi: 10.4103/1673-5374.241463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vestergaard C., Yoneyama H., Murai M., Nakamura K., Tamaki K., Terashima Y., Imai T., Yoshie O., Irimura T., Mizutani H., et al. Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J. Clin. Investig. 1999;104:1097–1105. doi: 10.1172/JCI7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martel B.C., Lovato P., Baumer W., Olivry T. Translational Animal Models of Atopic Dermatitis for Preclinical Studies. Yale J. Biol. Med. 2017;90:389–402. [PMC free article] [PubMed] [Google Scholar]
- 28.Teplitsky V., Mumcuoglu K.Y., Babai I., Dalal I., Cohen R., Tanay A. House dust mites on skin, clothes, and bedding of atopic dermatitis patients. Int. J. Dermatol. 2008;47:790–795. doi: 10.1111/j.1365-4632.2008.03657.x. [DOI] [PubMed] [Google Scholar]
- 29.Matsuoka H., Maki N., Yoshida S., Arai M., Wang J., Oikawa Y., Ikeda T., Hirota N., Nakagawa H., Ishii A. A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy. 2003;58:139–145. doi: 10.1034/j.1398-9995.2003.23790.x. [DOI] [PubMed] [Google Scholar]
- 30.Suto H., Matsuda H., Mitsuishi K., Hira K., Uchida T., Unno T., Ogawa H., Ra C. NC/Nga mice: A mouse model for atopic dermatitis. Int. Arch. Allergy Immunol. 1999;120((Suppl. S1)):70–75. doi: 10.1159/000053599. [DOI] [PubMed] [Google Scholar]
- 31.Houben E., De Paepe K., Rogiers V. A keratinocyte’s course of life. Skin Pharmacol. Physiol. 2007;20:122–132. doi: 10.1159/000098163. [DOI] [PubMed] [Google Scholar]
- 32.Chieosilapatham P., Kiatsurayanon C., Umehara Y., Trujillo-Paez J.V., Peng G., Yue H., Nguyen L.T.H., Niyonsaba F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021;204:296–309. doi: 10.1111/cei.13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koga C., Kabashima K., Shiraishi N., Kobayashi M., Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto M., Haruna T., Yasui K., Takahashi H., Iduhara M., Takaki S., Deguchi M., Arimura A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. 2007;56:139–148. doi: 10.2332/allergolint.O-06-458. [DOI] [PubMed] [Google Scholar]
- 35.Ding Y., Ma T., Zhang Y., Zhao C., Wang C., Wang Z. Rosmarinic acid ameliorates skin inflammation and pruritus in allergic contact dermatitis by inhibiting mast cell-mediated MRGPRX2/PLCgamma1 signaling pathway. Int. Immunopharmacol. 2023;117:110003. doi: 10.1016/j.intimp.2023.110003. [DOI] [PubMed] [Google Scholar]
- 36.Helou D.G., Martin S.F., Pallardy M., Chollet-Martin S., Kerdine-Romer S. Nrf2 Involvement in Chemical-Induced Skin Innate Immunity. Front. Immunol. 2019;10:1004. doi: 10.3389/fimmu.2019.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaburagi Y., Shimada Y., Nagaoka T., Hasegawa M., Takehara K., Sato S. Enhanced production of CC-chemokines (RANTES, MCP-1, MIP-1alpha, MIP-1beta, and eotaxin) in patients with atopic dermatitis. Arch. Dermatol. Res. 2001;293:350–355. doi: 10.1007/s004030100230. [DOI] [PubMed] [Google Scholar]
- 38.Mitamura Y., Nunomura S., Nanri Y., Ogawa M., Yoshihara T., Masuoka M., Tsuji G., Nakahara T., Hashimoto-Hachiya A., Conway S.J., et al. The IL-13/periostin/IL-24 pathway causes epidermal barrier dysfunction in allergic skin inflammation. Allergy. 2018;73:1881–1891. doi: 10.1111/all.13437. [DOI] [PubMed] [Google Scholar]
- 39.Danso M.O., van Drongelen V., Mulder A., van Esch J., Scott H., van Smeden J., El Ghalbzouri A., Bouwstra J.A. TNF-alpha and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J. Investig. Dermatol. 2014;134:1941–1950. doi: 10.1038/jid.2014.83. [DOI] [PubMed] [Google Scholar]
- 40.Oshio T., Sasaki Y., Funakoshi-Tago M., Aizu-Yokota E., Sonoda Y., Matsuoka H., Kasahara T. Dermatophagoides farinae extract induces severe atopic dermatitis in NC/Nga mice, which is effectively suppressed by the administration of tacrolimus ointment. Int. Immunopharmacol. 2009;9:403–411. doi: 10.1016/j.intimp.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Kim J.Y., Jeong M.S., Park M.K., Lee M.K., Seo S.J. Time-dependent progression from the acute to chronic phases in atopic dermatitis induced by epicutaneous allergen stimulation in NC/Nga mice. Exp. Dermatol. 2014;23:53–57. doi: 10.1111/exd.12297. [DOI] [PubMed] [Google Scholar]
- 42.Ohmen J.D., Hanifin J.M., Nickoloff B.J., Rea T.H., Wyzykowski R., Kim J., Jullien D., McHugh T., Nassif A.S., Chan S.C., et al. Overexpression of IL-10 in atopic dermatitis. Contrasting cytokine patterns with delayed-type hypersensitivity reactions. J. Immunol. 1995;154:1956–1963. doi: 10.4049/jimmunol.154.4.1956. [DOI] [PubMed] [Google Scholar]
- 43.Wilson S.R., The L., Batia L.M., Beattie K., Katibah G.E., McClain S.P., Pellegrino M., Estandian D.M., Bautista D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song H.K., Park S.H., Kim H.J., Jang S., Choo B.K., Kim H.K., Kim T. Inhibitory effect of Sanguisorba hakusanensis Makino ethanol extract on atopic dermatitis-like responses in NC/Nga mice and human keratinocytes. Sci. Rep. 2023;13:14594. doi: 10.1038/s41598-023-41676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shim K.S., Song H.K., Park M., Kim H.J., Jang S., Kim T., Kim K.M. Reynoutria japonica consisted of emodin-8-beta-D-glucoside ameliorates Dermatophagoides farinae extract-induced atopic dermatitis-like skin inflammation in mice by inhibiting JAK/STAT signaling. Biomed. Pharmacother. 2024;176:116765. doi: 10.1016/j.biopha.2024.116765. [DOI] [PubMed] [Google Scholar]
- 46.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 47.Magesh S., Chen Y., Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012;32:687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eggler A.L., Small E., Hannink M., Mesecar A.D. Cul3-mediated Nrf2 ubiquitination and antioxidant response element (ARE) activation are dependent on the partial molar volume at position 151 of Keap1. Biochem. J. 2009;422:171–180. doi: 10.1042/BJ20090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo S.C., Li X., Henzl M.T., Beamer L.J., Hannink M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006;25:3605–3617. doi: 10.1038/sj.emboj.7601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Z.Y., Lu M.C., Xu L.L., Yang T.T., Xi M.Y., Xu X.L., Guo X.K., Zhang X.J., You Q.D., Sun H.P. Discovery of potent Keap1-Nrf2 protein-protein interaction inhibitor based on molecular binding determinants analysis. J. Med. Chem. 2014;57:2736–2745. doi: 10.1021/jm5000529. [DOI] [PubMed] [Google Scholar]
- 51.Londhe A.M., Gadhe C.G., Lim S.M., Pae A.N. Investigation of Molecular Details of Keap1-Nrf2 Inhibitors Using Molecular Dynamics and Umbrella Sampling Techniques. Molecules. 2019;24:4085. doi: 10.3390/molecules24224085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z., Peng L., Fu Y., Wang W., Wang P., Zhou F. Ginnalin A Binds to the Subpockets of Keap1 Kelch Domain To Activate the Nrf2-Regulated Antioxidant Defense System in SH-SY5Y Cells. ACS Chem. Neurosci. 2021;12:872–882. doi: 10.1021/acschemneuro.0c00713. [DOI] [PubMed] [Google Scholar]
- 53.Fernando P.M., Piao M.J., Kang K.A., Ryu Y.S., Hewage S.R., Chae S.W., Hyun J.W. Rosmarinic Acid Attenuates Cell Damage against UVB Radiation-Induced Oxidative Stress via Enhancing Antioxidant Effects in Human HaCaT Cells. Biomol. Ther. 2016;24:75–84. doi: 10.4062/biomolther.2015.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang M., Li N., Cai R., Gu J., Xie F., Wei H., Lu C., Wu D. Rosmarinic acid protects mice from imiquimod induced psoriasis-like skin lesions by inhibiting the IL-23/Th17 axis via regulating Jak2/Stat3 signaling pathway. Phytother. Res. 2021;35:4526–4537. doi: 10.1002/ptr.7155. [DOI] [PubMed] [Google Scholar]
- 55.Zhou M.W., Jiang R.H., Kim K.D., Lee J.H., Kim C.D., Yin W.T., Lee J.H. Rosmarinic acid inhibits poly(I:C)-induced inflammatory reaction of epidermal keratinocytes. Life Sci. 2016;155:189–194. doi: 10.1016/j.lfs.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 56.Mohd Kasim V.N.K., Noble S.M., Liew K.Y., Tan J.W., Israf D.A., Tham C.L. Management of Atopic Dermatitis Via Oral and Topical Administration of Herbs in Murine Model: A Systematic Review. Front. Pharmacol. 2022;13:785782. doi: 10.3389/fphar.2022.785782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee Y.S., Ryu H.W., Yang W.K., Park M.H., Park Y.C., Kim D.Y., Kwon H.J., Kim S.Y., Oh S.R., Kim S.H. A combination of Olea europaea leaf extract and Spirodela polyrhiza extract alleviates atopic dermatitis by modulating immune balance and skin barrier function in a 1-chloro-2,4-dinitrobenzene-induced murine model. Phytomedicine. 2021;82:153407. doi: 10.1016/j.phymed.2020.153407. [DOI] [PubMed] [Google Scholar]
- 58.Ji K.Y., Jung D.H., Pyun B.J., Kim Y.J., Lee J.Y., Choi S., Jung M.A., Song K.H., Kim T. Angelica gigas extract ameliorates allergic rhinitis in an ovalbumin-induced mouse model by inhibiting Th2 cell activation. Phytomedicine. 2021;93:153789. doi: 10.1016/j.phymed.2021.153789. [DOI] [PubMed] [Google Scholar]
- 59.Shim K.S., Park M., Yang W.K., Lee H., Kim S.H., Choo B.K., Chae S., Kim H.K., Kim T., Kim K.M. Veronica persica Ethanol Extract Ameliorates Dinitrochlorobenzene-Induced Atopic Dermatitis-like Skin Inflammation in Mice, Likely by Inducing Nrf2/HO-1 Signaling. Antioxidants. 2023;12:1267. doi: 10.3390/antiox12061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All collected data are presented in this article.