Abstract
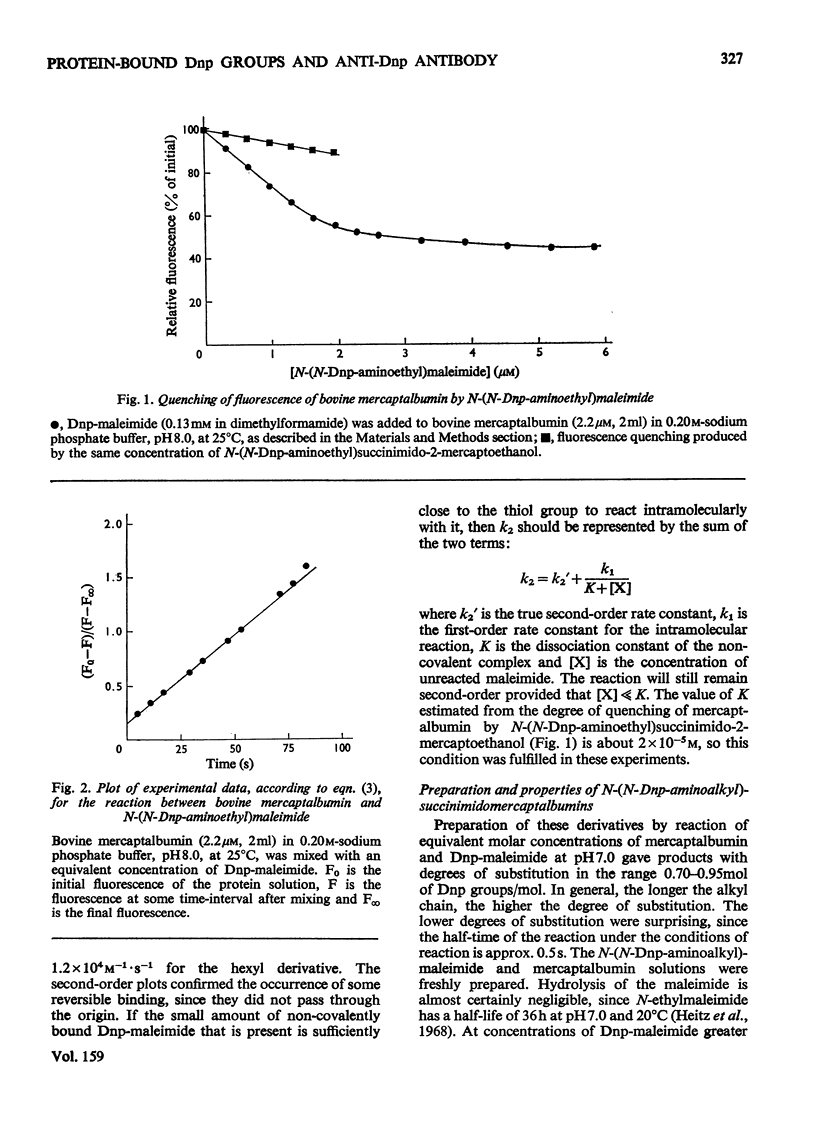
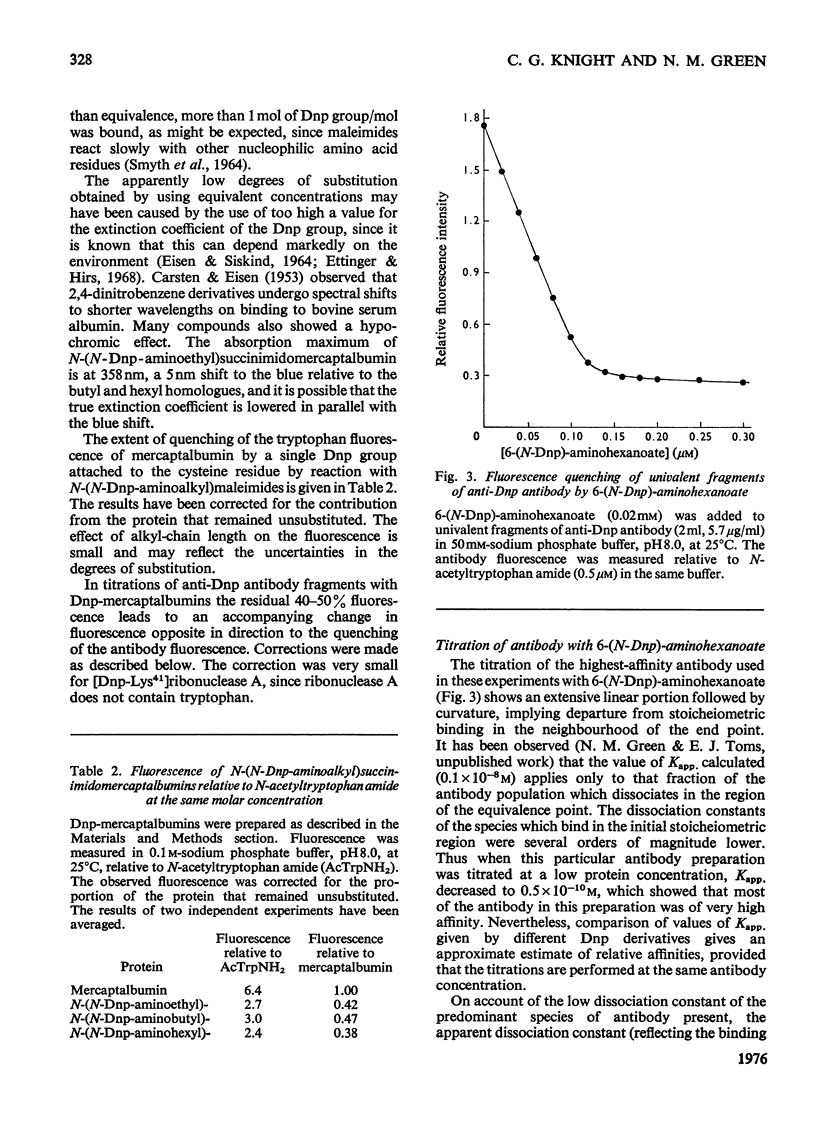
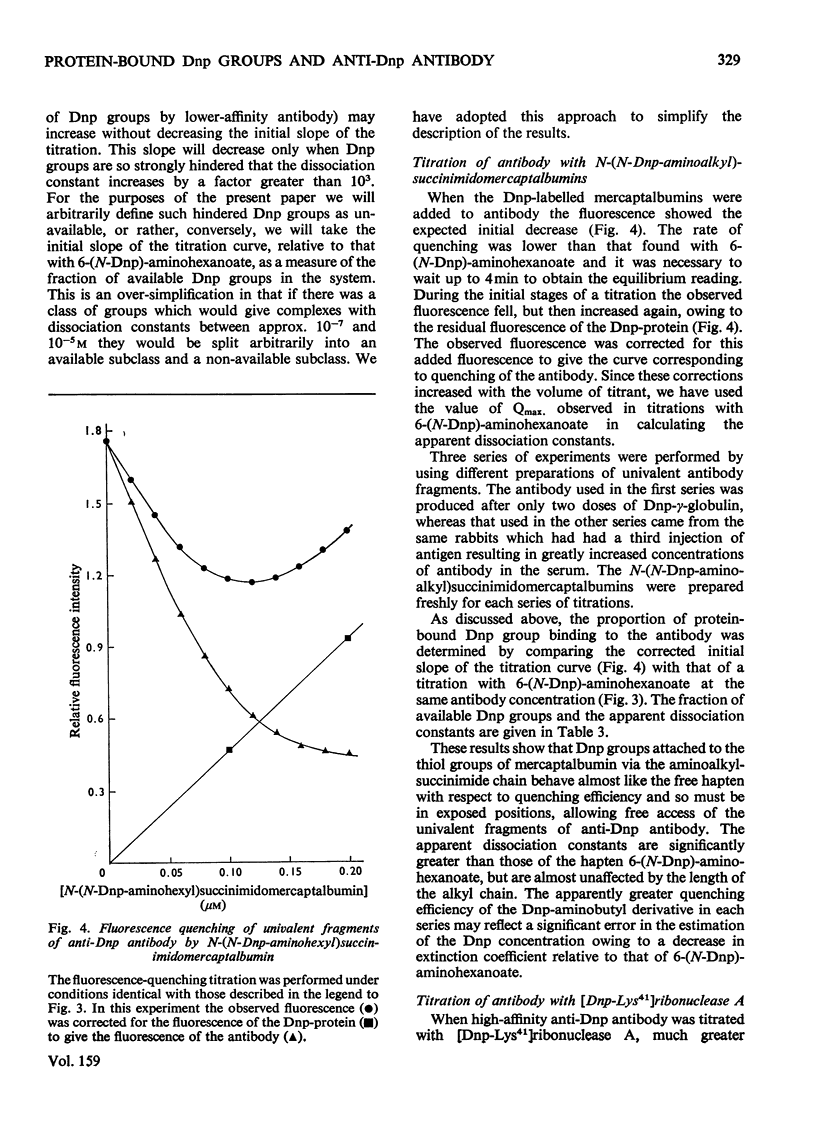
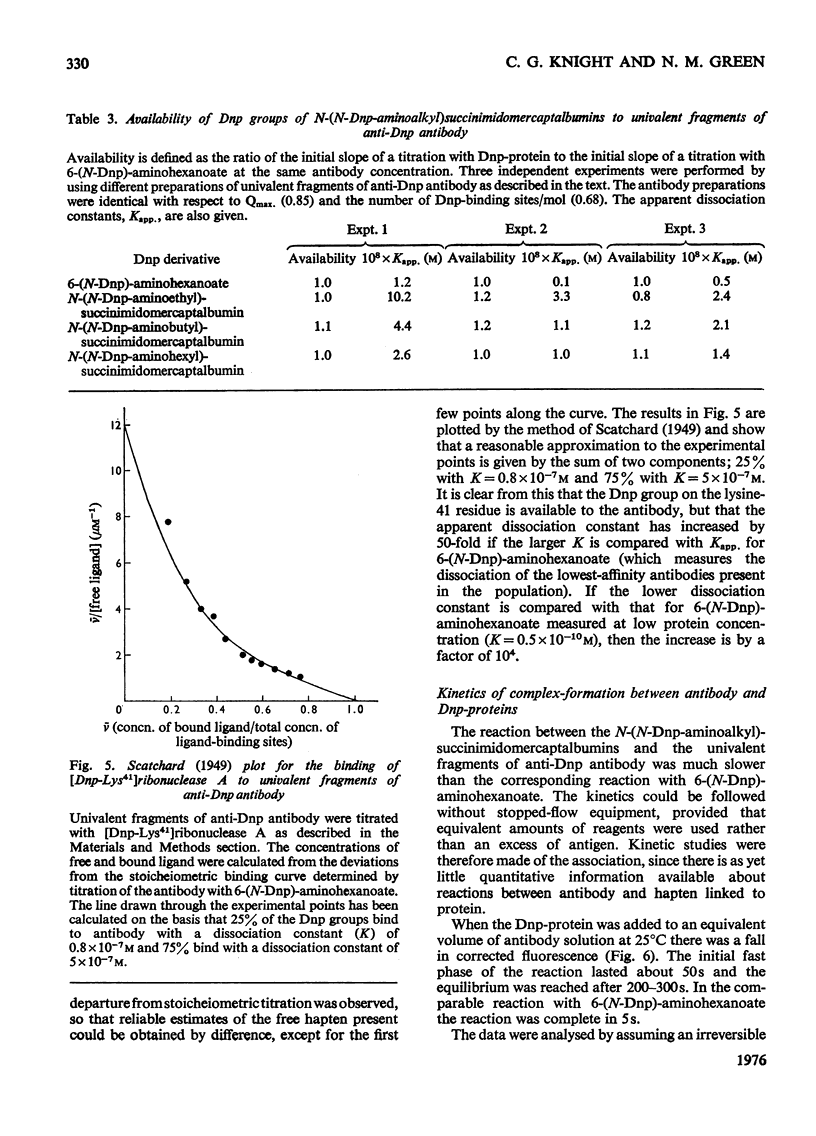
A series of N-(N-dinitrophenylaminoalkyl)maleimides were sythesized with alkyl-chain lengths of two, four and six carbon atoms. When these compounds reacted with the thiol group of mercaptalbumin, the tryptophan fluorescence of the protein was quenched. This change in fluorescence was used to determine the rate of reaction of the Dnp (dinitrophenyl)-maleimides with mercaptalbumin. The second-order rate constants were similar to those observed in reactions between low-molecular-weight thiol compounds and maleimides. When N-(N-Dnp-aminoalkyl)succinimidomercaptalbumins were added to univalent fragments of anti-Dnp antibody the antibody fluorescence was quenched. Florescence-quenching titrations showed that the protein-bound Dnp groups were fully available to the antibody even when the alkyl chain was short. The apparent dissociation constants were significantly greater than that of the interaction between anti-Dnp antibody and the free hapten, 6-(N-Dnp)-aminohexanoate. The antibody fluorescence was quenched efficienty by [dnp-Lys41]ribonuclease A, also with an increased dissociation constant. It could be concluded from the increase in dissociation constant that the Dnp group spent no more than 0.1% of its time in the dissociated state, available to antibody. The second-order rate constants for the association between the Dnp-mercaptablumins and the antibody were determined and were similar in magnitude to those observed in other interactions between protein and anti-protein antibody.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allewell N. M., Mitsui Y., Wyckoff H. W. X-ray diffraction studies of epsilon-41-dinitropheny-ribonuclease-S. J Biol Chem. 1973 Aug 10;248(15):5291–5298. [PubMed] [Google Scholar]
- Anderson B. M., Kimsj, Wang C. N. Inactivation of rabbit muscle L-alpha-glycerophosphate dehydrogenase by N-alkylmaleimides. Arch Biochem Biophys. 1970 May;138(1):66–72. doi: 10.1016/0003-9861(70)90285-7. [DOI] [PubMed] [Google Scholar]
- Anderson B. M., Vasini E. C. Nonpolar effects in reactions of the sulfhydryl group of papain. Biochemistry. 1970 Aug 18;9(17):3348–3352. doi: 10.1021/bi00819a009. [DOI] [PubMed] [Google Scholar]
- Andersson L. O. The heterogeneity of bovine serum albumin. Biochim Biophys Acta. 1966 Mar 28;117(1):115–133. doi: 10.1016/0304-4165(66)90159-0. [DOI] [PubMed] [Google Scholar]
- BALDWIN R. L. Boundary spreading in sedimentation-velocity experiments. V. Measurement of the diffusion coefficient of bovine albumin by Fujita's equation. Biochem J. 1957 Mar;65(3):503–512. doi: 10.1042/bj0650503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. K., McEwan M., Mikoryak C. A., Polkowski J. Studies on the antigenic structure of ribonuclease. V. Dinitrophenylated ribonuclease. J Biol Chem. 1967 Jun 25;242(12):3007–3013. [PubMed] [Google Scholar]
- Dandliker W. B., Levison S. A. Investigation of antigen-antibody kinetics by fluorescence polarization. Immunochemistry. 1968 Mar;5(2):171–183. doi: 10.1016/0019-2791(68)90101-8. [DOI] [PubMed] [Google Scholar]
- EISEN H. N. PREPARATION OF PURIFIED ANTI-2,4-DINITROPHENYL ANTIBODIES. Methods Med Res. 1964;10:94–102. [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Ettinger M. J., Hirs C. H. On the structure of 41-Dinitrophenyl ribonuclease A. Solvent perturbation, thermal transition, optical rotatory dispersion, and binding studies. Biochemistry. 1968 Oct;7(10):3374–3380. doi: 10.1021/bi00850a010. [DOI] [PubMed] [Google Scholar]
- Fonda M. L., Anderson B. M. D-amino acid oxidase. IV. Inactivation by maleimides. J Biol Chem. 1969 Feb 25;244(4):666–674. [PubMed] [Google Scholar]
- Froese A. Kinetic and equilibrium studies on 2,4-Dinitrophenyl hapten-antibody systems. Immunochemistry. 1968 May;5(3):253–264. doi: 10.1016/0019-2791(68)90070-0. [DOI] [PubMed] [Google Scholar]
- GREEN N. M., WORK E. Pancreatic trypsin inhibitor. II. Reaction with trypsin. Biochem J. 1953 May;54(2):347–352. doi: 10.1042/bj0540347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin G., Martic P. A., Doughty G. Kinetics of the reaction of N-ethylmaleimide with cysteine and some congeners. Arch Biochem Biophys. 1966 Sep 9;115(3):593–597. doi: 10.1016/0003-9861(66)90079-8. [DOI] [PubMed] [Google Scholar]
- Green N. M. Electron microscopy of the immunoglobulins. Adv Immunol. 1969;11:1–30. doi: 10.1016/s0065-2776(08)60476-9. [DOI] [PubMed] [Google Scholar]
- HUGHES W. L., Jr Protein mercaptides. Cold Spring Harb Symp Quant Biol. 1950;14:79–84. doi: 10.1101/sqb.1950.014.01.011. [DOI] [PubMed] [Google Scholar]
- Hall P. L., Anderson C. D. Proflavine interactions with papain and ficin. I. Dye binding and its effects upon enzyme inactivation by N-alkylmaleimides. Biochemistry. 1974 May 7;13(10):2082–2087. doi: 10.1021/bi00707a013. [DOI] [PubMed] [Google Scholar]
- Heitmann P. Reactivity of sulfhydryl groups in micelles. A model for protein. Eur J Biochem. 1968 Aug;5(3):305–315. doi: 10.1111/j.1432-1033.1968.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Heitz J. R., Anderson C. D., Anderson B. M. Inactivation of yeast alcohol dehydrogenase by N-alkylmaleimides. Arch Biochem Biophys. 1968 Sep 20;127(1):627–636. doi: 10.1016/0003-9861(68)90271-3. [DOI] [PubMed] [Google Scholar]
- Hirs C. H., Halmann M., Kycia J. H. Dinitrophenylation and inactivation of bovine pancreatic ribonuclease A. Arch Biochem Biophys. 1965 Jul;111(1):209–222. doi: 10.1016/0003-9861(65)90343-7. [DOI] [PubMed] [Google Scholar]
- Hull H. H., Chang R., Kaplan L. J. On the location of the sulfhydryl group in bovine plasma albumin. Biochim Biophys Acta. 1975 Jul 21;400(1):132–136. doi: 10.1016/0005-2795(75)90133-6. [DOI] [PubMed] [Google Scholar]
- King T. P., Spencer E. M. Amino acid sequences of the amino and the carboxyl terminal cyanogen bromide peptides of bovine plasma albumin. Arch Biochem Biophys. 1972 Dec;153(2):627–640. doi: 10.1016/0003-9861(72)90382-7. [DOI] [PubMed] [Google Scholar]
- Lee F. H., Froese A. Kinetics of the reaction between DNP-insulin and homologous antibody. Immunol Commun. 1973;2(6):565–571. doi: 10.3109/08820137309022827. [DOI] [PubMed] [Google Scholar]
- McGuigan J. E., Eisen H. N. Differences in spectral properties and tryptophan content among rabbit anti-2,4-dinitrophenyl antibodies of the gamma G-immunologbulin class. Biochemistry. 1968 May;7(5):1919–1928. doi: 10.1021/bi00845a041. [DOI] [PubMed] [Google Scholar]
- Noble R. W., Reichlin M., Gibson Q. H. The reactions of antibodies with hemeprotein antgens. The measurement of reaction kinetics and stoichiometry by fluorescence quenching. J Biol Chem. 1969 May 10;244(9):2403–2411. [PubMed] [Google Scholar]
- Noel J. K., Hunter M. J. Bovine mercaptalbumin and non-mercaptalbumin monomers. Interconversions and structural differences. J Biol Chem. 1972 Nov 25;247(22):7391–7406. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs D. H., Schechter A. N., Eastlake A., Anfinsen C. B. Inactivation of staphylococcal nuclease by the binding of antibodies to a distinct antigenic determinant. Biochemistry. 1972 Nov 7;11(23):4268–4273. doi: 10.1021/bi00773a012. [DOI] [PubMed] [Google Scholar]
- Smyth D. G., Blumenfeld O. O., Konigsberg W. Reactions of N-ethylmaleimide with peptides and amino acids. Biochem J. 1964 Jun;91(3):589–595. doi: 10.1042/bj0910589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G. T., Eisen H. N., Jones R. H. The problem of hapten persistently bound to antibody. Biochem J. 1970 Jan;116(1):151–153. doi: 10.1042/bj1160151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMBS M. P., SOUTER F., MACLAGAN N. F. The spectrophotometric determination of protein at 210 millimicrons. Biochem J. 1959 Sep;73:167–171. doi: 10.1042/bj0730167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velick S. F., Parker C. W., Eisen H. N. EXCITATION ENERGY TRANSFER AND THE QUANTITATIVE STUDY OF THE ANTIBODY HAPTEN REACTION. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1470–1482. doi: 10.1073/pnas.46.11.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Green G., Schumaker V. N. Bivalent hapten-antibody interactions--1. A comparison of water soluble and water insoluble bivalent haptens. Immunochemistry. 1975 Jan;12(1):39–47. doi: 10.1016/0019-2791(75)90047-6. [DOI] [PubMed] [Google Scholar]


