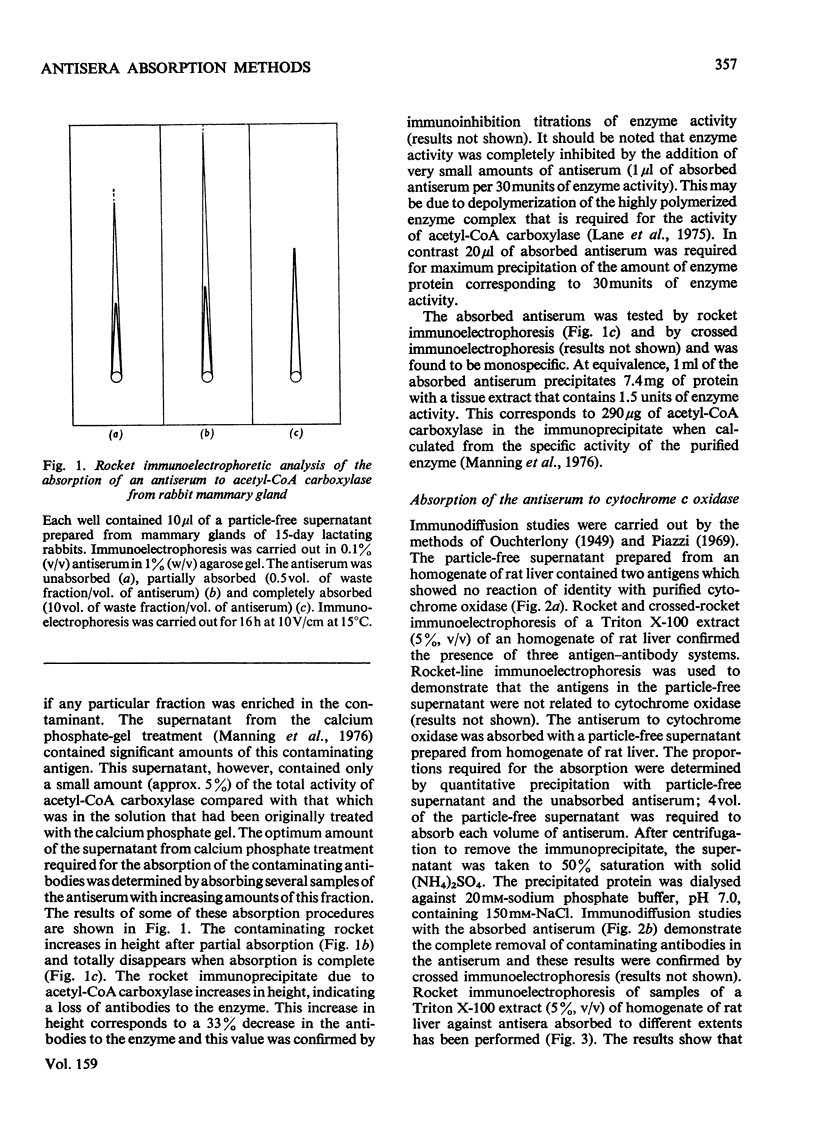
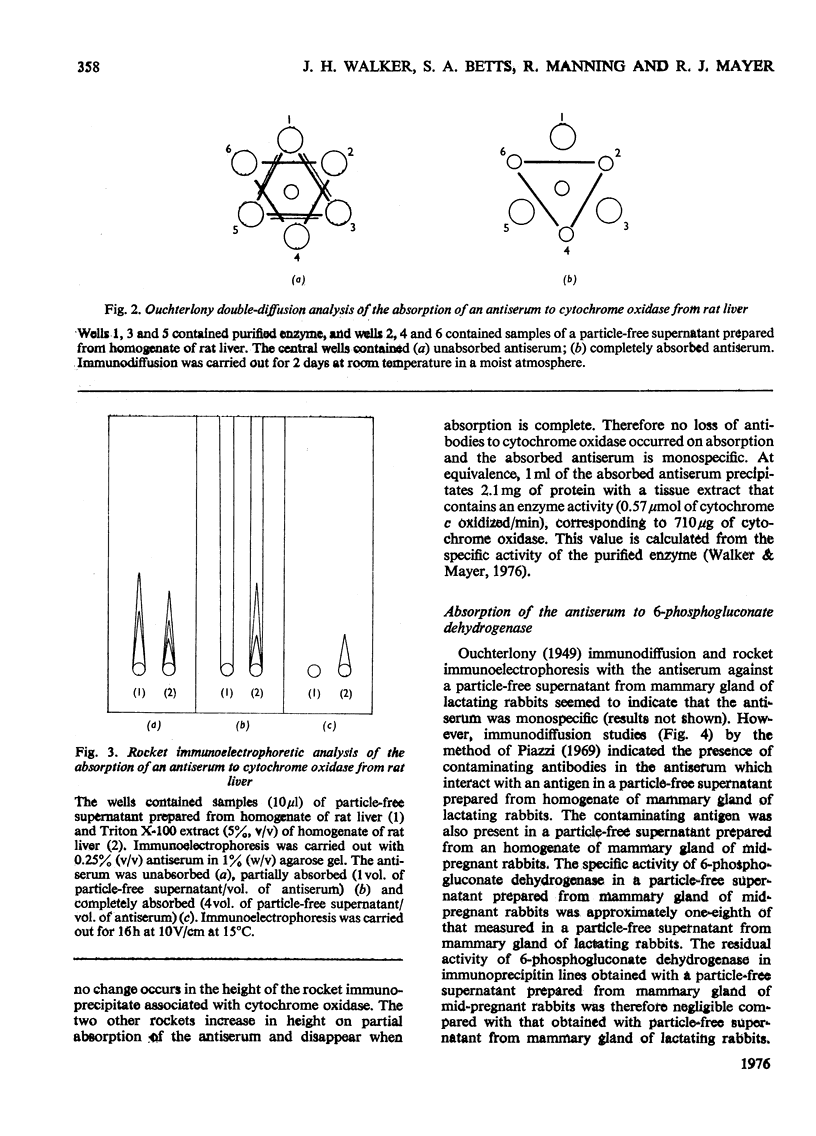
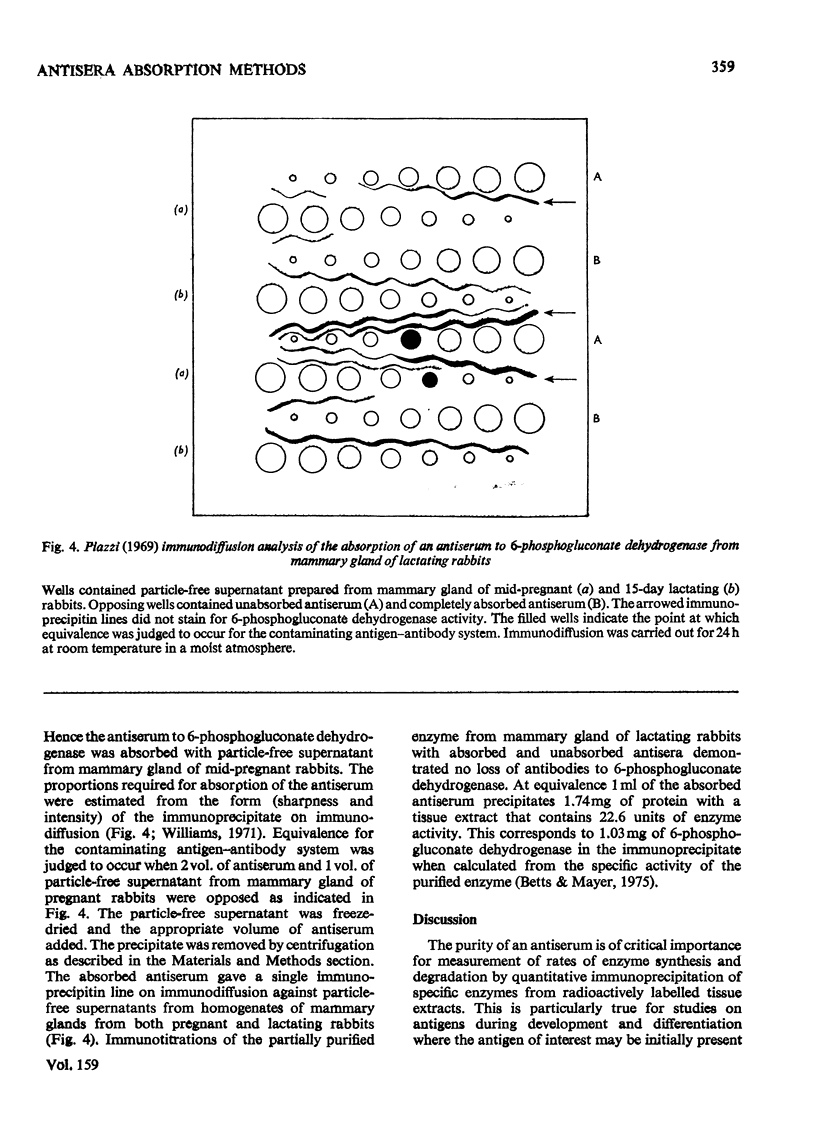
Abstract
Antisera were raised to acetyl-CoA carboxylase and 6-phosphogluconate dehydrogenase from mammary glands of lactating rabbits, and cytochrome oxidase from rat liver. The enzymes were all highly purified but gave rise to multispecific antisera when tested against tissue extracts. Absorption procedures were devised to free the antisera of contaminating antibodies. Antisera to acetyl-CoA carboxylase and cytochrome oxidase were absorbed with fractions discarded during enzyme purification. The antiserum to 6-phospho-gluconate dehydrogenase was absorbed with a tissue extract from an early stage in mammary-gland differentiation. Monospecific antisera are essential for enzyme turnover studies and therefore antisera should be extensively tested and absorbed before use. A general procedure for the absorption of antisera to purified enzymes has been devised on the basis of accepted principles of antisera absorption.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeley J. G. Active fragments obtained by cyanogen bromide cleavage of ovomucoid. Biochem J. 1976 May 1;155(2):345–351. doi: 10.1042/bj1550345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts S. A., Mayer R. J. Purification and properties of 6-phosphogluconate dehydrogenase from rabbit mammary gland. Biochem J. 1975 Nov;151(2):263–270. doi: 10.1042/bj1510263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M., Kamp P. F., Robinson N. C., Capaldi R. A. The subunit structure of the cytochrome c oxidase complex. Biochemistry. 1975 Nov 18;14(23):5123–5128. doi: 10.1021/bi00694a016. [DOI] [PubMed] [Google Scholar]
- Bucher J. R., Penniall R. The subunit composition of beef heart cytochrome c oxidase. FEBS Lett. 1975 Dec 1;60(1):180–184. doi: 10.1016/0014-5793(75)80447-9. [DOI] [PubMed] [Google Scholar]
- Chauvet J., Acher R. Isolation of a trypsin inhibitor (Kunitz inhibitor) from bovine ovary by affinity chromatography through trypsin-sepharose. FEBS Lett. 1972 Jul 1;23(3):317–320. doi: 10.1016/0014-5793(72)80305-3. [DOI] [PubMed] [Google Scholar]
- Cihak A., Lamar C., Jr, Pitot H. C. L-tryptophan inhibition of tyrosine aminotransferase degradation in rat liver in vivo. Arch Biochem Biophys. 1973 May;156(1):188–194. doi: 10.1016/0003-9861(73)90356-1. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Rabbit beta-glucuronidase. Subcellular distribution and immunochemical properties. Biochem J. 1974 Mar;138(3):407–413. doi: 10.1042/bj1380407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery R. S., Baldwin R. L. Turnover of several mammary enzymes during lactation. Biochim Biophys Acta. 1967 Mar 22;136(2):223–232. doi: 10.1016/0304-4165(67)90067-0. [DOI] [PubMed] [Google Scholar]
- Glass R. D., Doyle D. On the measurement of protein turnover in animal cells. J Biol Chem. 1972 Aug 25;247(16):5234–5242. [PubMed] [Google Scholar]
- Hizi A., Yagil G. On the mechanism of glucose-6-phosphate dehydrogenase regulation in mouse liver. 3. The rate of enzyme synthesis and degradation. Eur J Biochem. 1974 Jun 1;45(1):211–221. doi: 10.1111/j.1432-1033.1974.tb03545.x. [DOI] [PubMed] [Google Scholar]
- JENNINGS R. K., KAPLAN M. A. Gel diffusion studies of serologically related antigens. 2. Patterns of partial identity. Int Arch Allergy Appl Immunol. 1960;17:267–278. doi: 10.1159/000229136. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Manning R., Dils R., Mayer R. J. Purification and some properties of acetyl-coenzyme A carboxylase from rabbit mammary gland. Biochem J. 1976 Feb 1;153(2):463–468. doi: 10.1042/bj1530463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. E., Hansen R. J. Immunological titration of rat liver glucose-6-phosphate dehydrogenase from animals fed high and low carbohydrate diets. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1106–1111. doi: 10.1016/0006-291x(75)90471-4. [DOI] [PubMed] [Google Scholar]
- Penniall R., Holbrook J. P., Elliott W. B. Studies on a cytochrome oxidase antibody. IV. An immunologic impurity. Immunol Commun. 1974;3(4):391–399. doi: 10.3109/08820137409061118. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Mahler H. R. Studies on cytochrome oxidase. Partial resolution of enzymes containing seven or six subunits, from yeast and beef heart, respectively. J Biol Chem. 1976 Jan 25;251(2):257–269. [PubMed] [Google Scholar]
- Phan S. H., Mahler H. R. Studies on cytochrome oxidase. Preliminary characterization of an enzyme containing only four subunits. J Biol Chem. 1976 Jan 25;251(2):270–276. [PubMed] [Google Scholar]
- Philippidis H., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. The initial synthesis of proteins during development. Phosphoenolpyruvate carboxylase in rat liver at birth. Biochem J. 1972 Mar;126(5):1127–1134. doi: 10.1042/bj1261127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzi S. E. A simple method for preliminary immunodiffusion test of antigen-antibody systems having unknown ratios of reaction. Anal Biochem. 1969 Feb;27(2):281–284. doi: 10.1016/0003-2697(69)90033-5. [DOI] [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. IV. Immunological evidence for the participation of a mitochondrially synthesized subunit in enzymatic activity. J Biol Chem. 1975 Jan 25;250(2):762–766. [PubMed] [Google Scholar]
- Rietra P. J., Molenaar J. L., Hamers M. N., Tager J. M., Borst P. Investigation of the alpha-galactosidase deficiency in Fabry's disease using antibodies against the purified enzyme. Eur J Biochem. 1974 Jul 1;46(1):89–98. doi: 10.1111/j.1432-1033.1974.tb03600.x. [DOI] [PubMed] [Google Scholar]
- Robinson N. C., Tye R. W., Neurath H., Walsh K. A. Isolation of trypsins by affinity chromatography. Biochemistry. 1971 Jul 6;10(14):2743–2747. doi: 10.1021/bi00790a014. [DOI] [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differention. Studies on the effects of hormones on the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1975 May;148(2):309–320. doi: 10.1042/bj1480309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. H., Mayer R. J. Purification and subunit structure of cytochrome oxidase from rat liver. Biochem Soc Trans. 1976;4(2):342–344. doi: 10.1042/bst0040342. [DOI] [PubMed] [Google Scholar]
- Werner S. Isolation and characterisation of a mitochondrially synthesized precursor protein of cytochrome oxidase. Eur J Biochem. 1974 Mar 15;43(1):39–48. doi: 10.1111/j.1432-1033.1974.tb03382.x. [DOI] [PubMed] [Google Scholar]


