Abstract
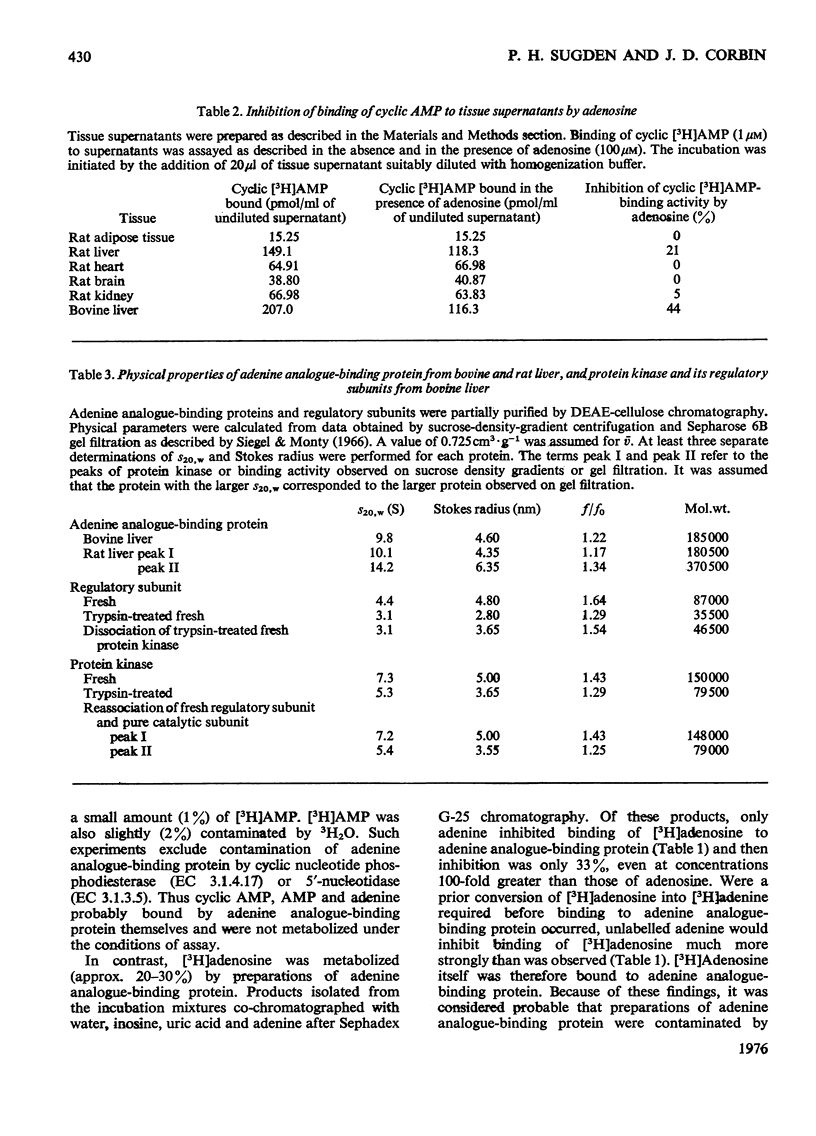
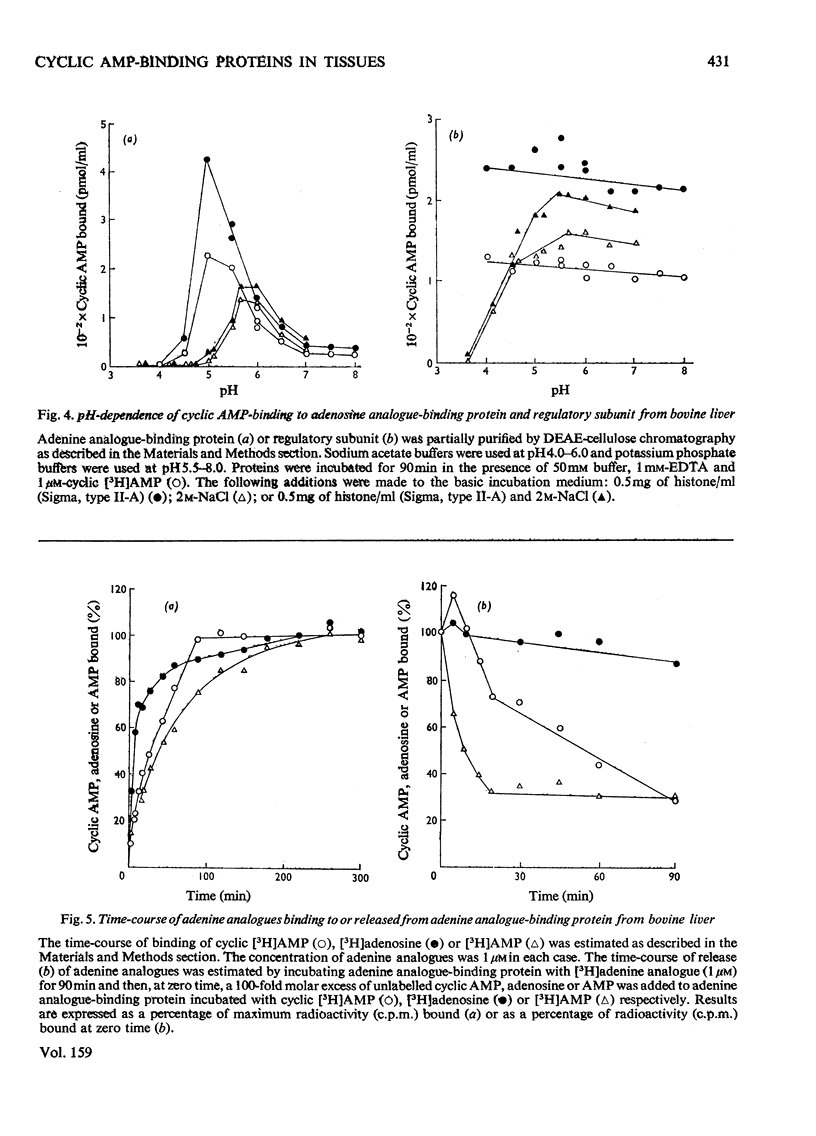
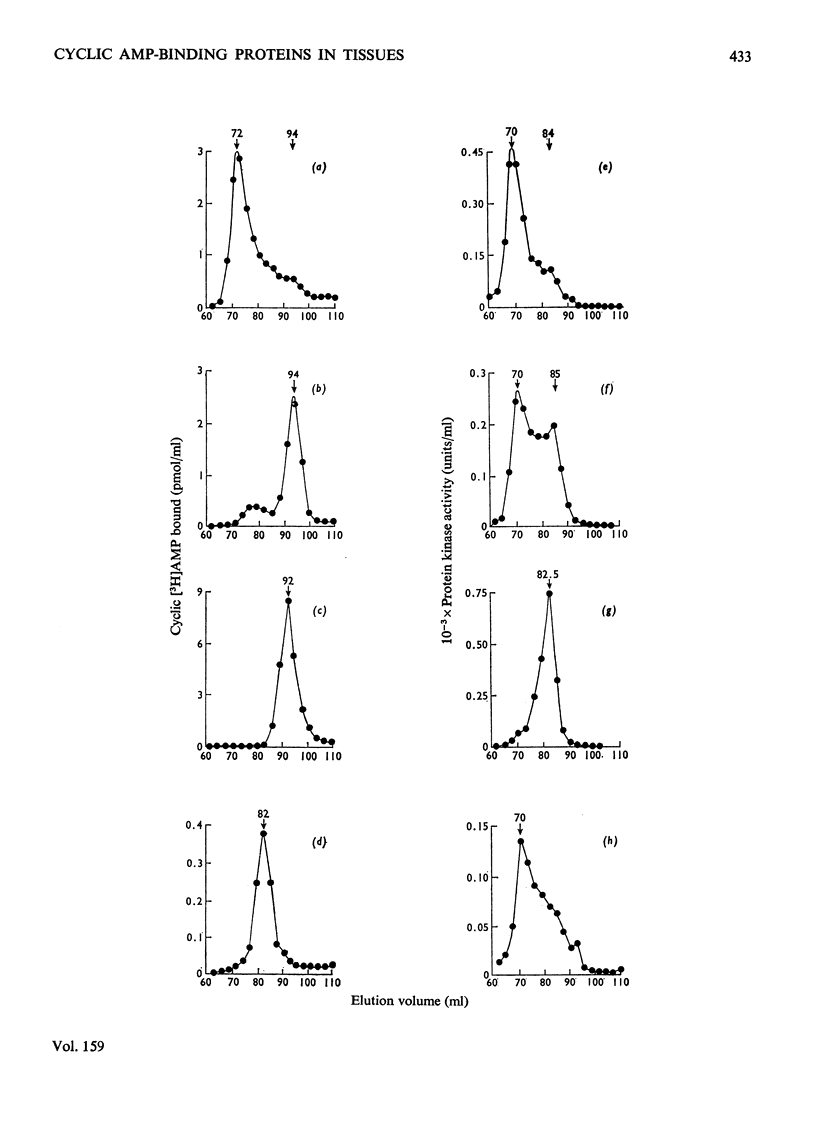
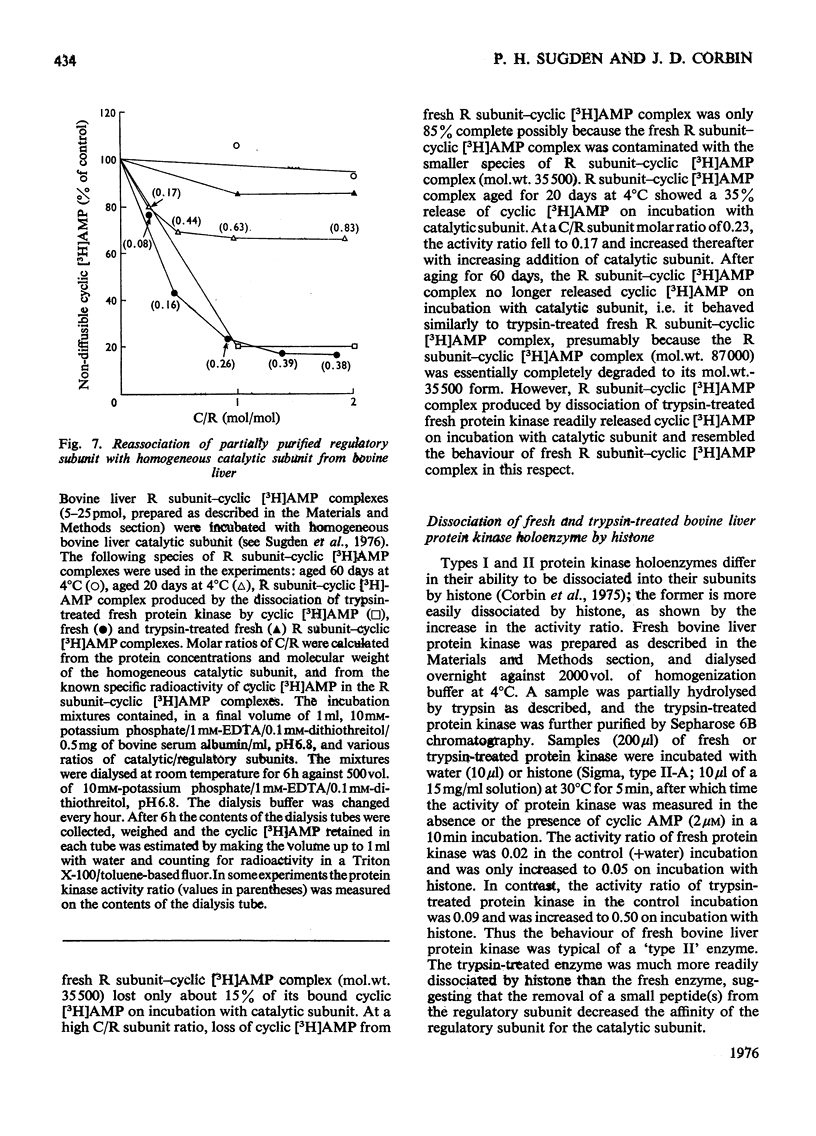
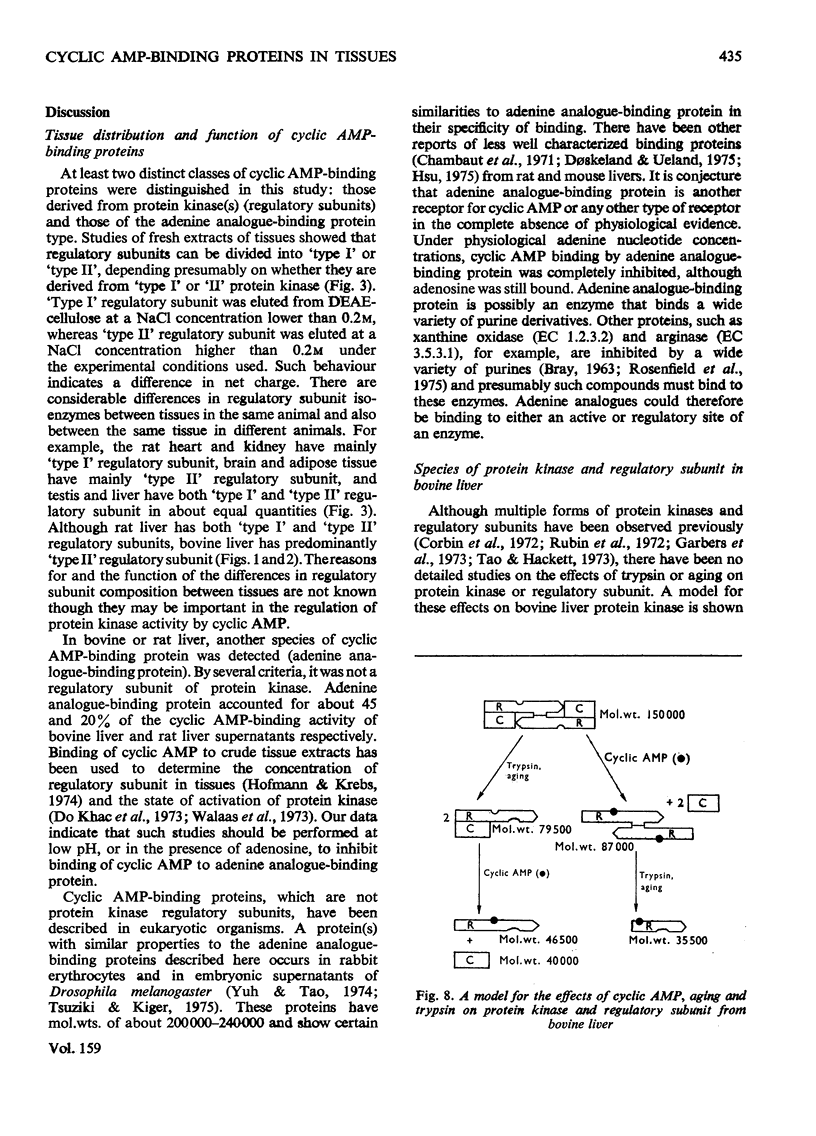
1. At least two classes of high-affinity cyclic AMP-binding proteins have been identified: those derived from cyclic AMP-dependent protein kinases (regulatory subunits) and those that bind a wide range of adenine analogues (adenine analogue-binding proteins). 2. In fresh-tissue extracts, regulatory subunits could be further subdivided into 'type I or 'type II' depending on whether they were derived from 'type I' or 'type II' protein kinase [see Corbin et al. (1975) J. Biol. Chem. 250, 218-225]. 3. The adenine analogue-binding protein was detected in crude tissue supernatant fractions of bovine and rat liver. It differed from the regulatory subunit of cyclic AMP-dependent protein kinase in many of its properties. Under the conditions of assay used, the protein accounted for about 45% of the binding of cyclic AMP to bovine liver supernatants. 4. The adenine analogue-binding protein from bovine liver was partially purified by DEAE-cellulose and Sepharose 6B chromatography. It had mol.wt. 185000 and was trypsin-sensitive. As shown by competition and direct binding experiments, it bound adenosine and AMP in addition to cyclic AMP. At intracellular concentrations of adenine nucleotides, binding of cyclic AMP was essentially completely inhibited in vitro. Adenosine binding was inhibited by only 30% under similar conditions. 5. Rat tissues were examined for the presence of the adenine analogue-binding protein, and, of those examined (adipose tissue, heart, brain, testis, kidney and liver), significant amounts were only found in the liver. The possible physiological role of the adenine analogue-binding protein is discussed. 6. Because the adenine analogue-binding protein or other cyclic AMP-binding proteins in tissues may be products of partial proteolysis of the regulatory subunit of cyclic AMP-dependent protein kinase, the effects of trypsin and aging on partially purified protein kinase and its regulatory subunit from bovine liver were investigated. In all studies, the effects of trypsin and aging were similar. 7. In fresh preparations, the cyclic AMP-dependent protein kinase had mol.wt. 150000. Trypsin treatment converted it into a form of mol.wt 79500. 8. The regulatory subunit of the protein kinase had mol.wt. 87000. It would reassociate with and inhibit the catalytic subunit of the enzyme. Trypsin treatment of the regulatory subunit produced a species of mol.wt. 35500 which bound cyclic AMP but did not reassociate with the catalytic subunit. Trypsin treatment of the protein kinase and dissociation of the product by cyclic AMP produced a regulatory subunit of mol.wt. 46500 which reassociated with the catalytic subunit. 9. These results may be explained by at least two trypsin-sensitive sites on the regulatory subunit. A model for the effects of trypsin is described.
Full text
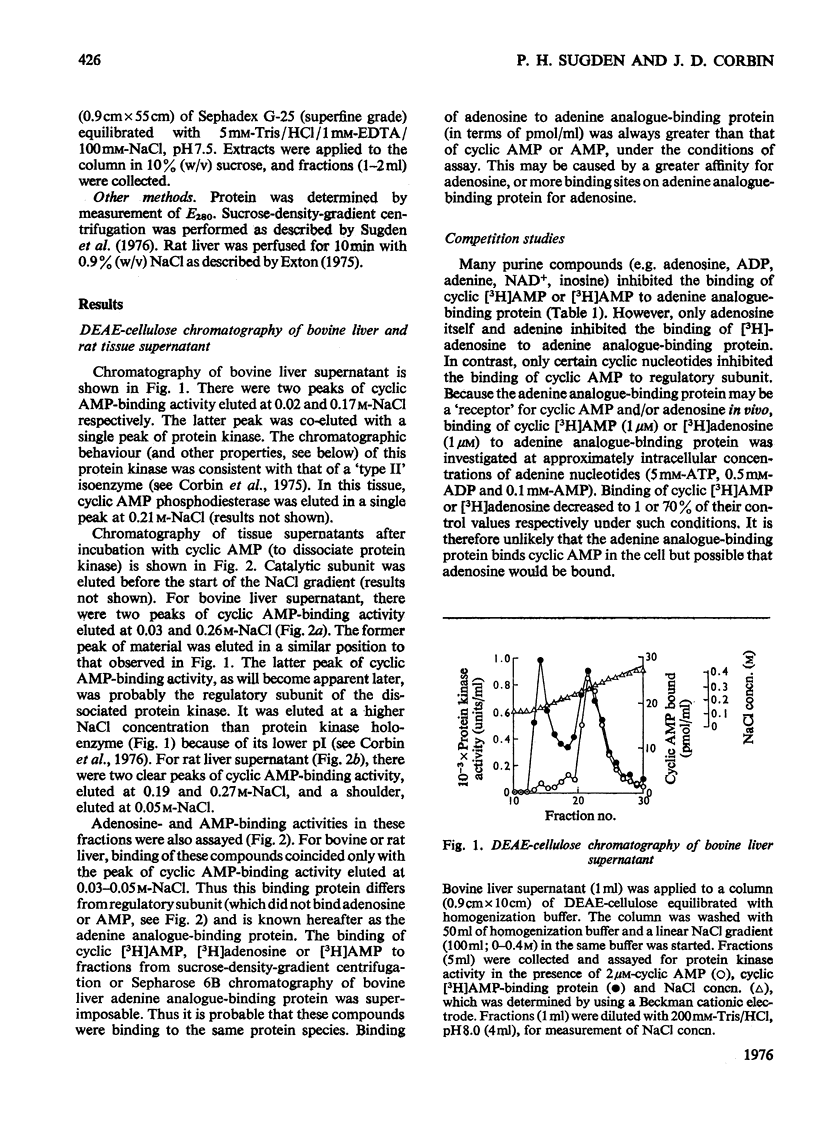
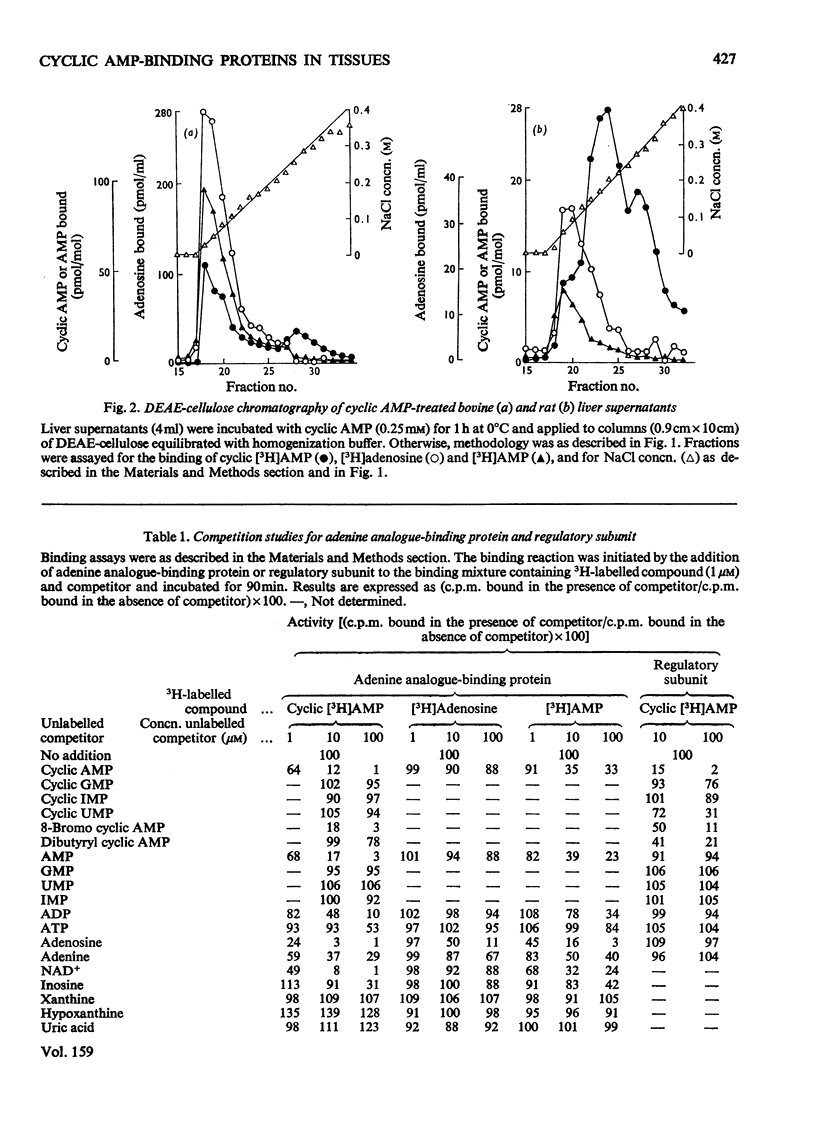
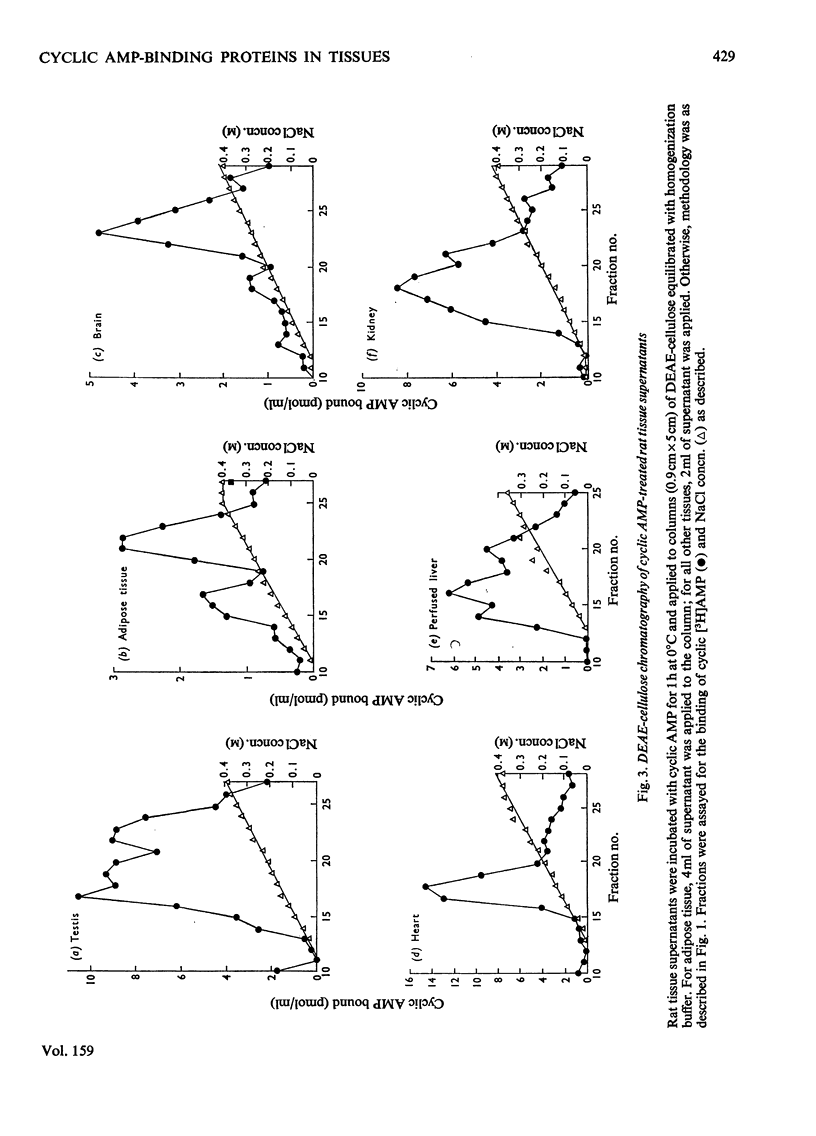
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Hardman J. G., Sutherland E. W. Hydrolysis of cyclic guanosine and adenosine 3',5'-monophosphates by rat and bovine tissues. J Biol Chem. 1970 Nov 10;245(21):5649–5655. [PubMed] [Google Scholar]
- Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Interaction of the subunits of adenosine 3':5'-cyclic monophosphate-dependent protein kinase of muscle. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2444–2447. doi: 10.1073/pnas.68.10.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREETH J. M. The use of the Gouy diffusiometer with dilute protein solutions; an assessment of the accuracy of the method. Biochem J. 1952 Apr;51(1):10–17. doi: 10.1042/bj0510010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F. J., Barker R. Examination of the dissociation of multichain proteins in guanidine hydrochloride by membrane osmometry. Biochemistry. 1968 Jun;7(6):2207–2217. doi: 10.1021/bi00846a025. [DOI] [PubMed] [Google Scholar]
- Chambaut A. M., Leray F., Hanoune J. Relationship between cyclic AMP dependent protein kinase(s) and cyclic AMP binding protein(s) in rat liver. FEBS Lett. 1971 Jul 8;15(5):328–334. doi: 10.1016/0014-5793(71)80327-7. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Brostrom C. O., King C. A., Krebs E. G. Studies on the adenosine 3',5'-monophosphate-dependent protein kinases of rabbit skeletal muscle. J Biol Chem. 1972 Dec 10;247(23):7790–7798. [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- Donovan G., Oliver I. T. Purification and properties of a microsomal cyclic adenosine monophosphate binding protein required for the release of tyrosine aminotransferase from polysomes. Biochemistry. 1972 Oct 10;11(21):3904–3910. doi: 10.1021/bi00771a011. [DOI] [PubMed] [Google Scholar]
- Doskeland S. O., Ueland P. M. A cAMP receptor from mouse liver cytosol whose binding capacity is enhanced by Mg++-ATP. Biochem Biophys Res Commun. 1975 Sep 16;66(2):606–613. doi: 10.1016/0006-291x(75)90553-7. [DOI] [PubMed] [Google Scholar]
- Exton J. H. The perfused rat liver. Methods Enzymol. 1975;39:25–36. doi: 10.1016/s0076-6879(75)39006-x. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., First N. L., Lardy H. A. Properties of adenosine 3',5'-monophosphate-dependent protein kinases isolated from bovine epididymal spermatozoa. J Biol Chem. 1973 Feb 10;248(3):875–879. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES W. L., Jr Protein mercaptides. Cold Spring Harb Symp Quant Biol. 1950;14:79–84. doi: 10.1101/sqb.1950.014.01.011. [DOI] [PubMed] [Google Scholar]
- Hsu H. H., Archibald R. M. Specific adenosine binding proteins from rat liver. Proc Soc Exp Biol Med. 1975 Jul;149(3):698–701. doi: 10.3181/00379727-149-38882. [DOI] [PubMed] [Google Scholar]
- Keely S. L., Corbin J. D., Park C. R. Regulation of adenosine 3:5-monophosphate-dependent protein kinase. J Biol Chem. 1975 Jul 10;250(13):4832–4840. [PubMed] [Google Scholar]
- Khac L. D., Harbon S., Clauser H. J. Intracellular titration of cyclic AMP bound to receptor proteins and correlation with cyclic-AMP levels in the surviving rat diaphragm. Eur J Biochem. 1973 Dec 3;40(1):177–185. doi: 10.1111/j.1432-1033.1973.tb03183.x. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- RACKIS J. J., SASAME H. A., MANN R. K., ANDERSON R. L., SMITH A. K. Soybean trypsin inhibitors: isolation, purification and physical properties. Arch Biochem Biophys. 1962 Sep;98:471–478. doi: 10.1016/0003-9861(62)90213-8. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J. L., Dutta S. P., Chheda G. B., Tritsch G. L. Purine and pyrimidine inhibitors of arginase. Biochim Biophys Acta. 1975 Nov 20;410(1):164–166. doi: 10.1016/0005-2744(75)90217-x. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Molecular forms and subunit composition of a cyclic adenosine 3',5'-monophosphate-dependent protein kinase purified from bovine heart muscle. J Biol Chem. 1972 Jan 10;247(1):36–44. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Soderling T. R., Corbin J. D., Park C. R. Regulation of adenosine 3',5'-monophosphate-dependent protein kinase. II. Hormonal regulation of the adipose tissue enzyme. J Biol Chem. 1973 Mar 10;248(5):1822–1829. [PubMed] [Google Scholar]
- Sugden P. H., Holladay L. A., Reimann E. M., Corbin J. D. Purification and characterization of the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J. 1976 Nov;159(2):409–422. doi: 10.1042/bj1590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy J., Richter D. Separation of a cyclic 3',5'-adenosine monophosphate binding protein from yeast. Biochemistry. 1972 Jul 18;11(15):2784–2787. doi: 10.1021/bi00765a008. [DOI] [PubMed] [Google Scholar]
- Tao M., Hackett P. Adenosine cyclic 3':5'-monophosphate-dependent protein kinase from rabbit erythrocytes. Purification and characterization of multiple forms. J Biol Chem. 1973 Aug 10;248(15):5324–5332. [PubMed] [Google Scholar]
- Tsuzuki J., Kiger J. A., Jr Cyclic AMP binding proteins in early embryos of Drosophila melanogaster. Biochim Biophys Acta. 1975 May 30;393(1):225–235. doi: 10.1016/0005-2795(75)90235-4. [DOI] [PubMed] [Google Scholar]
- Walaas O., Walaas E., Gronnerod O. Hormonal regulation of cyclic-AMP-dependent protein kinase of rat diaphragm by epinephrine and insulin. Eur J Biochem. 1973 Dec 17;40(2):465–477. doi: 10.1111/j.1432-1033.1973.tb03215.x. [DOI] [PubMed] [Google Scholar]
- Yuh K. C., Tao M. Purification and characterization of adenosine-adenosine cyclic 3',5'-monophosphate binding protein factors from rabbit erythrocytes. Biochemistry. 1974 Dec 3;13(25):5220–5226. doi: 10.1021/bi00722a027. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]


