Abstract
Purpose:
Incomplete polymerisation processes produce several leachable substances. The aim of this work was to review, through existing research and published literature, the genotoxic effect of residual monomers of polymers used in restorative dentistry.
Materials and Methods:
The selection of published studies was performed on six databases from January 2000 to June 2020. The keywords used were: ‘genotoxicity’ or ‘DNA damage’ and ‘dental resin’ or ‘methacrylates’ or ‘residual monomers’. The selection was carried out according to the parameters and guidelines of the Preferred Reporting Items for Systematic Review and Metanalyses (PRISMA) and was based on patient, intervention, comparison, and outcome (PICO). The inclusion criteria were: in vitro and in vivo studies published in English that evaluated genotoxicity for residual monomers leached from polymers related to restorative dentistry. Case reports and review articles were excluded.
Results:
Twenty-seven studies met the eligibility criteria. Two categories were constructed based on the experimental design, in vivo and in vitro reports. For the in vitro research, two main methods of assessing DNA damage were reported in selected studies: micronucleus (MN) counting and alkaline comet assay. For in vivo reports, the main method for assessing genotoxic damage was MN counting.
Conclusion:
From the electronic search, structured data extraction, and analysis by different independent reviewers, results from the present systematic review allow us to conclude that DNA damage is induced by monomers/co-monomers (triethylene glycol dimethacrylate, bisphenol-A-glycidyl methacrylate, urethane dimethacrylate, and 2-hydroxyethyl methacrylate) that are used in restorative dentistry. This systematic review highlights the need for more research on the use of monomers/co-monomers to properly assess clinical biocompatibility.
Key words: dental resin, genotoxicity, methacrylates, residual monomers
Dentistry uses various polymer materials based on methacrylates. The matrix of these dental materials contains highly viscous major monomers such as bisphenol-A-glycidyl methacrylate (bis-GMA) or urethane dimethacrylate (UDMA), as well as dilutive monomers such as 2-hydroxyethyl methacrylate (HEMA) or the comonomer triethylene glycol dimethacrylate (TEG-DMA).9 The curing of restorative materials and adhesives is initiated chemically by mixing two components or by light. In both cases, polymerisation is incomplete, so varying amounts of free and unreacted monomers remain in the polymerised resin.4,12,20 The initial release of free monomers may occur during monomer–polymer conversion, and the long-term release of leachable substances is caused by erosion and degradation over time. Degradation of composites and polymers in the oral environment is caused by thermal changes, the components of saliva, chewing forces, chemical dietary changes, and oral microorganisms.14,22,38
Furthermore, monomers/co-monomers have the potential to increase the levels of reactive oxygen species (ROS). ROS are known mediators of signaling cascades, but elevated levels of ROS can disrupt the cellular redox balance, resulting in oxidative DNA damage and apoptosis in mammalian cells. Along this line, the ROS attack on DNA might induce adverse toxic effects such as mutagenicity and genotoxicity in the affected cells and organisms.13,20,37 A genotoxic agent is one that induces point mutations, deletions, insertions, gene amplification, chromosomal rearrangements, or numerical chromosomal changes. In the context of short-term tests for mutagenicity and genotoxicity, tests are designed to detect one or more types of genetic alterations. Since such biological properties result directly or indirectly from DNA damage, no single assay, no matter how extensive the protocol, can detect all genotoxic chemicals. Therefore, it is generally accepted that several tests must be conducted to evaluate whether a chemical is genotoxic or not, and often the weight-of-evidence approach must be taken to evaluate the results.33
Genomic damage is probably the most important fundamental cause of developmental and degenerative diseases. It is also well established that genomic damage is produced by environmental exposure to genotoxins, medical procedures (e.g. radiation and chemicals), micronutrient deficiency (e.g. folate), lifestyle factors (e.g. alcohol, smoking, drugs, and stress), and genetic factors such as inherited defects in DNA metabolism and/or repair.7,16,43 Several studies have investigated and identified the cytotoxicity and genotoxicity of some of these methacrylates during the last two decades.17,19,32,34,35 Resin monomers such as TEG-DMA or HEMA induced cytotoxicity via apoptosis in various cell types, including pulp and gingiva cells; genotoxic or mutagenic effects caused by monomers were reported as well.18 However, some studies report non-significant DNA damage and nuclear changes with the use of more dilute concentrations, which would resemble clinical conditions.8,42
This review evaluated, through existing research and publish literature, the genotoxic effect of residual monomers of polymers used in restorative dentistry.
Materials and Methods
This study was carried out according to the parameters and guidelines of the Preferred Reporting Items for Systematic Review and Metanalyses (PRISMA).27
Search Strategy
The search strategy was based on patient, intervention, comparison, and outcome (PICO).15 A structured PICO question was developed, where P stood for human or animal cells and tissues, I for the exposition to residual monomers contained in polymers used in dentistry, C for unexposed cells or tissues, and O for DNA damage or chromosomal aberrations. The full question read as follows: ‘Do any residual monomers contained in polymers used in dentistry generate DNA damage or chromosomal aberrations?’
Databases and Data Collection Process
An electronic search was carried out in the databases MEDLINE, EBSCO, SCiELO, BVS LILACS, COCHRANE, and ScienceDirect, from January 1, 2000 to June 30, 2020, using the terms and their combinations: ‘Micronucleus’ OR ‘Genotoxicity’ OR ‘DNA damage’ AND ‘Dental Resin’ OR ‘Poly-Methyl Methacrylate’ OR ‘Residual Monomers’. The search was carried out in the English language using the MeSH terms described, with the help of Boolean operators (OR, AND) to combine the searches (Table 1).
Table 1.
Databases and data collection process
| Databases | Records Identified |
|---|---|
| MEDLINE | 366 |
| EBSCO | 115 |
| SCiELO | 1 |
| COCHRANE | 1 |
| BVS LILACS | 14 |
| SCIENCE DIRECT | 285 |
| Total | 782 |
Inclusion and Exclusion Criteria
The search was carried out in the databases of the last 20 years of publications, including in vitro studies in human or animal cells, clinical trials in humans or animals that measured or evaluated the genotoxic potential of some of the residual monomers and restorative materials used in dentistry. The exclusion criteria were case reports, review articles, studies, or trials without a measurement of genetic damage or chromosomal alterations, polymers or monomers used outside the dental area, and studies or trials without control groups.
Selection of Studies
The titles and summaries of the reports identified in the searches were read independently by two authors. For the studies that met the inclusion criteria or whose title and abstract information were not sufficient, a complete reading of the study was performed for decision making. Disagreements were discussed and resolved between the two authors. In case of not reaching an agreement, the analysis of a third reviewer was considered. Finally, the approved articles were contemplated for inclusion in this study. Aspects such as cell lineage, type of monomer or co-monomer, and genotoxicity assay were treated in a manner analogous to the PICO components of a trial.15
Quality Evaluation
This study was limited by the absence in the published literature of randomised clinical trials evaluating the genotoxicity of residual monomers used in restorative interventions, the lack of specific validated guidelines for systematic reviews of nonclinical studies, and the lack of external validity among in vitro studies.39 To overcome these limitations and promote quality and transparency of evidence among the in vitro studies, the Checklist for Reporting In Vitro Study Guidelines was used to evaluate each study according to the article’s description of the following five parameters for study quality assessment: description of sample size calculation (sample size calculation was one of the steps in methodology), description of the sample preparation and handling (detailed explanation of sample preparation and handling, and information on sample loss), randomisation and blinding (two or more independent observers or researchers, allocating samples, and maintaining a certain degree of blinding of samples), statistical analysis (use of the appropriate statistical method for analysing data), and meaningful differences between groups (measure that would make a difference clinically or scientifically).22 If the author reported the parameter the article received a ‘Y’ (yes); if it was not possible to find the information, the article received an ‘N’ (no). Articles that reported one or two parameters were classified as having a high risk of bias, three to four items as medium risk of bias, and five items as low risk of bias.
Results
A total of 782 publications were located, and 371 duplicate articles were identified. Of the remaining 411, titles and abstracts were analysed regarding the objective of the study and its relevance to dentistry. Three hundred fifty-nine were eliminated, and 52 potentially relevant articles were selected and downloaded for complete reading and analysis by the two authors. Twenty-seven studies were selected for analysis of this systematic review, according to the inclusion criteria (Fig 1). Results are shown in Table 2 for in vitro studies and in Table 3 for in vivo studies, to facilitate the distribution and visualisation of the data.
Fig 1.
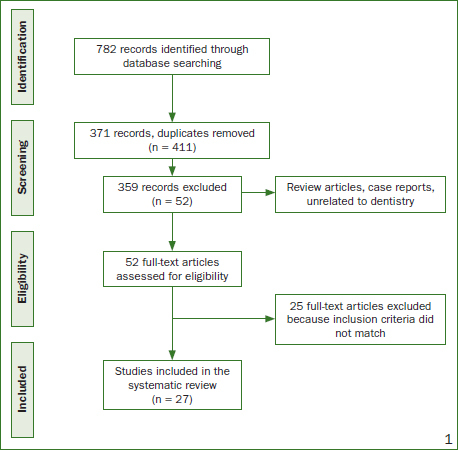
Study flowchart.
Table 2.
In vitro reports included in this review showing genotoxic effects of monomers used in restorative dentistry
| Assay | Groups | Results | Reference |
|---|---|---|---|
| Micronucleus test in hamster lung fibroblasts | HEMA at 0 mM at 4000 mM Bis-GMA at 0 mM at 75 mM UDMA at 0 mM at 75 mM TEG-DMA At 0 mM At 750 mM MMA At 0 mM At 2 x 104 mM |
4.3 MN/1000 cells 22.7 MN/1000 cells 8.3 MN/1000 cells 15.3 MN/1000 cells 12.7 MN/1000 cells 21 MN/1000 cells 9.3 MN/1000 cells 78.3 MN/1000 cells 7.3 MN/1000 cells 9.7 MN/1000 cells |
Schweilk, 2001 Germany |
| DNA synthesis inhibition assay and sister-chromatic exchange (SCE) in hamster ovary cells | MMA: 9.33x10-1 mg/ml 9.33x10-2 mg/ml 9.33x10-3 mg/ml 9.33x10-4 mg/ml Negative control: untreated cells |
90% inhibition – 6.70 SCE/cell* 80% inhibition – 8.33 SCE/cell* 63% inhibition* – 7.63 SCE/cell* 55% inhibition* – 8.25 SCE/cell* 100% inhibition – 4.23 SCE/cell |
Yang, 2003 Taiwan |
| Comet Assay in human peripheral lymphocytes | HEMA at 2.5x10-2 M Bis-GMA at 2.5x10-2 M UDMA at 2.5x10-2 M TEG-DMA at 2.5x10-2 M Negative control DMSO |
OTM 3.3 * OTM 7.4 * OTM 2.82 * OTM 4.5 * OTM 1.0-1.2 |
Kleinsasser, 2004 Germany |
| Comet Assay in human lymphocytes and parotid gland tissue | HEMA at 2.5 x 10-2 M TEG-DMA at 2.5 x 10-2 M UDMA at 2.5 x 10-2 M Negative Control DMSO |
Parotid: OTM 9.7 * Lymphocytes: OTM 6.1 * Parotid: OTM 10.7 * Lymphocytes: OTM 8.8 * Parotid: OTM 10.5 * Lymphocytes: OTM 6.4 * OTM 2.2 |
Kleinsasser, 2006 Germany |
| Micronucleus test in hamster fibroblasts | Clearfil SE Bond (Kuraray) Clearfil Protect Bond (Kuraray) AdheSE (Ivoclar Vivadent) Prompt L-Pop (3M Oral Care) Excite (Ivoclar Vivadent) Negative control: ethanol |
>10 MN/ 1000 cells * ≤10 MN/1000 cells >10 MN/1000 cells * >10 MN/1000 cells * >10 MN/1000 cells 10 MN/1000 cells |
Demirci, 2008 Germany |
| Sister-chromatic exchange (SCE) in human lymphocytes | Hibrid composites: Tetric Ceram (Ivoclar Vivadent) Filtek 250 (3M Oral Care) Nanohibrid composite: Simile (Pentron) Laboratory composite: Adoro (Ivoclar Vivadent) Negative control DMSO |
19.79 SCE/cell * 15.10 SCE/cell * 13.15 SCE/cell 11.45 SCE/cell 10.79 SCE/cell |
Bakopoulou, 2008 Greece |
| Fluorimetric Detection of Alkaline DNA Unwinding (FADU) in human gingival fibroblasts | HEMA at 2.5 mM Bis-GMA at 0.25 mM MMA at 2.5 mM TEG-DMA at 2.5 mM Negative control: untreated cells |
>60% DNA integrity 25% DNA integrity * >60% DNA integrity >60% DNA integrity 68% DNA integrity |
Durner, 2010 Germany |
| Comet Assay in human peripheral lymphocytes | HEMA at 2.5 mM HEMA at 5 mM HEMA at 10 mM Negative control: unexposed cells |
>60% tail DNA (DNA damage) >80% tail DNA (DNA damage) * 100% tail DNA (DNA damage) * <60% tail DNA (DNA damage) |
Pawlowska, 2010 Poland |
| Comet Assay in human peripheral lymphocytes | UDMA at 0.1 mM UDMA at 0.25 mM UDMA at 0.5 mM UDMA at 0.75 mM UDMA at 1 mM Negative control: unexposed cells |
<10% tail DNA (DNA damage) * >10% tail DNA (DNA damage) * >10% tail DNA (DNA damage) * 20% tail DNA (DNA damage) * >30% tail DNA (DNA damage) * <5% tail DNA (DNA damage) |
Poplawski, 2010 Poland |
| H2AX- Inmunofluorescence in human gingival fibroblasts | HEMA at 11.2 mM Bis-GMA at 0.09 mM UDMA at 0.1 mM TEG-DMA at 3.6 mM Negative control DMSO |
>2 foci/cell * 5 foci/cell * 3 foci/cell * >2 foci/cell * <1 foci/cell |
Ucran, 2010 Germany |
| Comet Assay in human gingival fibroblasts | Monomer mixture bis-GMA/TEG-DMA (55/45) At 0.05 mM At 0.10 mM At 0.20 mM Negative control: unexposed cell |
>20% tail DNA (DNA damage) * 30% tail DNA (DNA damage) * <40% tail DNA (DNA damage) * <5% tail DNA (DNA damage |
Blasiak, 2012 Poland |
| Comet Assay in human leukocyte cells | Elutes of freshly cured: Tetric Evoceram (Ivoclar Vivadent) Tetric Evoflow (Ivoclar Vivadent) Gradia Direct Posterior (GC) Gradia Direct flow (GC) Filtek z250 (3M Oral Care Filtek Supreme XT flow (3M Oral Care) Negative control: Saline solution |
1.5% tail DNA (DNA damage) * >1.8% tail DNA (DNA damage) * 1.8% tail DNA (DNA damage) * 1.5% tail DNA (DNA damage) * >0.8% tail DNA (DNA damage) 0.6% tail DNA (DNA damage) 0.8 tail DNA (DNA damage) |
Tadin, 2013 Croatia |
| H2AX- Inmunofluorescence in human gingival fibroblasts | Bis-GMA at 90 µM Bis-GMA at 30 µM Bis-GMA negative control UDMA at 100 µM UDMA at 33.5 µM UDMA negative control GMA at 2500 µM GMA negative control |
4.05 foci/cell * 2.12 foci/cell * 1.39 foci/cell 2.5 foci/cell * 2.21 foci/cell * 1.39 foci/cell 2.57 foci/cell * 1.39 foci/cell |
Lottner, 2013 Germany |
| Comet Assay in human gingival and pulp fibroblasts | Versatile flow, Kerr (self-adhering flowable composite) Kalore, GC (nano-hybrid resin composite) Negative control: medium only |
(Gingival) 2.5 tail intensity (% DNA) * (Pulp) 1.6 tail intensity (% DNA) * (Gingival) 1.2 tail intensity (% DNA) (Pulp) 4.0 tail intensity (% DNA) * (Gingival) 1.1 tail intensity (% DNA) (Pulp) 0.4 – 0.6 tail intensity (% DNA) |
Tadin, 2014 Croatia |
| Comet Assay and micronucleus test in human lymphocytes | HEMA at 10 µM HEMA at 100 µM HEMA at 1 mM TEG-DMA at 1 µM TEG-DMA at 10 µM TEG-DMA at 100 µM Negative control: DMSO |
OTM 0.4 and 1.3 M/N 1000 cells OTM 0.5 and 1.4 M/N 1000 cells OTM 1.5 * and 2.5 M/N 1000 cells OTM 0.4 and 1.4 M/N 1000 cells OTM 0.5 and 1.5 M/N 1000 cells OTM 1.2 * and 1.4 M/N 1000 cells OTM 0.5 and 1.1-1.2 M/N 1000 cells |
Ginzkey, 2015 Germany |
| H2AX/53BP1 focus assay in human gingival fibroblasts | Bis-GMA: 0.012 mM 0.03 mM 0.1 mM 0.3 mM GMA: 0.0036 mM 0.009 mM 0.03 mM 0.09 mM Negative control DMSO |
.7 av. Foci/cell * 2.2 av. Foci/cell * 2.8 av. Foci/cell * 3.8 av. Foci/cell * 0.7 av. Foci/cell * 0.9 av. Foci/cell * 1.9 av. Foci/cell * 2.7 av. Foci/cell * 0.4 av. Foci/cell |
Styllou, 2015 Germany |
| Comet Assay and micronucleus test in human lymphocytes | Bulk-fill composite SDR (Dentsply) Bulk-fill composite Venus (Heraeus Kulzer) Bulk-fill composite X-tra Base (Voco) Conventional composite Tetric Evoflow (Ivoclar) Negative control: untreated cells |
2.6 (% of DNA in tail) 7 MN/1000 cells 2.8 (% of DNA in tail) 7 MN/1000 cells 2.9 (% of DNA in tail) 7 MN/1000 cells 1.8 (% of DNA in tail) 10 MN/1000 cells 1.4 (% of DNA in tail) 6.5 MN/1000 cells |
Taubock, 2016 Switzerland |
| H2AX- Inmunofluorescence in human gingival fibroblasts | Micro-hybrid composite Esthet.X (Dentsply) Micro-hybrid composite Venus (Heraeus Kulzer) Mulit-hybrid composite X-tra Fil (Voco) Micro-hybrid composite Clearfil AP-X(Kuraray) Nano-hybrid ormocer Admira (Voco) Micro-hybrid QuiXfil (Dentsply) Negative control: médium |
0.43 foci/cell * 0.39 foci/cell * 0.26 foci/cell 0.28 foci/cell 0.20 foci/cell 0.23 foci/cell 0.22 foci/cell |
Yang, 2018 Germany |
| Comet Assay in mouse fibroblasts | Self-adhesive resin cement G-Cem (GC). UDMA and TEG-DMA Self-adhesive resin cement Speed-Cem (Ivoclar). UDMA and TEG-DMA Self-adhesive resin cement Relyx U200 (3M Oral Care) UDMA and TEG-DMA Negative control DMSO |
250 tail intensity (% control) 200 tail intensity (% control) * 340 tail intensity (% control) * 100 tail intensity (% control) |
Kurt, 2018 Turkey |
| Micronucleus assay in human lymphocytes | Eluate of Tetric EvoCeram (Ivoclar) At 0.007 g/ml after 4 h At 0.013 g/ml after 4 h At 0.007 g/ml after 24 h At 0.013 g/ml after 24 h Eluate of Tetric EvoFlow (Ivoclar) At 0.007 g/ml after 4 h At 0.013 g/ml after 4 h At 0.007 g/ml after 24 h At 0.013 g/ml after 24 h Eluate of Filtek Ultimate (3M Oral Care) At 0.007 g/ml after 4 h At 0.013 g/ml after 4 h At 0.007 g/ml after 24 h At 0.013 g/ml after 24 h Eluate of Filtek Ulimtate Flow (3M Oral Care) At 0.007 g/ml after 4 h At 0.013 g/ml after 4 h At 0.007 g/ml after 24 h At 0.013 g/ml after 24 h Eluate of G-aenial (GC) At 0.007 g/ml after 4 h At 0.013 g/ml after 4 h At 0.007 g/ml after 24 h At 0.013 g/ml after 24 h Eluate of G-aenial Flo (GC) At 0.007 g/ml after 4 h At 0.013 g/ml after 4 h At 0.007 g/ml after 24 h At 0.013 g/ml after 24 h Negative control: exposed only to medium |
7 MN/1000 cells 10 MN/1000 cells 5 MN/1000 cells 8 MN/1000 cells 5 MN/1000 cells 6 MN/1000 cells 9 MN/1000 cells 4 MN/1000 cells 3 MN/1000 cells 2 MN/1000 cells 2 MN/1000 cells 4 MN/1000 cells 1 MN/1000 cells 2 MN/1000 cells 5 MN/1000 cells 5 MN/1000 cells 3 MN/1000 cells 4 MN/1000 cells 4 MN/1000 cells 4 MN/1000 cells 2 MN/1000 cells 4 MN/1000 cells 4 MN/1000 cells 3 MN/1000 cells 2 MN/1000 cells |
Brzovic, 2018 Croatia |
*Statistically significant compared to control (p<0.05).
Table 3.
In vivo reports included in this review showing genotoxic effects of monomers used in restorative dentistry
| Assay | Groups | Results | Reference |
|---|---|---|---|
| Comet Assay in human lymphocytes | 40 subjects carrying dental fillings (20 males, 24 females) Control group: 24 individuals (13 males, 11 females) |
OTM 65. 8 * 42.1 tail intensity (% tail DNA) * OTM 35.4 28.5 tail intensity (% tail DNA) |
Dipietro, 2008 Italy |
| Micronucleus test in Wistar rat erythrocytes | 16 rats exposed to MMA vapor for 8 h 8 rats receiving cyclophosphamide (positive control) 8 rats receiving water and food ad libitum (negative control) |
7 MN/1000 cells after 24 h * 2 MN/1000 cells after 5 days. 9 MN/1000 cells * 0.75 MN/1000 cells |
Araujo, 2012 Brazil |
| Micronucleus assay in human lymphocytes | 54 male dental technicians Control group: 38 male clerical workers, not exposed to metal alloys or other chemicals during work or leisure time |
8.5 MN/1000 cells * 4.1 MN/1000 cells |
Ishikawa, 2012 Japan |
| Micronuleus test and Comet Assay in human exfoliative cells from oral mucosa | 43 subjects with restorative fillings (males and females, age interval of 18-28) Control group: 20 subjects with no restorative fillings (males and females, age interval of 18-28) |
0.25% MN/1000 cells * >80% of DNA in the tail (TDNA) * 0.12% MN/1000 cells 60% of DNA in the tail (TDNA) |
Visalli, 2012 Italy |
| Micronucleus test in human bucal mucosal cells | 13 dental technicians working in a prosthetic production laboratory for at least 1 year Control group. 14 students and doctors |
5.21 MN/1000 cells 6.23 MN/1000 cells |
Azhar, 2013 Kindom of Saudi Arabia |
| Micronucleus test and Comet assay in human gingival epithelial cells | 15 patients (38-59 years of age) receiving restorations with nanohybrid composite Kalore (GC) 15 patients (38-59 years of age) receiving restorations with self-adhering composite Vertise Flow (Kerr) Negative control: immediately before restoration |
7 days: 4% tail DNA* 7 MN/1000 cells * 30 days: 5% tail DNA* 6.5 MN/1000 cells * 180 days: 5% tail DNA* 4.5 MN/1000 cells 7 days: 4% tail DNA* 6 MN/1000 cells * 30 days: 5% tail DNA* 6 MN/1000 cells * 180 days: 5% tail DNA* 4 MN/1000 cells 0 days: 2% tail DNA 4 MN/1000 cells |
Tadin, 2013 Croatia |
| Micronucleus assay in human exfoliated epithelial cells from oral mucosa | 26 Dental surgeons Control group: 26 individuals not related to the profession 19 dental technicians Control group: 19 individuals not related to the profession |
1.6 MN/1000 cells * 0.6 MN/1000 cells 1.7 MN/1000 cells * 0.7 MN/1000 cells |
Molina, 2019 Mexico |
* Statistically significant compared to control (p<0.05).
Assessment of Risk of Bias
Of the 27 studies included in this work, six studies presented a high risk of bias and 21 studies showed a medium risk of bias. None of the studies possessed all five parameters to be considered as having a low risk of bias. The results are described in Tables 4 and 5 according to the parameters considered in the analyses.
Table 4.
Quality of evidence and assessment of risk of bias
| Sample size calculation | Sample preparation and handling | Randomisation and blinding | Statistical analysis | Meaningful differences between groups | Risk of bias | |
|---|---|---|---|---|---|---|
| Schweilk, 2001 | N | Y | N | N | Y | High |
| Yang, 2003 | N | Y | N | Y | Y | Medium |
| Kleinsasser, 2004 | N | Y | N | Y | Y | Medium |
| Kleinsasser, 2006 | N | Y | N | Y | Y | Medium |
| Demirci, 2008 | N | Y | Y | Y | Y | Medium |
| Bakopoulou, 2008 | N | Y | Y | Y | Y | Medium |
| Durner, 2010 | N | Y | N | Y | N | High |
| Pawloska, 2010 | N | Y | N | Y | Y | Medium |
| Poplawski, 2010 | N | Y | N | Y | Y | Medium |
| Ucran, 2010 | N | Y | N | Y | Y | Medium |
| Blasiak, 2012 | N | Y | N | Y | Y | Medium |
| Tadin, 2013 | N | Y | N | Y | Y | Medium |
| Lottner, 2013 | N | Y | N | Y | Y | Medium |
| Tadin, 2014 | N | Y | N | Y | Y | Medium |
| Ginzkey, 2015 | N | Y | N | Y | N | High |
| Styllou, 2015 | N | Y | N | Y | Y | Medium |
| Taubock, 2016 | N | Y | N | Y | N | High |
| Yang, 2018 | N | Y | N | Y | Y | Medium |
| Kurt, 2018 | N | Y | N | Y | Y | Medium |
| Brzovic, 2018 | N | Y | N | Y | N | High |
| Dipietro, 2008 | N | Y | Y | Y | Y | Medium |
| Araujo, 2012 | N | Y | Y | Y | Y | Medium |
| Ishikawa, 2012 | N | Y | N | Y | Y | Medium |
| Visalli, 2012 | N | Y | Y | Y | Y | Medium |
| Azhar, 2013 | N | Y | N | Y | N | High |
| Tadin, 2013 | N | Y | Y | Y | Y | Medium |
| Molina, 2019 | N | Y | N | Y | Y | Medium |
Table 5.
Risk of bias results of studies included in this review
| Risk of bias | Number of reports |
|---|---|
| Low risk of bias | 0 |
| Medium risk of bias | 21 |
| High risk of bias | 6 |
| Total number of reports | 27 |
Discussion
From the initial electronic research, 27 studies fulfilled all the required criteria for eligibility. Using these, two categories were constructed based on the experimental design, in vivo and in vitro reports. As shown in Table 2 for the in vitro research, two main methods of assessing DNA damage were reported: micronucleus (MN) counting and alkaline comet assay.4,5,6,8,10,12,14,18,19,22,23,32,34,36,38,40,41,42,44,47,48 As shown in Table 3 for in vivo reports, the main method for assessing genotoxic damage was MN counting.1,3,11,17,28,39,46
The MN assay is a mutagenic test system that is frequently used in in vitro and in vivo toxicological screening for detecting potential genotoxic compounds that lead to the induction of small DNA fragments (micronuclei) in the cytoplasm of the dividing cells. Micronuclei can be observed as chromosome fragments produced by DNA strand breakage, or as whole chromosomes that have been formed during the anaphase of mitosis or meiosis when they were not able to migrate with the rest of the chromosomes.6 The Comet assay is a microgel technique involving electrophoresis under alkaline (pH >13) conditions for detecting DNA damage in single cells. At this pH, increased DNA migration is associated with raised levels of frank single-strand breaks (SSBs). Because genotoxic agents induce different magnitudes of SSB, this assay provides great sensitivity for identifying genotoxic agents.10 Irrespective of the method, however, the analysis of DNA damage performed in most of the studies included in this systematic review (23 out of 27) (Tables 2 and 3) revealed alterations in DNA stability by MN assay, comet assay, sister-chromatic exchange, and immunofluorescence.
In the in vitro studies, the cells most commonly used to show a genotoxic effect with exposure to monomers were peripheral lymphocytes (9 of 20) and gingival fibroblasts (7 of 20) (Table 2). The collection of buccal cells is arguably the least invasive method available for measuring DNA damage in humans, especially in comparison to obtaining blood samples for lymphocyte and erythrocyte assays, or tissue biopsies.43 Because the clinical use of these dental materials involves direct contact with oral tissues, the information collected from in vitro studies using gingival cells can be considered more accurate and less invasive in terms of analysing a local genotoxic effect. Only one of these studies performed on gingival cells did not show an association between the use of composites and genotoxicity measured with the MN assay.8
Several reviews are available on the application of the MN assay in exfoliated cells,16,24,35 showing the usefulness of the MN test applied in buccal cells to assess the genotoxic impact of environmental and occupational exposure, lifestyle, and malnutrition in intervention studies. Moreover, the strong correlation of MN frequency in exfoliated buccal cells with MN frequency in lymphocytes implies that systemic genotoxic effects within the bloodstream may also impact on and be detectable in buccal cells. One of the main limitations of the assay, which needs to be addressed in the context of practical application, is the large variability in MN frequency observed across laboratories, patients, and control groups. Nevertheless, our review has some limitations, such as heterogeneity in the study design, with different schemes of subject recruitment, and the use of different experimental protocols.6
Through electronic research, it is notable that the most common monomers/co-monomers associated with increased levels of reactive oxygen species (ROS) and DNA damage (genotoxic effect) are bis-GMA, HEMA, TEG-DMA, and UDMA (Tables 2 and 3), which are used as bonding resins and direct restoration materials, and are present in some cements, dentin adhesives, and sealing agents, as well as in bonding of orthodontic brackets. Several studies have shown that these monomers and other components were released from restorative materials into the oral environment either from incomplete polymerisation or because of resin degradation. This degradation can occur through a variety of physical and chemical mechanisms, such as dissolution and disintegration in saliva, mechanical wear through chewing forces, bacterial activity and erosion by food.2,11,25,26,49
Nevertheless, results regarding an association with a genotoxic effect from in vitro studies should be viewed with caution, due to an absence of correlation between the concentrations used in in vitro studies and actual clinical situations. How high is the concentration of monomers leached from a class-I cavity restored with composite resin? Some studies mention that the surface area of the sample used (220 mm2) is four times larger than that of typical restorations (52 mm2).48 It has been demonstrated that a larger surface area of the sample increases the release of components. Furthermore, the presence of an oxygen inhibition layer also contributes to an increased number of released components. However, in a clinical situation, the exposed surface area is limited, and the oxygen inhibition layer will be removed by grinding and polishing.48 Other authors mention that composites eluted into 75% ethanol/25% water solution should be no cause for alarm for several reasons: first, normal alcoholic drinks have lower concentrations of ethanol. Second, the contact time between ethanol, the composite, and then the oral cells is exceptionally low; therefore, the elution time is much shorter than 24 h as used in experiments.12
In most in vivo studies (4 of 7), the samples were taken from the oral mucosa and the DNA damage was registered with the MN test. Some of these reports even use both methods, MN assay and comet assay, at the same time.11,39,46 The combined comet/MN assay protocol has proven to be a sensitive and effective method for detecting multiple classes of genotoxins across a wide range of target organs within the same patient.45 As for studies that analysed patients with resin restorations (3 of 7), a genotoxic effect was demonstrated by comparing the measurements obtained from the group with resin restorations and their negative controls using the MN and the Comet assay.11,39,46
Of the studies that evaluated a genotoxic effect caused by occupational exposure to monomers, only one did not report a genotoxic effect measured with the MN test. However, this type of exposure entails a series of larger variables in the study subjects, such as workplace ventilation, the type and use of face masks, the use of gloves, and the use of protective glasses.1,3,17,28
For in vivo studies, it may be a limitation that the oral cavity is a multifactorial environment and that each patient bears his/her specific biological variation. Several biological, ecological, demographic, and lifestyle factors can influence in vivo analyses, which are therefore difficult to standardise.7,43
It must also be mentioned that data revealing the potential genotoxic effect are based upon dental material, composites, and adhesives used in each study. New dental materials, composites, self-adhesive composites and techniques are constantly introduced in the field of restorative dentistry, in which monomer leaching and monomer elution may differ from the materials analysed in this study. Data regarding a possible genotoxic effect of the most recent materials are not yet available.29,30,31
Conclusion
From the electronic search, structured data extraction, and analysis by different independent reviewers, results of this systematic review allow us to conclude that DNA damage is induced by monomers/co-monomers (TEG-DMA, bis-GMA, UDMA, and HEMA) used in restorative dentistry.
However, this finding should be interpreted with caution. The concentrations used in in vitro studies are heterogeneous; therefore, making comparisons between them is difficult. Furthermore, the general lack of correlation between the dosage of leachable monomers used for experiments and actual clinical situations is illustrated. As for the validation of existing in vivo studies, large-scale prospective studies in patients with dental restorations are still required, since evidence from available research is still insufficient. Further efforts are needed to carry out controlled randomised clinical trials, which are urgently required to explore the extent to which genotoxicity can be observed for these materials in clinical situations.
Acknowledgments
This publication was supported by grants from COECYTJAL, project number 9277-2021, and the Universidad Autónoma de Guadalajara, México.
Funding Statement
This publication was supported by grants from COECYTJAL, project number 9277-2021, and the Universidad Autónoma de Guadalajara, México.
References
- Araújo AM, Alves GR, Avanço GT, Parizi JL, Nai GA. Assessment of methyl methacrylate genotoxicity by the micronucleus test. Braz Oral Res. 2013;27:31–36. doi: 10.1590/s1806-83242013000100006. [DOI] [PubMed] [Google Scholar]
- Attin T, Becker K, Wiegand A, Tauböck TT, Wegehaupt FJ. Impact of laminar flow velocity of different acids on enamel calcium loss. Clin Oral Investig. 2012;17:595–600. doi: 10.1007/s00784-012-0731-3. [DOI] [PubMed] [Google Scholar]
- Azhar DA, Syed S, Luqman M, Ali AA. Evaluation of methyl methacrylate monomer cytotoxicity in dental lab technicians using buccal micronucleus cytome assay. Dent Mater J. 2013;32:519–521. doi: 10.4012/dmj.2012-322. [DOI] [PubMed] [Google Scholar]
- Bakopoulou A, Mourelatos D, Tsiftsoglou AS, Mioglou E, Garefis P. Sister-chromatid exchange, chromosomal aberrations and delays in cell-cycle kinetics in human lymphocytes induced by dental composite resin eluates. Mutat Res. 2008;649:79–90. doi: 10.1016/j.mrgentox.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Blasiak J, Synowiec E, Tarnawska J, Czarny P, Poplawski T, Reiter RJ. Dental methacrylates may exert genotoxic effects via the oxidative induction of DNA double strand breaks and the inhibition of their repair. Mol Biol Rep. 2012;39:7487–7496. doi: 10.1007/s11033-012-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi C, Bonassi S, Knasmueller S, Fenech M, Bruzzone M, Lando C, Ceppi M. Clinical application of micronucleus test in exfoliated buccal cells: A systematic review and metanalysis. Mutat Res Rev Mutat Res. 2015;766:20–31. doi: 10.1016/j.mrrev.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Coskun E, Ceppi M, Lando C, Bolognesi C, Burgaz S, et al. The Human MicroNucleus project on exfoliated buccal cells (HUMNXL): The role of life-style, host factors, occupational exposures, health status, and assay protocol. Mutat Res. 2011;728:88–97. doi: 10.1016/j.mrrev.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Brzović Rajić V, Želježić D, Malčić Ivanišević A, Verzak Ž, Baraba A, Miletić I. Cytotoxicity and genotoxicity of resin based dental materials in human lymphocytes in vitro. Acta Clin Croat. 2018;57:278–285. doi: 10.20471/acc.2018.57.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res. 2011;90:402–416. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci M, Hiller KA, Bosl C, Galler K, Schmalz G, Schweikl H. The induction of oxidative stress, cytotoxicity, and genotoxicity by dental adhesives. Dent Mater. 2008;24:362–371. doi: 10.1016/j.dental.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Di Pietro A, Visalli G, La Maestra S, Micale R, Baluce B, Matarese G, Cingano L, Scoglio ME. Biomonitoring of DNA damage in peripheral blood lymphocytes of subjects with dental restorative fillings. Mutat Res. 2008;650:115–122. doi: 10.1016/j.mrgentox.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Durner J, Dębiak M, Bürkle A, Hickel R, Reichl FX. Induction of DNA strand breaks by dental composite components compared to X-ray exposure in human gingival fibroblasts. Arch Toxicol. 2011;85:143–148. doi: 10.1007/s00204-010-0558-0. [DOI] [PubMed] [Google Scholar]
- Gallorini M, Cataldi A, di Giacomo V. HEMA-induced cytotoxicity: oxidative stress, genotoxicity and apoptosis. Int Endod J. 2014;47:813–818. doi: 10.1111/iej.12232. [DOI] [PubMed] [Google Scholar]
- Ginzkey C, Zinnitsch S, Steussloff G, Koehler C, Hackenberg S, Hagen R, Kleinsasser NH, Froelich K. Assessment of HEMA and TEG-DMA induced DNA damage by multiple genotoxicological endpoints in human lymphocytes. Dent Mater. 2015;31:865–876. doi: 10.1016/j.dental.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane: 2021. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) pp. 16–20. Available from www.training.cochrane.org/handbook . [Google Scholar]
- Holland N, Bolognesi C, Kirschvolders M, Bonassi S, Zeiger E, Knasmueller S, et al. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: The HUMN project perspective on current status and knowledge gaps. Mutat Res. 2008;659:93–108. doi: 10.1016/j.mrrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Ishikawa H, Shindo T, Yoshida T, Shimoyama Y, Satomi T, et al. Effects of occupational environmental controls and work management on chromosomal damage in dental technicians in Japan. Int J Hyg Environ Health. 2013;216:100–107. doi: 10.1016/j.ijheh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Kleinsasser N, Schmid K, Sassen AW. Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (Comet) assay. Biomaterials. 2006;27:1762–1770. doi: 10.1016/j.biomaterials.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Kleinsasser NH, Wallner BC, Harréus UA, Kleinjung T, Folwaczny M, Hickel R, et al. Genotoxicity and cytotoxicity of dental materials in human lymphocytes as assessed by the single cell microgel electrophoresis (comet) assay. J Dent. 2004;32:229–234. doi: 10.1016/j.jdent.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Krifka S, Spagnuolo G, Schmalz G, Schweikl H. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials. 2013;34:4555–4563. doi: 10.1016/j.biomaterials.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Krithikadatta J, Gopikrishna V, Datta M. CRIS Guidelines (Checklist for Reporting In vitro Studies): A concept note on the need for standardized guidelines for improving quality and transparency in reporting in vitro studies in experimental dental research. J Conserv Dent. 2014;17:301–304. doi: 10.4103/0972-0707.136338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt A, Altintas SH, Kiziltas MV, Tekkeli SE, Guler EM. Evaluation of residual monomer release and toxicity of self-adhesive resin cements. Dent Mater J. 2018;37:40–48. doi: 10.4012/dmj.2016-380. [DOI] [PubMed] [Google Scholar]
- Lottner S, Shehata M, Hickel R, Reichl F-X, Durner J. Effects of antioxidants on DNA-double strand breaks in human gingival fibroblasts exposed to methacrylate based monomers. Dent Mater. 2013;29:991–998. doi: 10.1016/j.dental.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Majer BJ, Laky B, Knasmüller S, Kassie F. Use of the micronucleus assay with exfoliated epithelial cells as a biomarker for monitoring individuals at elevated risk of genetic damage and in chemoprevention trials. Mutat Res. 2001;489:147–172. doi: 10.1016/s1383-5742(01)00068-0. [DOI] [PubMed] [Google Scholar]
- Manojlovic D, Radisic M, Vasiljevic T, Zivkovic S, Lausevic M, Miletic V. Monomer elution from nanohybrid and ormocer-based composites cured with different light sources. Dent Mater. 2011;27:371–378. doi: 10.1016/j.dental.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Michelsen VB, Moe G, Strøm MB, Jensen E, Lygre H. Quantitative analysis of TEG-DMA and HEMA eluted into saliva from two dental composites by use of GC/MS and tailor-made internal standards. Dent Mater. 2008;24:724–731. doi: 10.1016/j.dental.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009:6. [PMC free article] [PubMed] [Google Scholar]
- Molina D, Coronado E, Vázquez J, Izaguirre E, Arellano E, Flores-García A, Torres O. Evaluation of genotoxicity and cytotoxicity amongst in dental surgeons and technicians by micronucleus assay. Dent Oral Craniofac Res. 2019;5:1–5. [Google Scholar]
- Mourouzis P, Andreasidou E, Samanidou V, Tolidis K. Short-term and long-term release of monomers from newly developed resin-modified ceramics and composite resin CAD-CAM blocks. J Prosth Dent. 2020;123:339–348. doi: 10.1016/j.prosdent.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Oliveira NG, Lima ASLC, da Silveira MT, de Souza Araújo PR, de Melo Monteiro GQ, de Vasconcelos Carvalho M. Evaluation of postoperative sensitivity in restorations with self-adhesive resin: a randomized split-mouth design controlled study. Clin Oral Investig. 2020;24:1829–1835. doi: 10.1007/s00784-019-03046-0. [DOI] [PubMed] [Google Scholar]
- Par M, Spanovic N, Tauböck TT, Attin T, Tarle Z. Degree of conversion of experimental resin composites containing bioactive glass 45S5: the effect of post-cure heating. Sci Rep. 2019;9:17245. doi: 10.1038/s41598-019-54035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowska E, Poplawski T, Ksiazek D, Szczepanska J, Blasiak J. Genotoxicity and cytotoxicity of 2-hydroxyethyl methacrylate. Mutat Res. 2010;696:122–129. doi: 10.1016/j.mrgentox.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Phillips DH, Arlt VM. Genotoxicity: damage to DNA and its consequences. EXS. 2009;99:87–110. doi: 10.1007/978-3-7643-8336-7_4. [DOI] [PubMed] [Google Scholar]
- Poplawski T, Loba K, Pawlowska E, Szczepanska J, Blasiak J. Genotoxicity of urethane dimethacrylate, a tooth restoration component. Toxicol in Vitro. 2010;24:854–862. doi: 10.1016/j.tiv.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Salama SA, Serrana M, Au WW. Biomonitoring using accessible human cells for exposure and health risk assessment. Mutat Res. 1999;436:99–112. doi: 10.1016/s1383-5742(98)00021-0. [DOI] [PubMed] [Google Scholar]
- Schweikl H, Schmalz G, Spruss T. The induction of micronuclei in vitro by unpolymerized resin monomers. J Dent Res. 2001;80:1615–1620. doi: 10.1177/00220345010800070401. [DOI] [PubMed] [Google Scholar]
- Schweikl H, Spagnuolo G, Schmalz G. Genetic and cellular toxicology of dental resin monomers. J Dent Res. 2006;85:870–877. doi: 10.1177/154405910608501001. [DOI] [PubMed] [Google Scholar]
- Styllou M, Reichl FX, Styllou P, Urcan E, Rothmund L, Hickel R, et al. Dental composite components induce DNA-damage and altered nuclear morphology in gingiva fibroblasts. Dent Mater. 2015;31:1335–1344. doi: 10.1016/j.dental.2015.08.156. [DOI] [PubMed] [Google Scholar]
- Tadin A, Galic N, Mladinic M, Marovic D, Kovacic I, Zeljezic D. Genotoxicity in gingival cells of patients undergoing tooth restoration with two different dental composite materials. Clin Oral Investig. 2013;18:87–96. doi: 10.1007/s00784-013-0933-3. [DOI] [PubMed] [Google Scholar]
- Tadin A, Marovic D, Galic N, Kovacic I, Zeljezic D. Composite-induced toxicity in human gingival and pulp fibroblast cells. Acta Odontol Scand. 2014;72:304–311. doi: 10.3109/00016357.2013.824607. [DOI] [PubMed] [Google Scholar]
- Tadin A, Marovic D, Galic N, Milevoj A, Medvedec Mikic I, Zeljezic D. Genotoxic biomonitoring of flowable and non-flowable composite resins in peripheral blood leukocytes. Acta Odontol Scand. 2013;l71:923–929. doi: 10.3109/00016357.2012.734419. [DOI] [PubMed] [Google Scholar]
- Tauböck TT, Marovic D, Zeljezic D, Steingruber AD, Attin T, Tarle Z. Genotoxic potential of dental bulk-fill resin composites. Dent Mater. 2017;33:788–795. doi: 10.1016/j.dental.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Thomas P, Fenech M. Buccal micronucleus cytome assay. Methods Mol Biol. 2011;682:235–248. doi: 10.1007/978-1-60327-409-8_17. [DOI] [PubMed] [Google Scholar]
- Urcan E, Scherthan H, Styllou M, Haertel U. Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials. 2010;31:2010–2014. doi: 10.1016/j.biomaterials.2009.11.065. [DOI] [PubMed] [Google Scholar]
- Vasquez MZ. Combining the in vivo comet and micronucleus assays: a practical approach to genotoxicity testing and data interpretation. Mutagenesis. 2010;25:187–199. doi: 10.1093/mutage/gep060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalli G, Baluce B, La Maestra S, Micale RT, Cingano L, De Flora S, et al. Genotoxic damage in the oral mucosa cells of subjects carrying restorative dental fillings. Arch Toxicol. 2013;87:179–187. doi: 10.1007/s00204-012-0915-2. [DOI] [PubMed] [Google Scholar]
- Yang H-W, Chou LS-S, Chou M-Y, Chang Y-C. Assessment of genetic damage by methyl methacrylate employing in vitro mammalian test system. Biomaterials. 2003;24:2909–2914. doi: 10.1016/s0142-9612(03)00132-7. [DOI] [PubMed] [Google Scholar]
- Yang Y, Reichl FX, Shi J, He X, Hickel R, Högg C. Cytotoxicity and DNA double-strand breaks in human gingival fibroblasts exposed to eluates of dental composites. Dent Mater. 2018;34:201–208. doi: 10.1016/j.dental.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Wiegand A, Credé A, Tschammler C, Attin T, Tauböck TT. Enamel wear by antagonistic restorative materials under erosive conditions. Clin Oral Investig. 2017;21:2689–2693. doi: 10.1007/s00784-017-2071-9. [DOI] [PubMed] [Google Scholar]


