Abstract
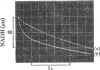
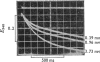
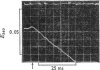
D-Glyceraldehyde 3-phosphate forms adducts with thiols. These adducts, which are presumed to be hemithioacetals, equilibrate rapidly with the unhydrated form of the aldehyde, which is the subtrate for D-glyceraldehyde 3-phosphate dehydrogenase. The adduct provides a substrate buffer system whereby a constant low free aldehyde concentration can be maintained during the oxidation of aldehyde by the enzyme and NAD+. With this system, the kinetics of the association of the aldehyde with the enzyme were examined. The rate profile for this reaction is a single exponential process, showing that all four active sites of the enzyme have equivalent and independent reactivity towards the aldehyde, with an apparent second-order rate constant of 5 X 10(7)M-1-S-1 at pH8.0 and 21 degrees C. The second-order rate constant becomes 8 X 10(7)M-1-S-1 when account is taken of the forward and reverse catalytic rate constants of the dehydrogenase. The pH-dependence of the observed rate constant is consistent with a requirement for the unprotonated form of a group of pK 6.1, which is the pK observed for second ionization of glyceraldehyde 3-phosphate. The rate of phosphorolysis of the acyl-enzyme intermediate during the steady-state oxidative phosphorylation of the aldehyde was studied, and is proportional to the total Pi concentration up to at least 1 mM-Pi at pH 7.5. The pH-dependence of the rate of NADH generation under these conditions can be explained by the rate law d[NADA]/dt = k[acy] holoenzyme][PO4(3-)-A1, where thioester bond, although kinetically indistinguishable rate equations for the reaction are possible. The rates of the phosphorolysis reaction and of the aldehyde-association reaction decrease with increasing ionic strength, suggesting that the active site of the enzyme has cationic groups which are involved in the reaction of the enzyme with anionic substrates.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buehner M., Ford G. C., Olsen K. W., Moras D., Rossman M. G. Three-dimensional structure of D-glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1974 Nov 25;90(1):25–49. doi: 10.1016/0022-2836(74)90254-x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FLUHARTY A. L., BALLOU C. E. D-Threose 2,4-diphosphate inhibiton of D-glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1959 Oct;234:2517–2522. [PubMed] [Google Scholar]
- Ferdinand W. The isolation and specific activity of rabbit-muscle glyceraldehyde phosphate dehydrogenase. Biochem J. 1964 Sep;92(3):578–585. doi: 10.1042/bj0920578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife T. H., Rikihisa T. Reaction of glyceraldehyde 3-phosphate dehydrogenase with aliphatic aldehydes. Biochemistry. 1970 Oct 13;9(21):4064–4067. doi: 10.1021/bi00823a006. [DOI] [PubMed] [Google Scholar]
- HARTING J., VELICK S. F. Acetyl phosphate formation catalyzed by glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1954 Apr;207(2):857–865. [PubMed] [Google Scholar]
- Harrigan P. J., Trentham D. R. Kinetic studies of the acylation of pig muscle D-glyceraldehyde 3-phosphate dehydrogenase by 1,3-diphosphoglycerate and of proton uptake and release in the overall enzyme mechanism. Biochem J. 1973 Dec;135(4):695–703. doi: 10.1042/bj1350695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan P. J., Trentham D. R. Kinetic studies on oxidized nicotinamide--adenine dinucleotide-facilitated reactions of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1974 Nov;143(2):353–363. doi: 10.1042/bj1430353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A. Half-of-the-sites and all-of-the-sites reactivity in rabbit muscle glyceraldehyde 3-phosphate dehydrogenase. J Mol Biol. 1974 Dec 15;90(3):451–468. doi: 10.1016/0022-2836(74)90227-7. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Jencks W. P. Thiol addition to the carbonyl group. Equilibria and kinetics. J Am Chem Soc. 1966 Sep 5;88(17):3982–3994. doi: 10.1021/ja00969a017. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., SUMNER J. B. D-Glyceraldehyde 3-phosphate dehydrogenase; a comparison with liver aldehyde dehydrogenase. Arch Biochem Biophys. 1952 Jul;39(1):119–128. doi: 10.1016/0003-9861(52)90266-x. [DOI] [PubMed] [Google Scholar]
- Peczon B. D., Spivey H. O. Catalytic sites in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Their number and their kinetic and spectral properties. Biochemistry. 1972 Jun 6;11(12):2209–2217. doi: 10.1021/bi00762a001. [DOI] [PubMed] [Google Scholar]
- SZEWCZUK A., WOLNY E., WOLNY M., BARANOWSKI T. [A new method for obtaining d-glyceraldehyde-3-phosphate]. Acta Biochim Pol. 1961;8:201–207. [PubMed] [Google Scholar]
- Seydoux F. J., Kelemen N., Kellershohn N., Roucous C. Specific interactions of 3-phosphoglyceroyl-glyceraldehyde-3-phosphate dehydrogenase with coenzymes. Eur J Biochem. 1976 May 1;64(2):481–489. doi: 10.1111/j.1432-1033.1976.tb10326.x. [DOI] [PubMed] [Google Scholar]
- Seydoux F., Bernhard S., Pfenninger O., Payne M., Malhotra O. P. Preparation and active-site specific properties of sturgeon muscle glyceraldehyde-3-phoshate dehydrogenase. Biochemistry. 1973 Oct 9;12(21):4290–4300. doi: 10.1021/bi00745a038. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Velick S. F. The glyceraldehyde 3-phosphate dehydrogenases of liver and muscle. Cooperative interactions and conditions for functional reversibility. J Biol Chem. 1972 Jan 10;247(1):273–284. [PubMed] [Google Scholar]
- Stinson R. A., Holbrook J. J. Equilibrium binding of nicotinamide nucleotides to lactate dehydrogenases. Biochem J. 1973 Apr;131(4):719–728. doi: 10.1042/bj1310719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R., McMurray C. H., Pogson C. I. The active chemical state of D-glyceraldehyde 3-phosphate in its reactions with D-glyceraldehyde 3-phosphate dehydrogenase, aldolase and triose phosphate isomerase. Biochem J. 1969 Aug;114(1):19–24. doi: 10.1042/bj1140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R. Rate-determining processes and the number of simultaneously active sties of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1971 Mar;122(1):71–77. doi: 10.1042/bj1220071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R. Reactions of D-glyceraldehyde 3-phosphate dehydrogenase facilitated by oxidized nicotinamide-adenine dinucleotide. Biochem J. 1971 Mar;122(1):59–69. doi: 10.1042/bj1220059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELICK S. F., HAYES J. E., Jr Phosphate binding and the glyceraldehyde-3-phosphate dehydrogenase reaction. J Biol Chem. 1953 Aug;203(2):545–562. [PubMed] [Google Scholar]
- WATSON H. C., BANASZAK L. J. STRUCTURE OF GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE. STRUCTURE SYMMETRY WITHIN THE MOLECULE. Nature. 1964 Dec 5;204:918–920. doi: 10.1038/204918a0. [DOI] [PubMed] [Google Scholar]
- Watson H. C., Duée E., Mercer W. D. Low resolution structure of glyceraldehyde 3-phosphate dehydrogenase. Nat New Biol. 1972 Nov 29;240(100):130–133. doi: 10.1038/newbio240130a0. [DOI] [PubMed] [Google Scholar]
- Weischet W. O., Kirschner K. The mechanism of the synthesis of indoleglycerol phosphate catalyzed by tryptophan synthase from Escherichia coli. Steady-state kinetic studies. Eur J Biochem. 1976 Jun 1;65(2):365–373. doi: 10.1111/j.1432-1033.1976.tb10350.x. [DOI] [PubMed] [Google Scholar]