Abstract
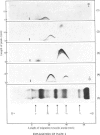
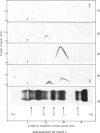
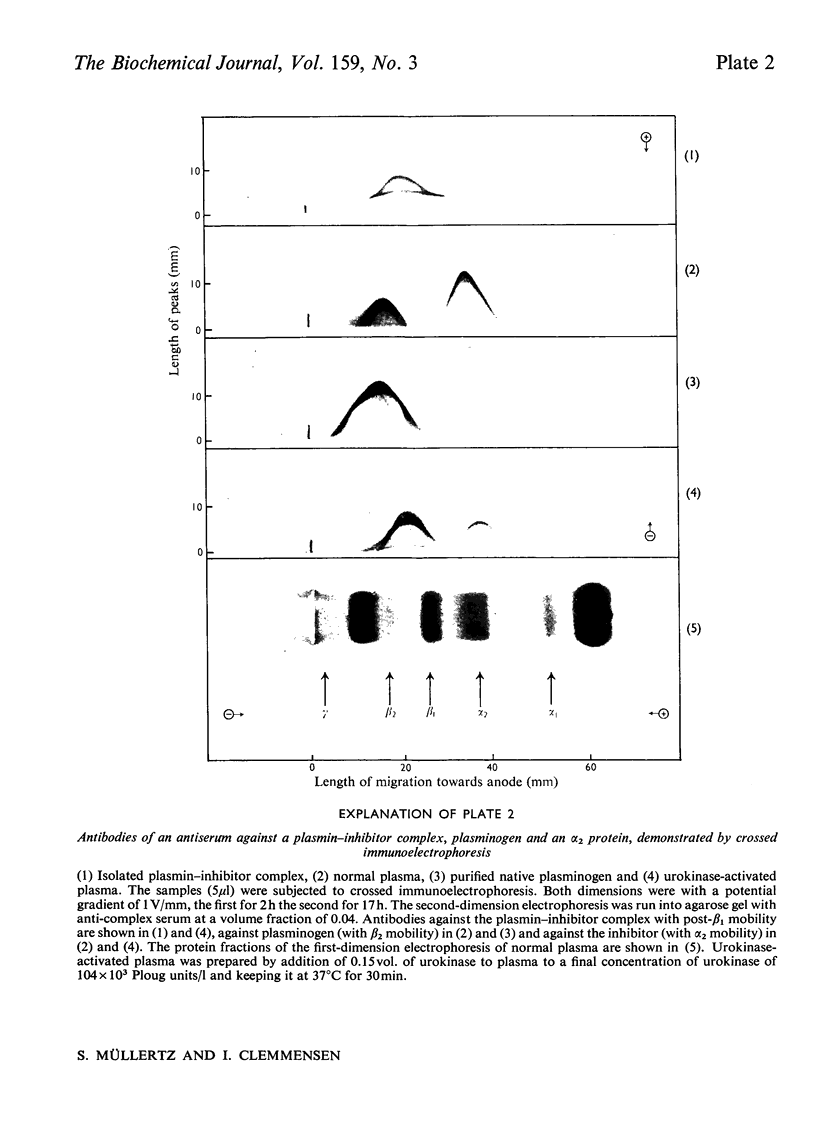
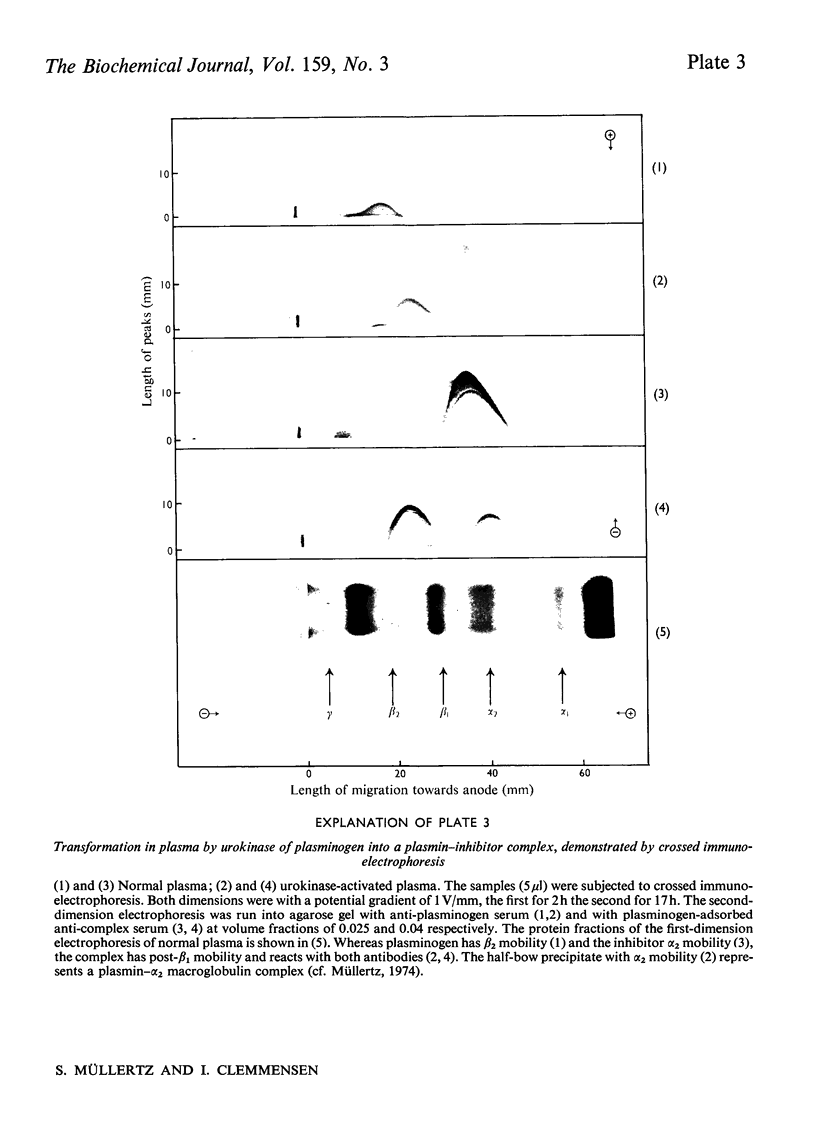
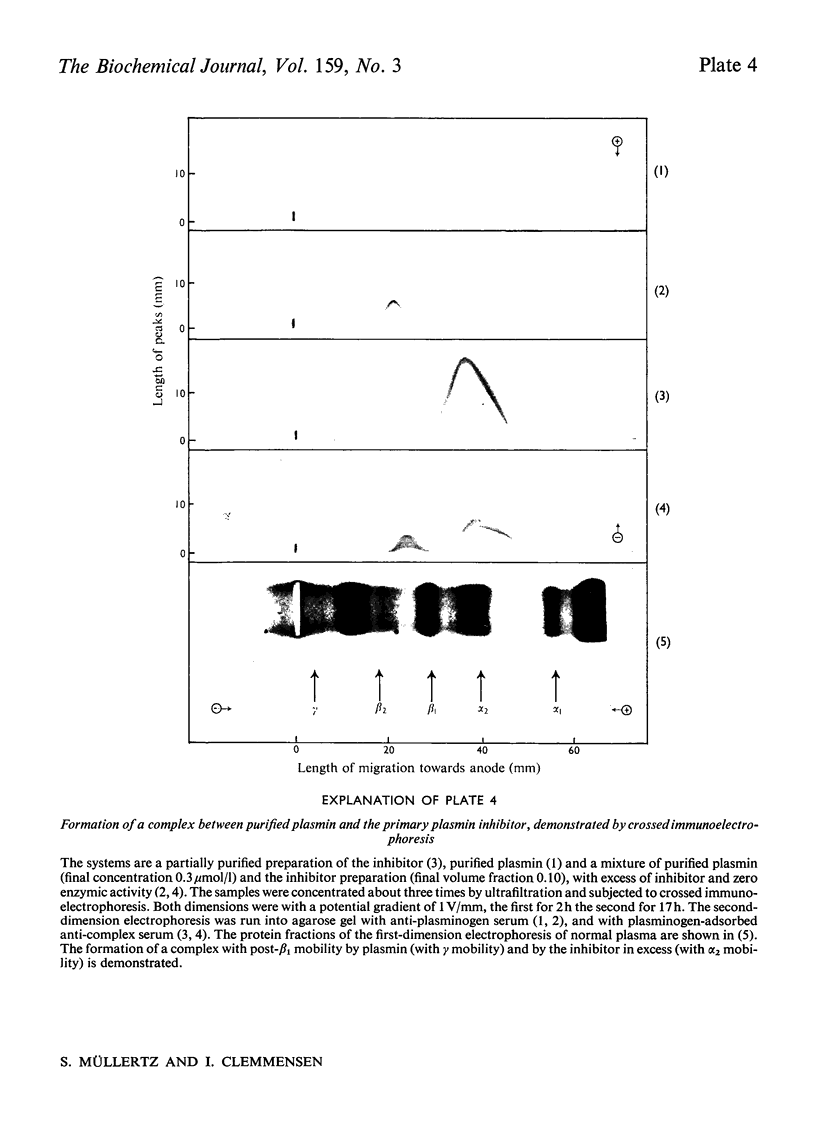
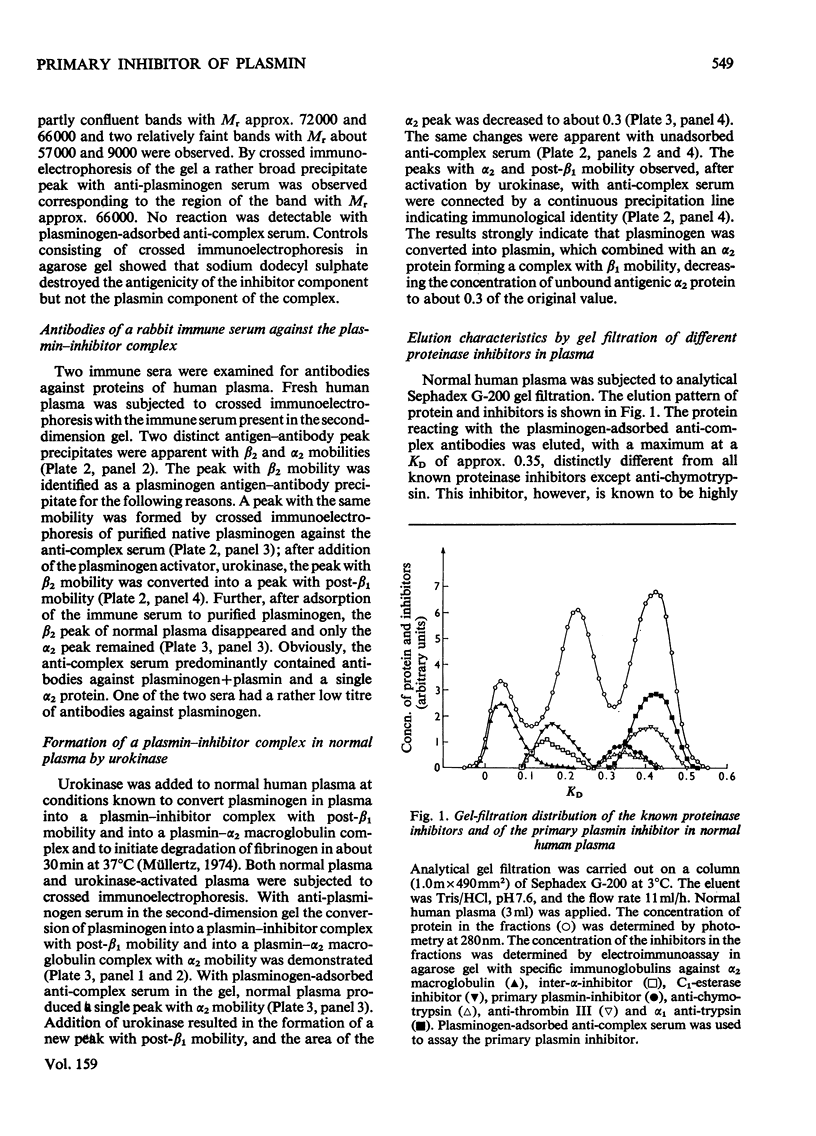
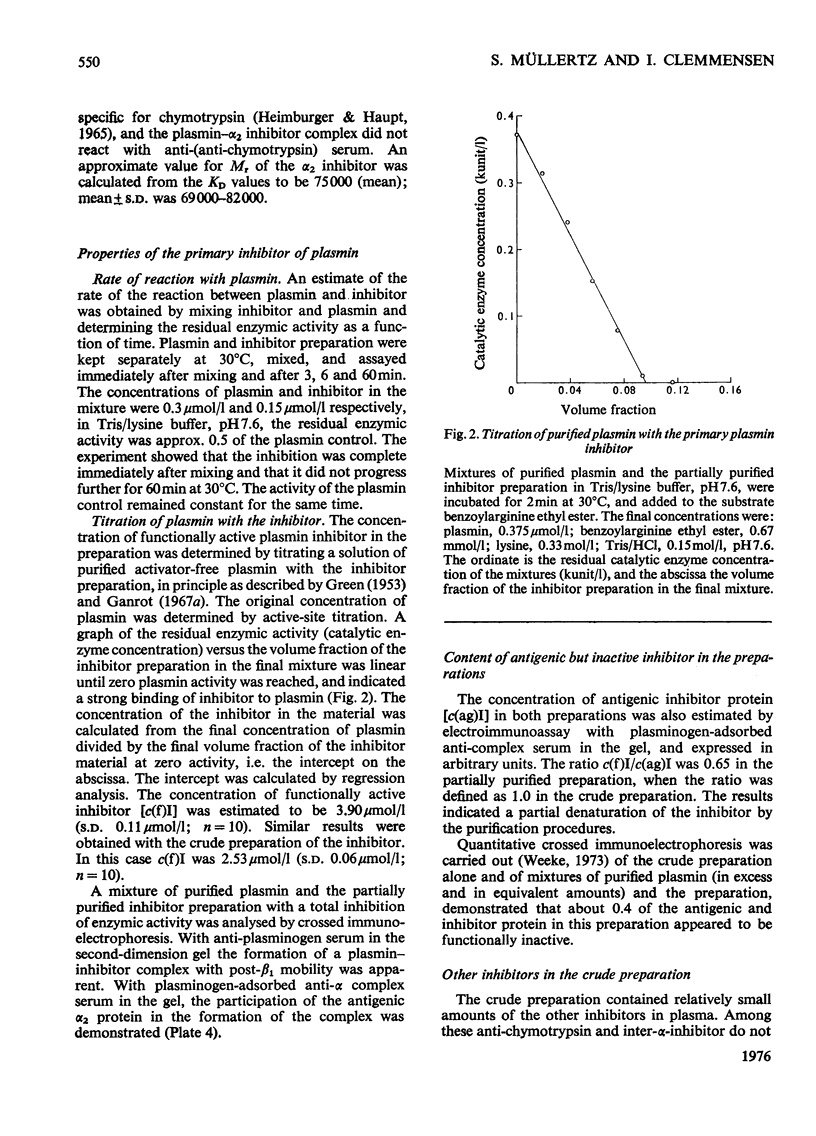
A complex between plasmin and an inhibitor was isolated by affinity chromatography from urokinase-activated human plasma. The complex did not react with antibodies against any of the known proteinase inhibitors in plasma. A rabbit antiserum against the complex was produced. It contained antibodies agianst plasminogen+plasmin and an alpha2 protein. By crossed immunoelectrophoresis the alpha2 protein was shown to form a complex with plasmin, when generated by urokinase in plasma, and with purified plasmin. The alpha2 protein was eluted by Sephadex G-200 gel filtration with KD approx. 0.35, different from the other inhibitors of plasmin in plasma, and corresponding to an apparent relative molecular mass (Mr) of about 75000. By sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, the Mr of the complex was found to be approx. 130000. After reduction of the complex two main bands of protein were observed, with Mr, about 72000 and 66000, probably representing an acyl-enzyme complex of plasmin-light chain and inhibitor-heavy chain, and a plasmin-heavy chain. A weak band with Mr 9000 was possibly an inhibitor-light chain. The inhibitor was partially purified and used to titrate purified plasmin of known active-site concentration. The inhibitor bound plasmin rapidly and strongly. Assuming an equimolar combining ratio, the concentration of active inhibitor in normal human plasma was estimated to be 1.1 mumol/1. A fraction about 0.3 of the antigenic inhibitor protein appeared to be functionally inactive. In plasma, plasmin is primarily bound to the inhibitor. Only after its saturation does lysis of fibrinogen and fibrin occur and a complex between plasmin and alpha2 macroglobulin appear.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N., Von Kaulla K. N. Human serum plasminogen antiactivator: its distinction from antiplasmin. Am J Physiol. 1971 Apr;220(4):1137–1145. doi: 10.1152/ajplegacy.1971.220.4.1137. [DOI] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- BENDER M. L., KEZDY J. MECHANISM OF ACTION OF PROTEOLYTIC ENZYMES. Annu Rev Biochem. 1965;34:49–76. doi: 10.1146/annurev.bi.34.070165.000405. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969 May;8(5):2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Collen D., De Cock, Verstraete M. Immunochemical distinction between antiplasmin and alpha-antitrypsin. Thromb Res. 1975 Jul;7(1):245–249. doi: 10.1016/0049-3848(75)90142-5. [DOI] [PubMed] [Google Scholar]
- Collen D. Emergence in plasma during activation of the coagulation or fibrinolytic systems of neoantigens, associated with the complexes of thrombin or plasmin with their inhibitors. Thromb Res. 1974 Dec;5(6):777–779. doi: 10.1016/0049-3848(74)90121-2. [DOI] [PubMed] [Google Scholar]
- Crawford G. P., Ogston D. The action of antithrombin III on plasmin and activators of plasminogen. Biochim Biophys Acta. 1975 May 23;391(1):189–192. doi: 10.1016/0005-2744(75)90165-5. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Fritz H., Wunderer G., Kummer K., Heimburger N., Werle E. -Antitrypsin und c 1-inaktivator: progressiv-inhibitoren für serumkallikreine von mensch und schwein. Hoppe Seylers Z Physiol Chem. 1972 Jun;353(6):906–910. [PubMed] [Google Scholar]
- GREEN N. M. Competition among trypsin inhibitors. J Biol Chem. 1953 Dec;205(2):535–551. [PubMed] [Google Scholar]
- Gallimore M. J. Serum inhibitors in fibrinolysis. Br J Haematol. 1975 Oct;31(2):217–231. doi: 10.1111/j.1365-2141.1975.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O. Crossed immunoelectrophoresis. Scand J Clin Lab Invest Suppl. 1972;124:39–47. doi: 10.3109/00365517209102749. [DOI] [PubMed] [Google Scholar]
- Harpel P. C., Cooper N. R. Studies on human plasma C1 inactivator-enzyme interactions. I. Mechanisms of interaction with C1s, plasmin, and trypsin. J Clin Invest. 1975 Mar;55(3):593–604. doi: 10.1172/JCI107967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner U. Studies on an inhibitor of plasminogen activation in human serum. Thromb Diath Haemorrh. 1973 Nov;30(2):414–424. [PubMed] [Google Scholar]
- Heimburger N., Haupt H. Charakterisierung von alpha-1-X-Glykoprotein als Chymotrypsin-Inhibitor des Humanplasmas. Clin Chim Acta. 1965 Jul;12(1):116–118. doi: 10.1016/0009-8981(65)90118-x. [DOI] [PubMed] [Google Scholar]
- Hercz A. The inhibition of proteinases by human alpha1-antitrypsin. Eur J Biochem. 1974 Nov 1;49(1):287–292. doi: 10.1111/j.1432-1033.1974.tb03833.x. [DOI] [PubMed] [Google Scholar]
- Highsmith R. F., Rosenberg R. D. The inhibition of human plasmin by human antithrombin-heparin cofactor. J Biol Chem. 1974 Jul 25;249(14):4335–4338. [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- McDonagh J., Messel H., McDonagh R. P., Jr, Murano G., Blombäck B. Molecular weight analysis of fibrinogen and fibrin chains by an improved sodium dodecyl sulfate gel electrophoresis method. Biochim Biophys Acta. 1972 Jan 26;257(1):135–142. doi: 10.1016/0005-2795(72)90262-0. [DOI] [PubMed] [Google Scholar]
- Müllertz S. Different molecular forms of plasminogen and plasmin produced by urokinase in human plasma and their relation to protease inhibitors and lysis of fibrinogen and fibrin. Biochem J. 1974 Nov;143(2):273–283. doi: 10.1042/bj1430273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllertz S. Molecular forms of plasmin and protease inhibitors in human fibrinolytic post-mortem plasma. Scand J Clin Lab Invest. 1972 Dec;30(4):369–379. doi: 10.3109/00365517209080272. [DOI] [PubMed] [Google Scholar]
- Rabiner S. F., Goldfine I. D., Hart A., Summaria L., Robbins K. C. Radioimmunoassay of human plasminogen and plasmin. J Lab Clin Med. 1969 Aug;74(2):265–273. [PubMed] [Google Scholar]
- Rickli E. E., Cuendet P. A. Isolation of plasmin-free human plasminogen with N-terminal glutamic acid. Biochim Biophys Acta. 1971 Nov 13;250(2):447–451. doi: 10.1016/0005-2744(71)90202-6. [DOI] [PubMed] [Google Scholar]
- Rimon A., Shamash Y., Shapiro B. The plasmin inhibitor of human plasma. IV. Its action on plasmin, trypsin, chymotrypsin, and thrombin. J Biol Chem. 1966 Nov 10;241(21):5102–5107. [PubMed] [Google Scholar]
- Robbins K. C., Bernabe P., Arzadon L., Summaria L. NH2-terminal sequences of mammalian plasminogens and plasmin S-carboxymethyl heavy (A) and light (B) chain derivatives. A re-evaluation of the mechanism of activation of plasminogen. J Biol Chem. 1973 Oct 25;248(20):7242–7246. [PubMed] [Google Scholar]
- Robbins K. C., Summaria L., Hsieh B., Shah R. J. The peptide chains of human plasmin. Mechanism of activation of human plasminogen to plasmin. J Biol Chem. 1967 May 25;242(10):2333–2342. [PubMed] [Google Scholar]
- Sjöholm I. Studies on the conformational changes of plasminogen induced during activation to plasmin and by 6-aminohexanoic acid. Eur J Biochem. 1973 Nov 15;39(2):471–479. doi: 10.1111/j.1432-1033.1973.tb03146.x. [DOI] [PubMed] [Google Scholar]
- Summaria L., Hsieh B., Groskopf W. R., Robbins K. C. The isolation and characterization of the S-carboxymethyl beta (light) chain derivative of human plasmin. The localization of the active site on the beta (light) chain. J Biol Chem. 1967 Nov 10;242(21):5046–5052. [PubMed] [Google Scholar]
- Thorsen S. The inhibition of tissue plasminogen activator and urokinase-induced fibrinolysis by some natural proteinase inhibitors and by plasma and serum from normal and pregnant subjects. Scand J Clin Lab Invest. 1973 Jan;31(1):51–59. doi: 10.3109/00365517309082418. [DOI] [PubMed] [Google Scholar]
- Walther P. J., Steinman H. M., Hill R. L., McKee P. A. Activation of human plasminogen by urokinase. Partial characterization of a pre-activation peptide. J Biol Chem. 1974 Feb 25;249(4):1173–1181. [PubMed] [Google Scholar]
- Wiman B., Wallén P. Activation of human plasminogen by an insoluble derivative of urokinase. Structural changes of plasminogen in the course of activation to plasmin and demonstration of a possible intermediate compound. Eur J Biochem. 1973 Jul 2;36(1):25–31. doi: 10.1111/j.1432-1033.1973.tb02880.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Wallén P. On the primary structure of human plasminogen and plasmin. Purification and characterization of cyanogen-bromide fragments. Eur J Biochem. 1975 Sep 15;57(2):387–394. doi: 10.1111/j.1432-1033.1975.tb02312.x. [DOI] [PubMed] [Google Scholar]