Abstract
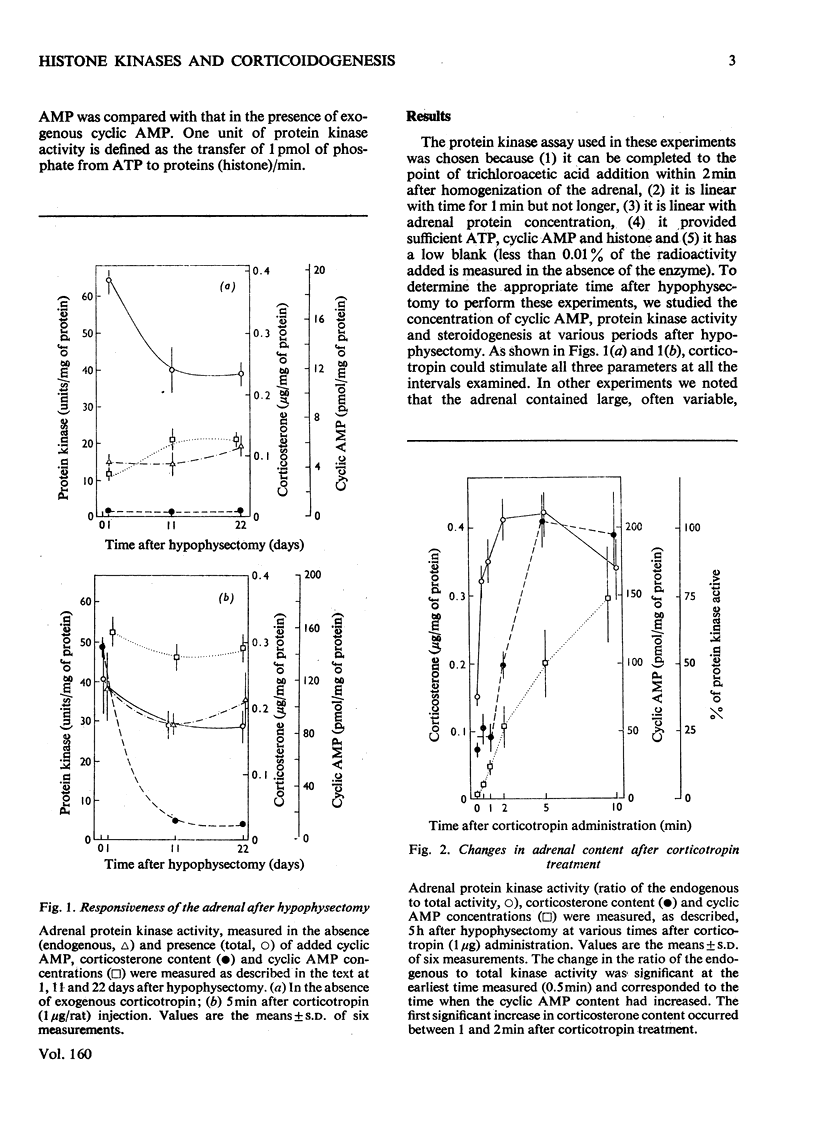
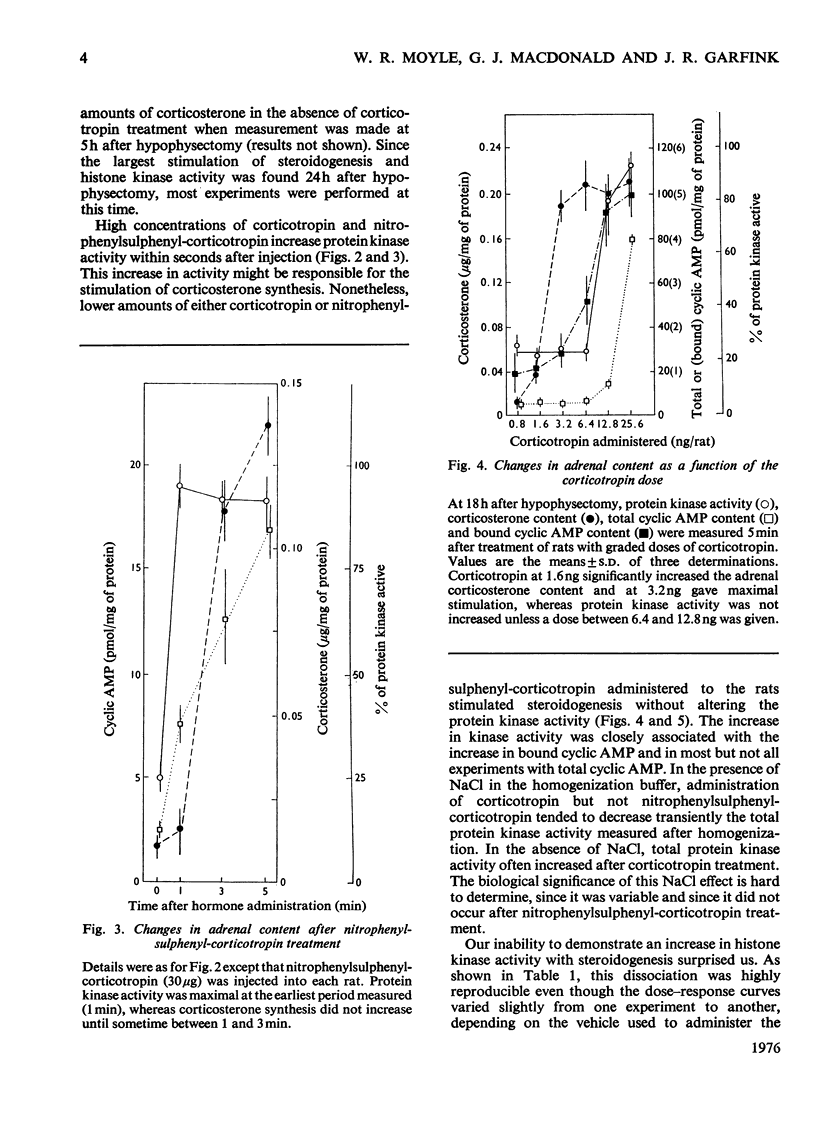
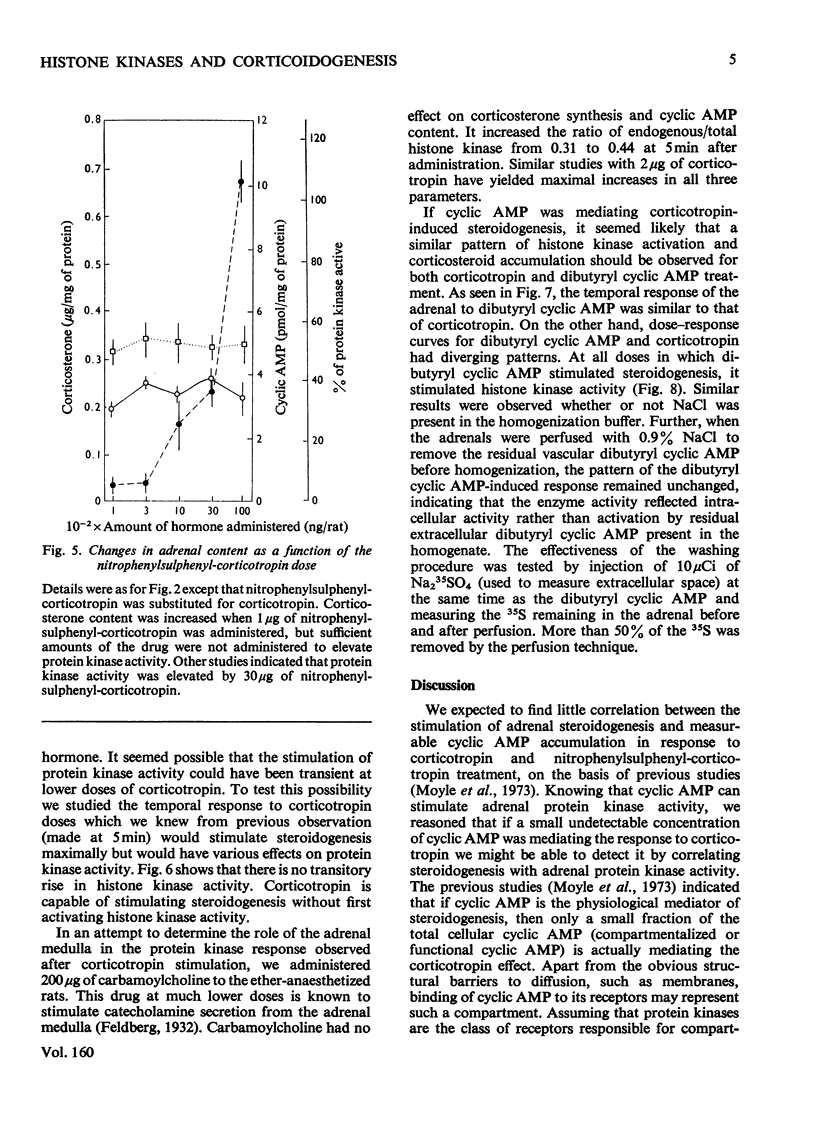
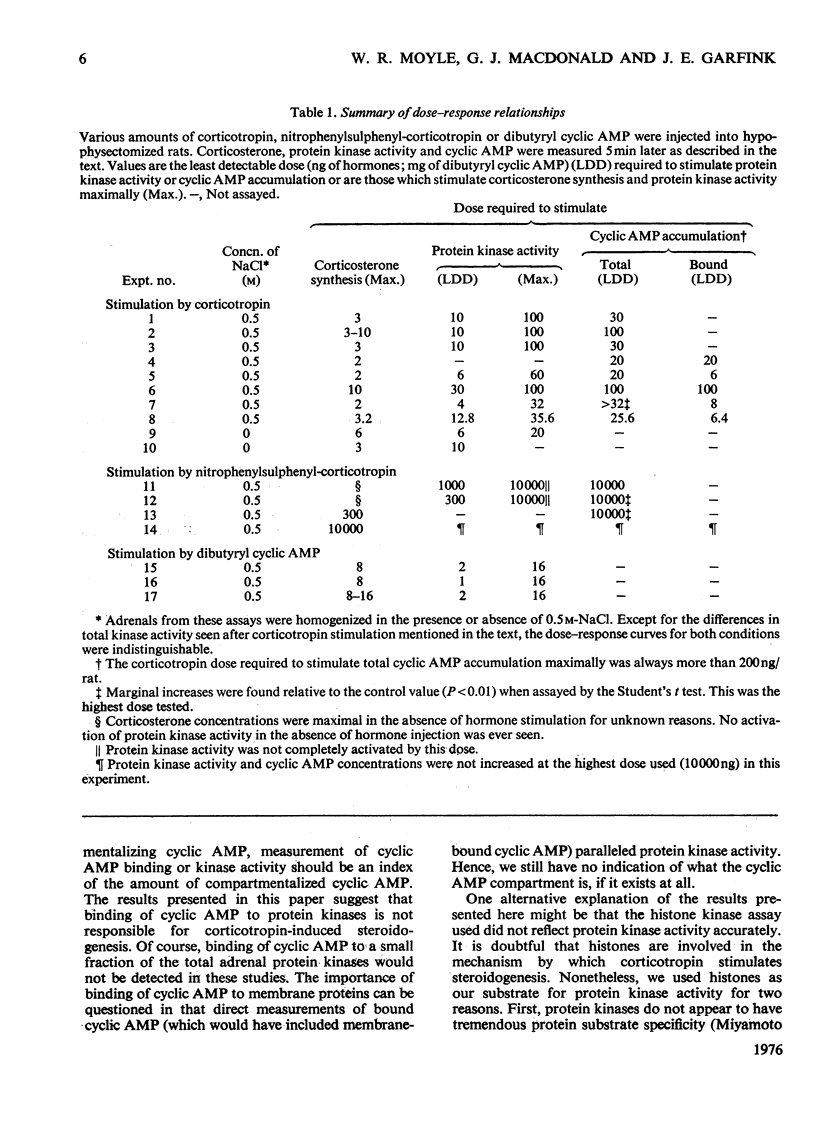
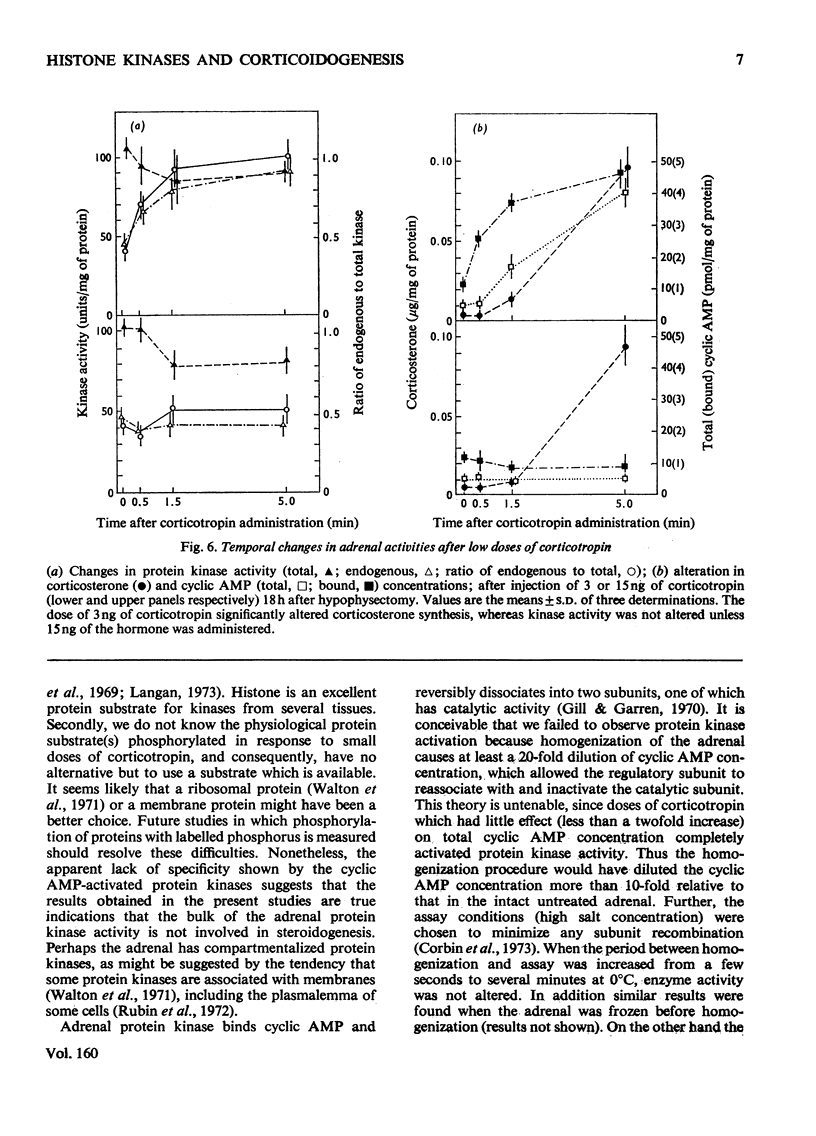
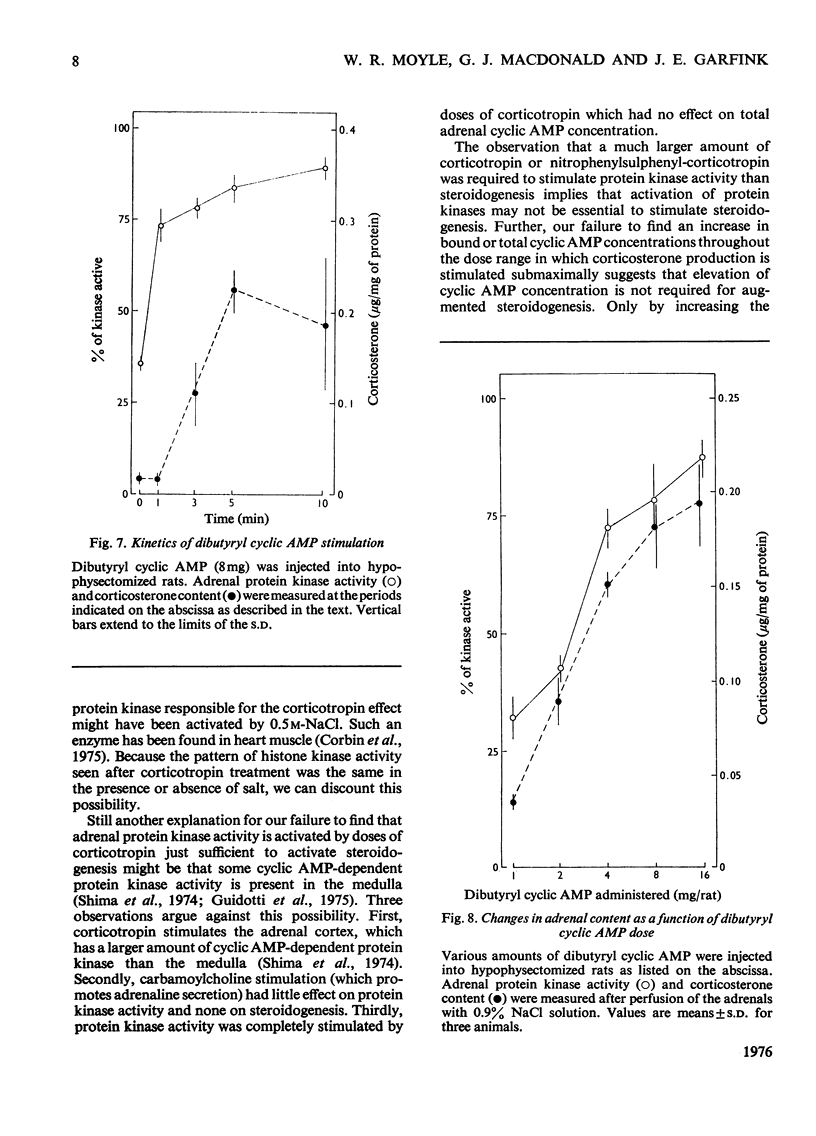
In an attempt to determine the role of protein (histone) kinases as mediators of corticotropin-induced corticosterone formation, the ability of homogenates, prepared from adrenals treated with various doses of corticotropin to catalyse the phosphorylation of calf thymus histones was measured. Although corticotropin promoted an increase in histone kinase activity, much more of the hormone was required to induce this response than to stimulate steroidogenesis maximally. In addition, a derivative, nitrophenylsulphenyl-corticotropin, which inhibits the stimulatory effect of corticotropin on cyclic AMP accumulation, stimulated corticosterone synthesis without altering histone kinase activity. Very high doses of nitrophenylsulphenyl-corticotropin were capable of stimulating histone kinase activity. In contrast, when dibutyryl cyclic AMP was used to stimulate steroidogenesis under the same conditions, any dose of the nucleotide which increased adrenal corticosteroid content also increased histone kinase activity. Assuming that histones serve as useful substrates for measurement of total adrenal protein kinase activity, the role of protein kinases as mediators of steroidogenesis is not supported by these studies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Corbin J. D., Soderling T. R., Park C. R. Regulation of adenosine 3',5'-monophosphate-dependent protein kinase. I. Preliminary characterization of the adipose tissue enzyme in crude extracts. J Biol Chem. 1973 Mar 10;248(5):1813–1821. [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. A cyclic-3',5'-adenosine monophosphate dependent protein kinase from the adrenal cortex: comparison with a cyclic AMP binding protein. Biochem Biophys Res Commun. 1970 May 11;39(3):335–343. doi: 10.1016/0006-291x(70)90581-4. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Kurosawa A., Chuang D. M., Costa E. Protein kinase activation as an early event in the trans-synaptic induction of tyrosine 3-monooxygenase in adrenal medulla. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1152–1156. doi: 10.1073/pnas.72.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYNES R. C., Jr, BERTHET L. Studies on the mechanism of action of the adrenocorticotropic hormone. J Biol Chem. 1957 Mar;225(1):115–124. [PubMed] [Google Scholar]
- HAYNES R. C., Jr, KORITZ S. B., PERON F. G. Influence of adenosine 3',5'-monophosphate on corticoid production by rat adrenal glands. J Biol Chem. 1959 Jun;234(6):1421–1423. [PubMed] [Google Scholar]
- Haksar A., Maudsley D. V., Péron F. G. Stimulation of cyclic adenosine 3':5'-monophosphate and corticosterone formation in isolated rat adrenal cells by cholera enterotoxin. Comparison with the effects of ACTH. Biochim Biophys Acta. 1975 Feb 13;381(2):308–323. doi: 10.1016/0304-4165(75)90237-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Protein kinases and protein kinase substrates. Adv Cyclic Nucleotide Res. 1973;3:99–153. [PubMed] [Google Scholar]
- Miyamoto E., Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. 3. Purification and properties of adenosine 3',5'-monophosphate-dependent protein kinase from bovine brain. J Biol Chem. 1969 Dec 10;244(23):6395–6402. [PubMed] [Google Scholar]
- Moyle W. R., Bahl O. P., März L. Role of carbohydrate of human chorionic gonadotropin in the mechanism of hormone action. J Biol Chem. 1975 Dec 10;250(23):9163–9169. [PubMed] [Google Scholar]
- Moyle W. R., Kong Y. C., Ramachandran J. Steroidogenesis and cyclic adenosine 3',5'-monophosphate accumulation in rat adrenal cells. Divergent effects of adrenocorticotropin and its o-nitrophenyl sulfenyl derivative. J Biol Chem. 1973 Apr 10;248(7):2409–2417. [PubMed] [Google Scholar]
- PETERSON R. E. The identification of corticosterone in human plasma and its assay by isotope dilution. J Biol Chem. 1957 Mar;225(1):25–37. [PubMed] [Google Scholar]
- PICKERING B. T., ANDERSEN R. N., LOHMAR P., BIRK Y., LI C. H. ADRENOCORTICOTROPIN. XXVII. ON THE PRESENCE OF PIG-TYPE ADRENOCORTICOTROPIN IN SHEEP PITUITARIES, AND A SIMPLE METHOD FOR THE ISOLATION OF ALPHA-S-ADRENOCORTICOTROPIN. Biochim Biophys Acta. 1963 Sep 10;74:763–773. doi: 10.1016/0006-3002(63)91428-8. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Lee V. Preparation and properties of the o-nitrophenyl sulfenyl derivative of ACTH: an inhibitor of the lipolytic action of the hormone. Biochem Biophys Res Commun. 1970 Feb 6;38(3):507–512. doi: 10.1016/0006-291x(70)90743-6. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Cyclic adenosine 3',5'-monophosphate-dependent protein kinase of human erythrocyte membranes. J Biol Chem. 1972 Oct 10;247(19):6135–6139. [PubMed] [Google Scholar]
- STONE D., HECHTER O. Studies on ACTH action in perfused bovine adrenals: the site of action of ACTH in corticosteroidogenesis. Arch Biochem Biophys. 1954 Aug;51(2):457–469. doi: 10.1016/0003-9861(54)90501-9. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Ahmed N. K., Sutliff L. S., Brush J. S. Metabolic regulation of steroidogenesis in isolated adrenal cells of the rat. ACTH regulation of cGMP and cAMP levels and steroidogenesis. FEBS Lett. 1974 Sep 1;45(1):107–110. doi: 10.1016/0014-5793(74)80822-7. [DOI] [PubMed] [Google Scholar]
- Shima S., Mitsunaga M., Kawashima Y., Taguchi S., Nakao T. Studies on cyclic nucleotides in the adrenal gland. 3. Properties of cyclic AMP- and GMP-dependent protein kinases in the adrenal gland. Biochim Biophys Acta. 1974 Mar 21;341(1):56–64. doi: 10.1016/0005-2744(74)90065-5. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- Walton G. M., Gill G. N., Abrass I. B., Garren L. D. Phosphorylation of ribosome-associated protein by an adenosine 3':5'-cyclic monophosphate-dependent protein kinase: location of the microsomal receptor and protein kinase. Proc Natl Acad Sci U S A. 1971 May;68(5):880–884. doi: 10.1073/pnas.68.5.880. [DOI] [PMC free article] [PubMed] [Google Scholar]


