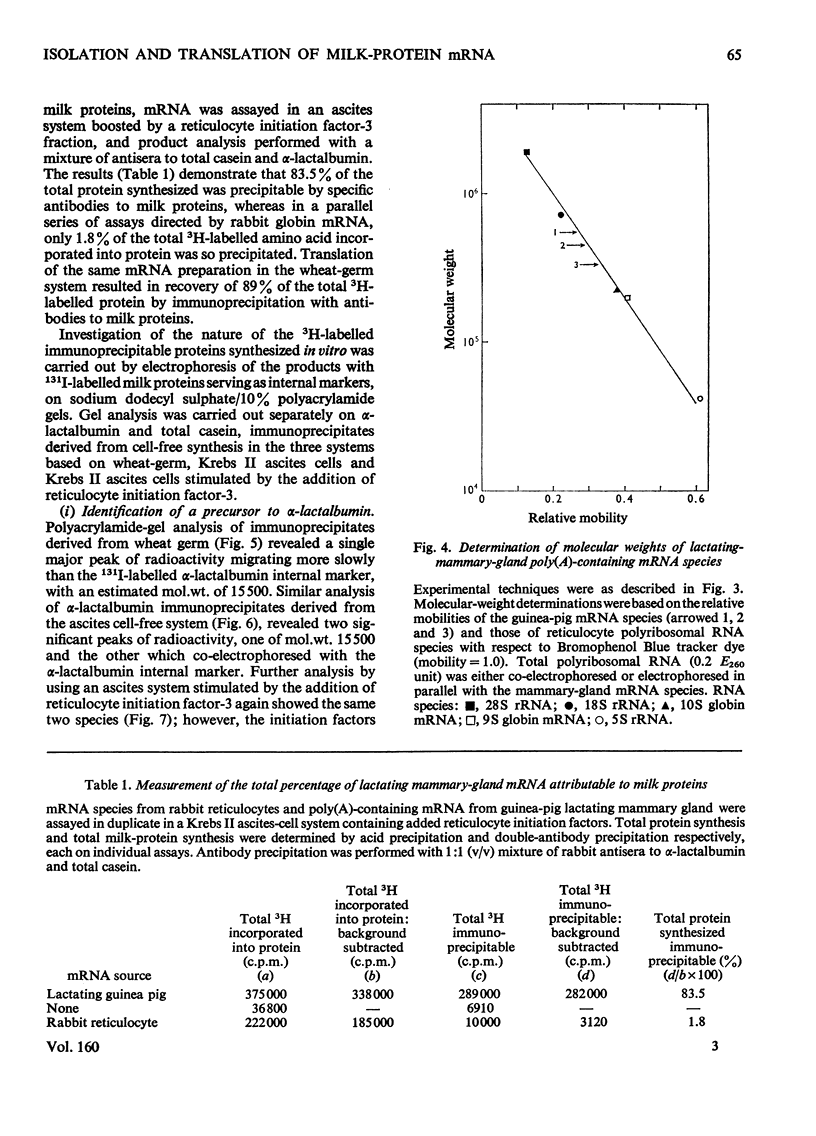
Abstract
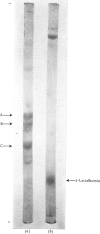
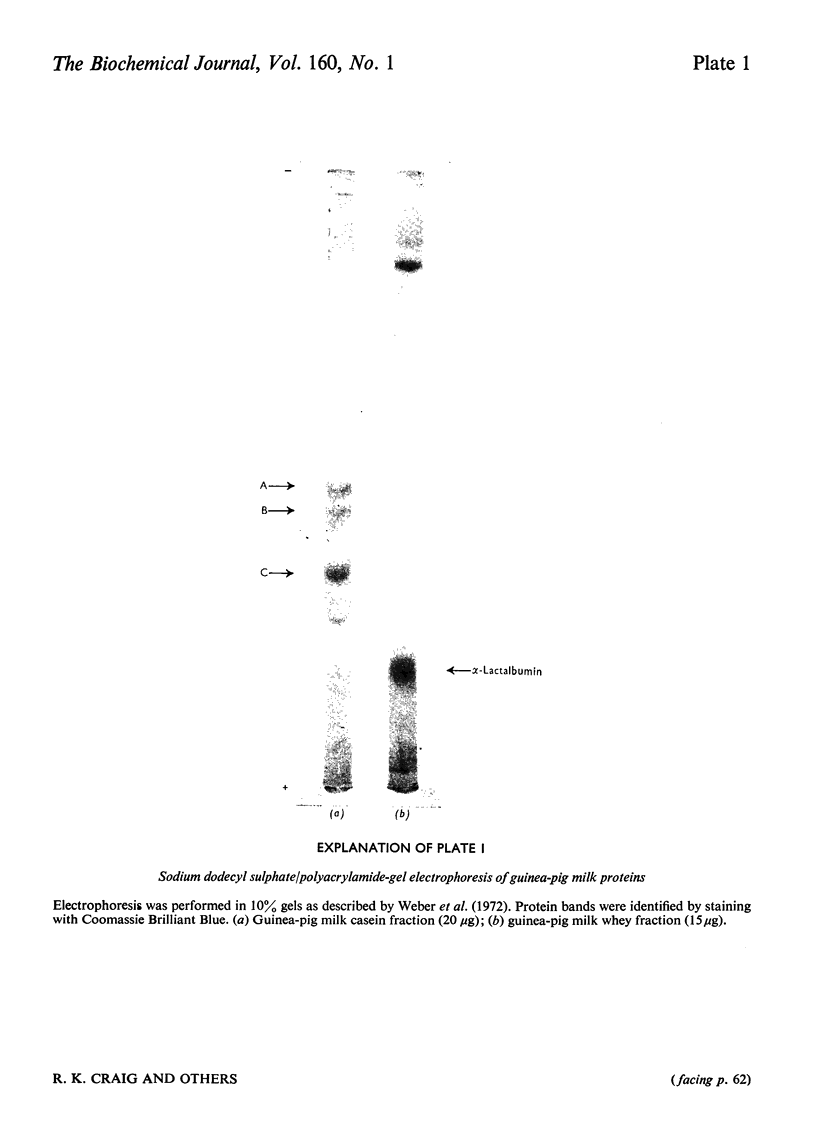
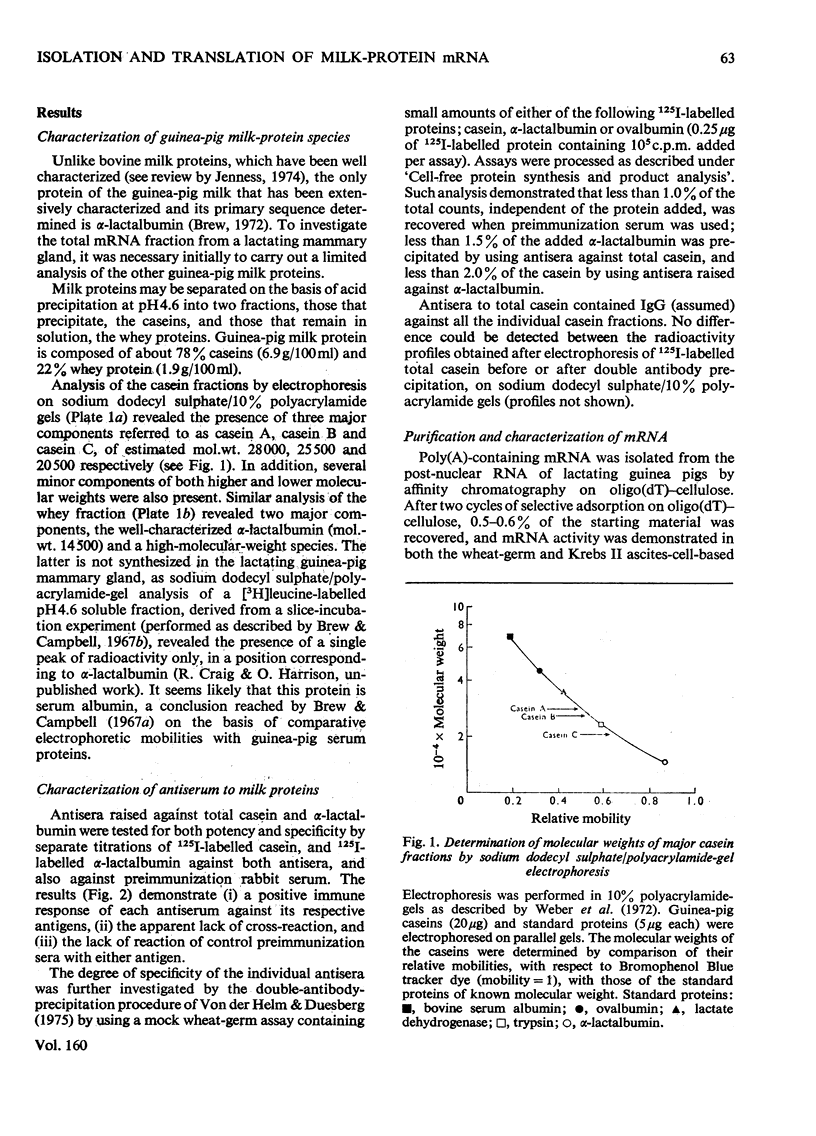
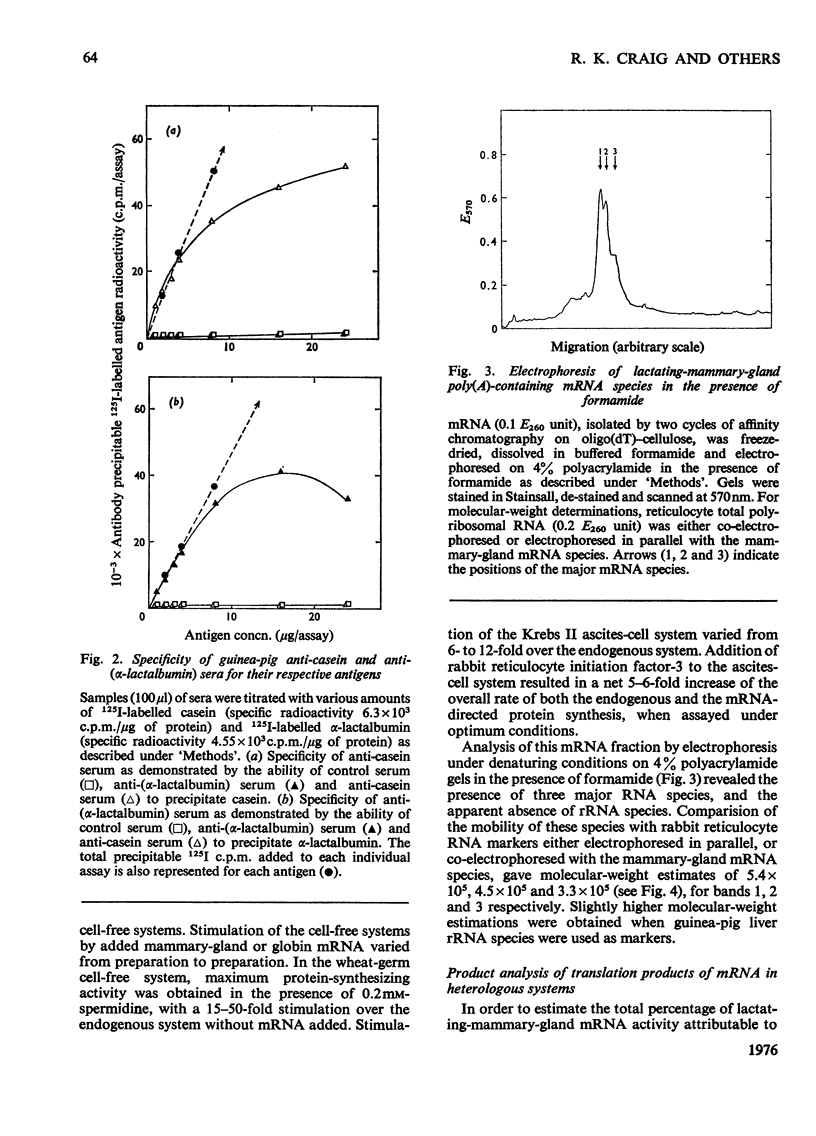
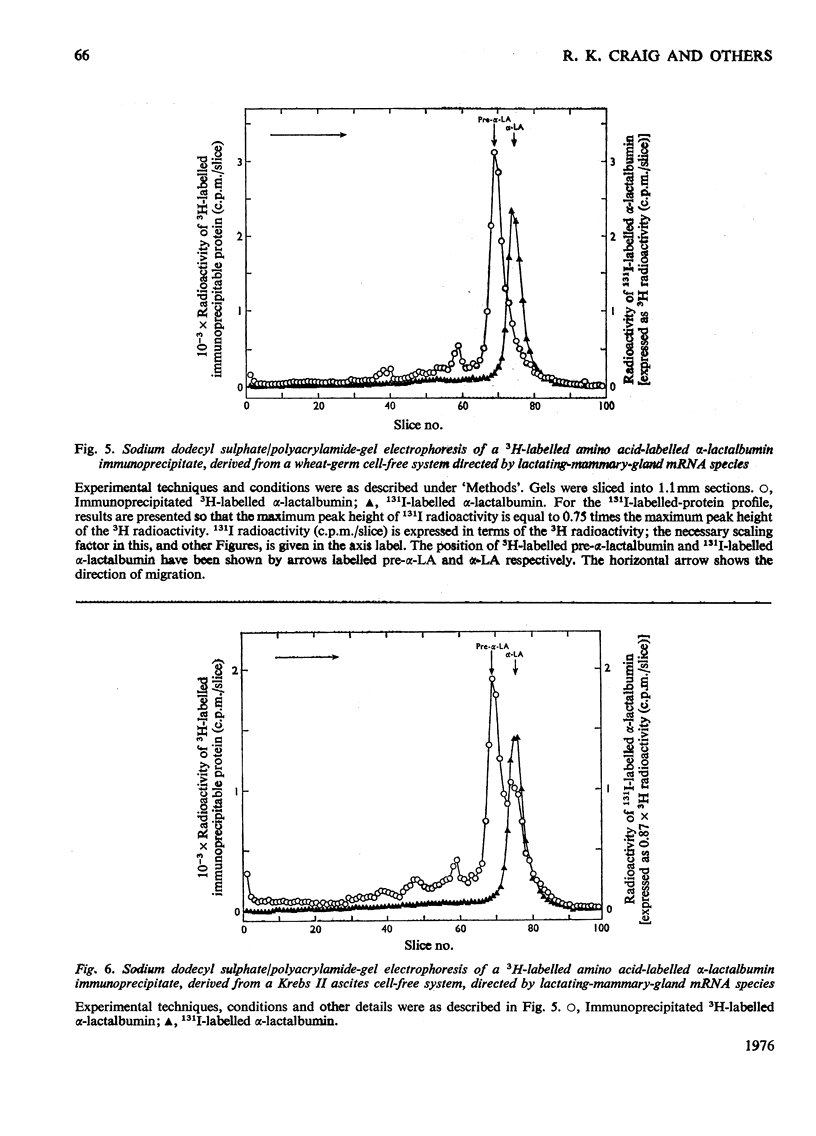
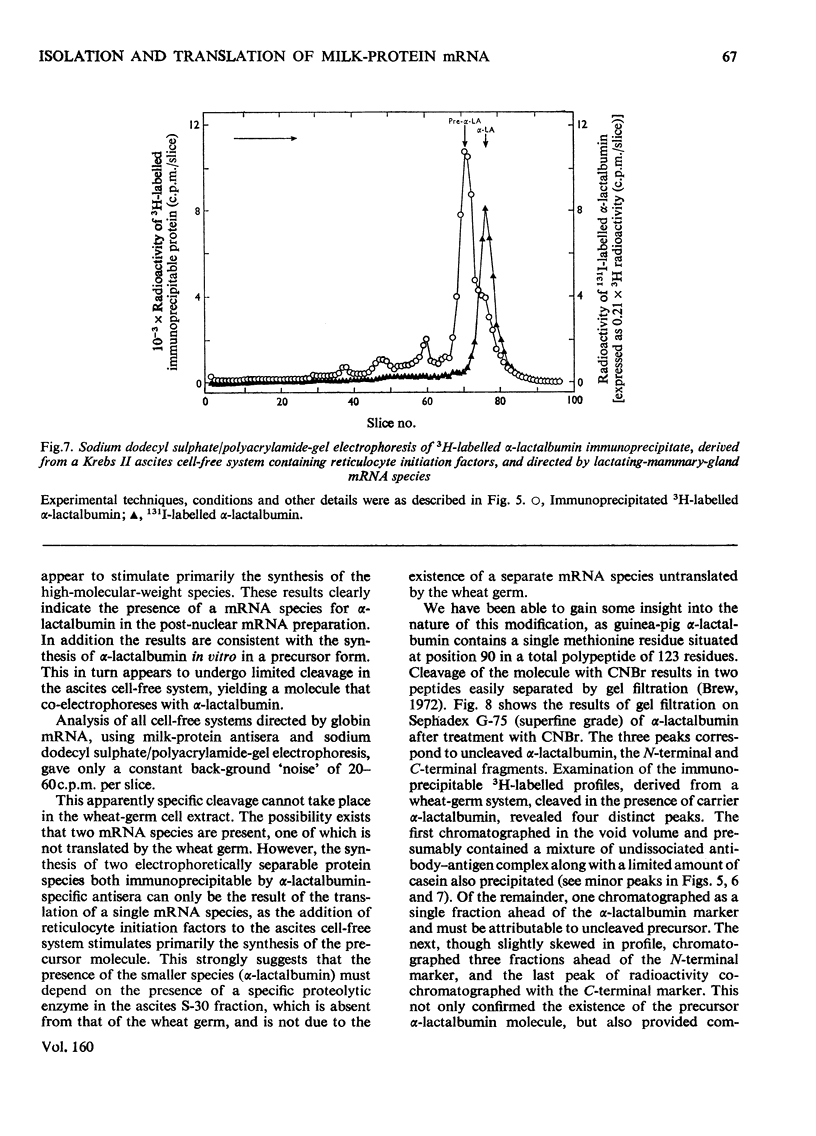
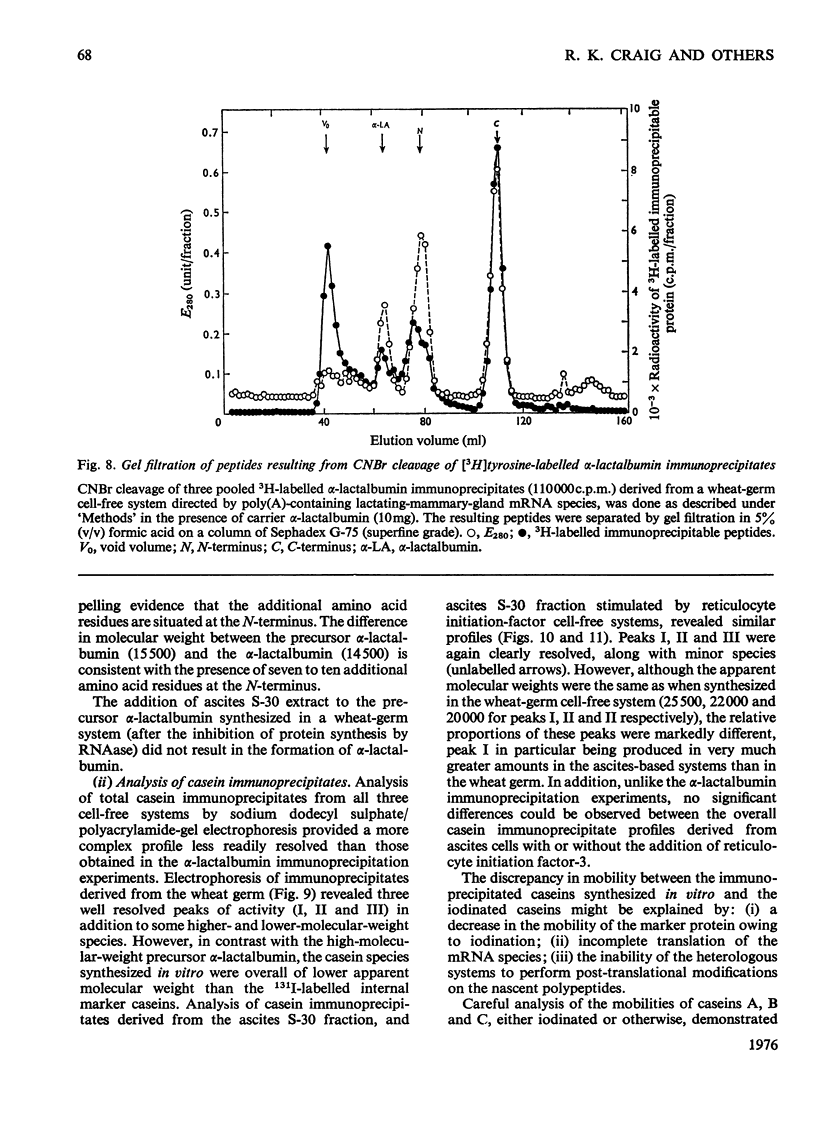
1. The major milk proteins synthesized by the lactating mammary gland of the guinea pig were identified and designated as caseins A, B and C and alpha-lactalbumin, with estimated mol.wts. of 28000, 25500, 20500 and 14500 respectively. 2. Antisera to the total casein fraction and to alpha-lactalbumin were prepared from rabbits. The milk proteins were also iodinated with either 131I or 125I. 3. A poly(A)-rich RNA fraction was isolated from lactating guinea-pig mammary glands. Isolation was by affinity chromatography on oligo(dT)-cellulose. 4. Examination of this RNA fraction by electrophoresis on polyacrylamide gels containing formamide indicated three major species 1, 2 and 3, with estimated wol.wts. of 5.4 X 10(5) and 3.3 X 10(5), and the apparent absence of rRNA species. 5. The poly(A)-rich RNA stimulated protein synthesis in heterologous cell-free systems based on wheat germ, Krebs II ascites-tumour cells, and the latter supplemented with an initiation factor-3 fraction from rabbit reticulocyte ribosomes. 6. Between 80 and 90% of the protein synthesis directed by the mRNA was for milk proteins. 7. Analysis of the proteins immunoprecipitated by the alpha-lactalbumin antiserum showed in the wheat-germ system that the product was a protein with a molecular weight greater than that of alpha-lactalbumin, whereas in the ascites-tumour-cell systems both this protein and alpha-lactalbumin were found. When the larger protein was treated with CNBr and the resulting peptides were examined, it was shown that the extra peptide was at the N-terminus. This and other evidence is adduced for the initial translation product of alpha-lactalbumin being a precursor with an addition of about ten amino acids at the N-terminus. 8. Similar analysis of the casein immlnospecific proteins produced under the direction of mRNA indicated that the products had a molecular weight that was apparently a littel smaller than that of the caseins synthesized in vivo. This was not consistent with higher-molecular weight casein precursors. 9. Possible explanations for the results obtained are discussed, especially in terms of the physiological significance of the pre-alpha-lactalbumin as a secretory protein.
Full text
PDF





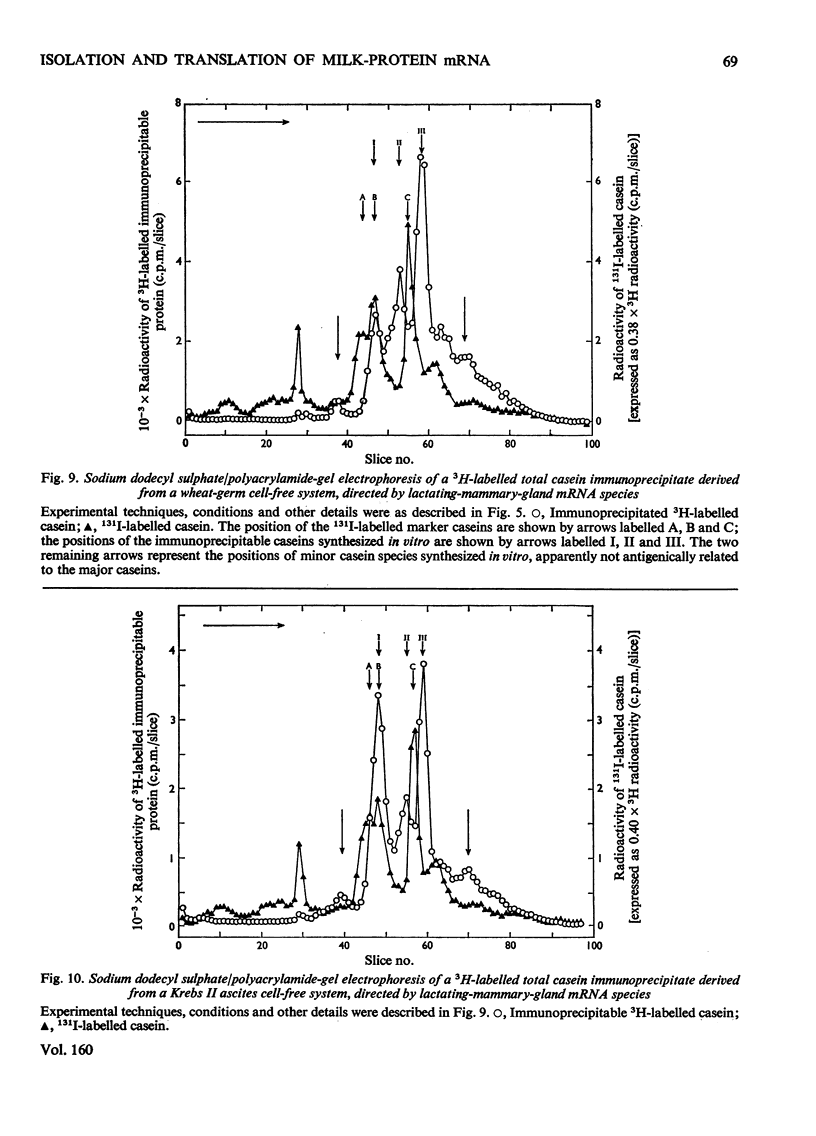
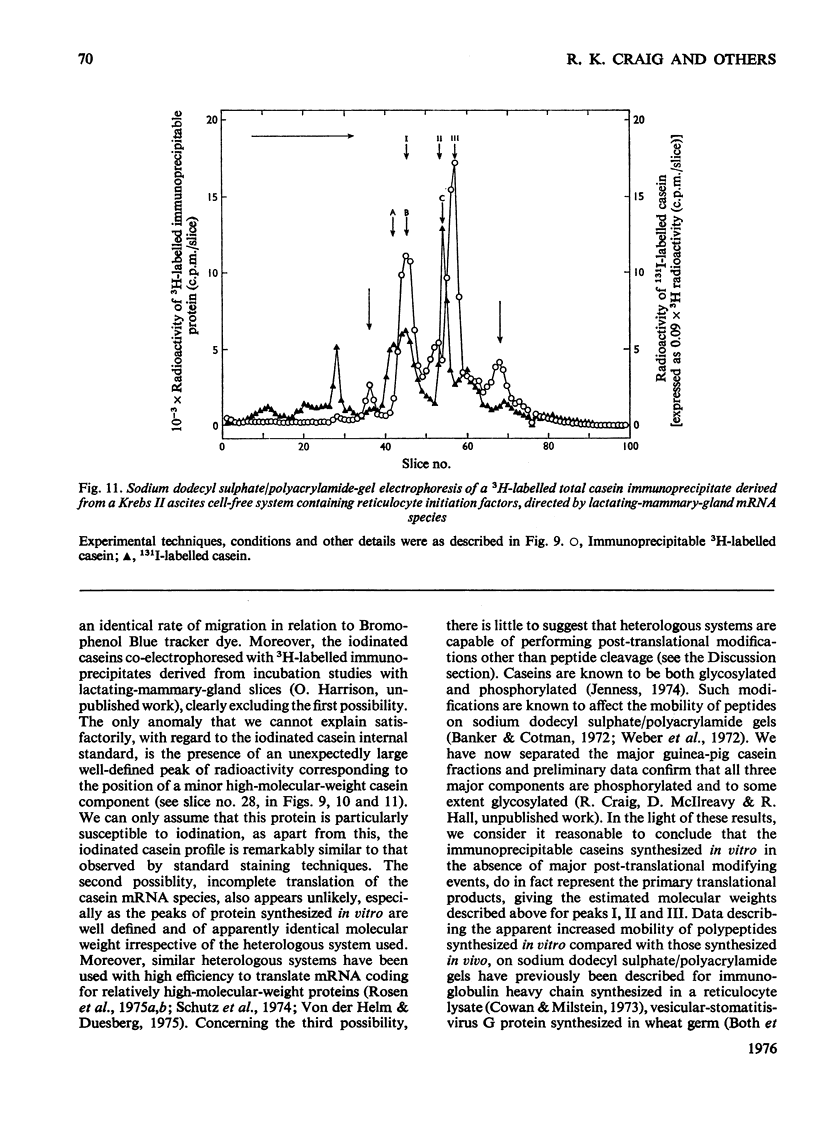




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft F. C., Wu G. J., Zubay G. Cell-free synthesis of rat growth hormone. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3646–3649. doi: 10.1073/pnas.70.12.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G. A., Cotman C. W. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972 Sep 25;247(18):5856–5861. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boime I., Boguslawski S., Caine J. The translation of a human placental lactogen mRNA fraction in heterologous cell-free systems: the synthesis of a possible precursor. Biochem Biophys Res Commun. 1975 Jan 6;62(1):103–109. doi: 10.1016/s0006-291x(75)80411-6. [DOI] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the mRNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1975 Jan;72(1):274–278. doi: 10.1073/pnas.72.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Campbell P. N. Studies on the biosynthesis of protein by lactating guinea-pig mammary gland. Characteristics of the synthesis of alpha-lactalbumin and total protein by slices and cell-free systems. Biochem J. 1967 Jan;102(1):265–274. doi: 10.1042/bj1020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Campbell P. N. The characterization of the whey proteins of guinea-pig milk. The isolation and properties of alpha-lactalbumin. Biochem J. 1967 Jan;102(1):258–264. doi: 10.1042/bj1020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Hill R. L. The isolation and characterization of the tryptic, chymotryptic, peptic, and cyanogen bromide peptides from bovine alpha-lactalbumin. J Biol Chem. 1970 Sep 10;245(17):4559–4569. [PubMed] [Google Scholar]
- Brew K. The complete amino-acid sequence of guinea-pig -lactalbumin. Eur J Biochem. 1972 May 23;27(2):341–353. doi: 10.1111/j.1432-1033.1972.tb01844.x. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM L. W., CLOUSE R. W., FORD J. D. HETEROGENEITY OF THE CARBOHYDRATE MOIETY OF CRYSTALLINE OVALBUMIN. Biochim Biophys Acta. 1963 Oct 29;78:379–381. doi: 10.1016/0006-3002(63)91652-4. [DOI] [PubMed] [Google Scholar]
- Campbell P. N., McIlreavy D., Tarin D. The detection of the messenger ribonucleic acid for the -lactalbumin of guinea-pig milk. Biochem J. 1973 May;134(1):345–347. doi: 10.1042/bj1340345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F. J., Barker R. Examination of the dissociation of multichain proteins in guanidine hydrochloride by membrane osmometry. Biochemistry. 1968 Jun;7(6):2207–2217. doi: 10.1021/bi00846a025. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Milstein C. The translation in vitro of mRNA for immunoglobulin heavy chains. Eur J Biochem. 1973 Jul 2;36(1):1–7. doi: 10.1111/j.1432-1033.1973.tb02877.x. [DOI] [PubMed] [Google Scholar]
- Cox R. F., Haines M. E., Emtage J. S. Quantitation of ovalbumin mRNA in hen and chick oviduct by hybridization to complementary DNA. Accumulation of specific mRNA in response to estradiol. Eur J Biochem. 1974 Nov 1;49(1):225–236. doi: 10.1111/j.1432-1033.1974.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Mullinix D. P., Wetekam W., Kronenberg H. M., Meyers M., Eldridge J. D., Goldberger R. F. Vitellogenin synthesis in the avian liver. Vitellogenin is the precursor of the egg yolk phosphoproteins. J Biol Chem. 1975 Dec 10;250(23):9060–9066. [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst E., McIlreavy D., Campbell P. N. The protein-synthesizing activity of ribosomes isolated from the mammary gland of lactating and pregnant guinea pigs. Biochem J. 1971 Aug;123(5):865–874. doi: 10.1042/bj1230865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. Nucleotide sequence of KB cell 5S RNA. Science. 1967 Dec 29;158(3809):1695–1699. doi: 10.1126/science.158.3809.1695. [DOI] [PubMed] [Google Scholar]
- Gaye P., Houdebine L. M. Isolation and characterization of casein mRNAs from lactating ewe mammary glands. Nucleic Acids Res. 1975 May;2(5):707–722. doi: 10.1093/nar/2.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D., Matzura H. An improved method of counting radioactive acrylamide gels. Anal Biochem. 1971 Aug;42(2):481–486. doi: 10.1016/0003-2697(71)90062-5. [DOI] [PubMed] [Google Scholar]
- Gould H. J., Hamlyn P. H. The molecular weight of rabbit globin messenger RNA's. FEBS Lett. 1973 Mar 15;30(3):301–304. doi: 10.1016/0014-5793(73)80674-x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Kemper B., Potts J. T., Jr, Rich A. Parathyroid mRNA directs the synthesis of pre-proparathyroid hormone and proparathyroid hormone in the Krebs ascites cell-free system. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1114–1121. doi: 10.1016/0006-291x(75)90789-5. [DOI] [PubMed] [Google Scholar]
- Haines M. E., Carey N. H., Palmiter R. S. Purification and properties of ovalbumin messenger RNA. Eur J Biochem. 1974 Apr 16;43(3):549–560. doi: 10.1111/j.1432-1033.1974.tb03442.x. [DOI] [PubMed] [Google Scholar]
- Harrison O. S., Craig R. K., Campbell P. N. Isolation and characterization of messenger ribonucleic acid species for guinea-pig milk proteins from free and membrane-bound polyribosomes. Biochem Soc Trans. 1976;4(2):340–341. doi: 10.1042/bst0040340. [DOI] [PubMed] [Google Scholar]
- Harrison P. R., Birnie G. D., Hell A., Humphries S., Young B. D., Paul J. Kinetic studies of gene frequency. I. Use of a DNA copy of reticulocyte 9 S RNA to estimate globin gene dosage in mouse tissues. J Mol Biol. 1974 Apr 25;84(4):539–554. doi: 10.1016/0022-2836(74)90115-6. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M., Gaye P. Absence of mRNA for casein in free polysomes of lactating ewe mammary gland. Nucleic Acids Res. 1975 Feb;2(2):165–178. doi: 10.1093/nar/2.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdebine L. M., Gaye P. Regulation of casein synthesis in the rabbit mammary gland. Titration of mRNA activity for casein under prolactin and progesterone treatments. Mol Cell Endocrinol. 1975 Jul;3(1):37–55. doi: 10.1016/0303-7207(75)90030-1. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Mulligan R. C., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: a direct translation product of parathyroid messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3731–3735. doi: 10.1073/pnas.71.9.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardi P. M., Williamson R., Brown D. D. The size of fibroin messenger RNA and its polyadenylic acid content. Cell. 1975 Mar;4(3):199–205. doi: 10.1016/0092-8674(75)90168-3. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Pennequin P., Schimke R. T. Induction of ovalbumin mRNA sequences by estrogen and progesterone in chick oviduct as measured by hybridization to complementary DNA. J Biol Chem. 1975 Oct 25;250(20):8105–8110. [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Nakazato H., Edmonds M. Purification of messenger RNA and heterogeneous nuclear RNA containing poly(a) sequences. Methods Enzymol. 1974;29:431–443. doi: 10.1016/0076-6879(74)29035-9. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Quinn P. S., Gamble M., Judah J. D. Biosynthesis of serum albumin in rat liver. Isolation and probable structure of 'proalbumin' from rat liver. Biochem J. 1975 Feb;146(2):389–393. doi: 10.1042/bj1460389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg H., Wehrli W., Staehelin M. Isolation of mammalian transfer RNA. Biochim Biophys Acta. 1969 Nov 19;195(1):13–15. doi: 10.1016/0005-2787(69)90597-8. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Comstock J. P. Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry. 1975 Jul;14(13):2895–2903. doi: 10.1021/bi00684a016. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Ross J., Gielen J., Packman S., Ikawa Y., Leder P. Globin gene expression in cultured erythroleukemic cells. J Mol Biol. 1974 Aug 25;87(4):697–714. doi: 10.1016/0022-2836(74)90079-5. [DOI] [PubMed] [Google Scholar]
- Russell J. H., Geller D. M. The structure of rat proalbumin. J Biol Chem. 1975 May 10;250(9):3409–3413. [PubMed] [Google Scholar]
- Schutz G., Beato M., Feigelson P. Isolation on cellulose of ovalbumin and globin mRNA and their translation in an ascites cell-free system. Methods Enzymol. 1974;30:701–708. doi: 10.1016/0076-6879(74)30067-5. [DOI] [PubMed] [Google Scholar]
- Soulier S., Ribadeau-Dumas B., Denamur R. Purification des caséines kappa de brebis. Analyse des parties glycanne et peptidique. Eur J Biochem. 1975 Jan 2;50(2):445–452. doi: 10.1111/j.1432-1033.1975.tb09822.x. [DOI] [PubMed] [Google Scholar]
- Stavis R. L., August J. T. The biochemistry of RNA bacteriophage replication. Annu Rev Biochem. 1970;39:527–560. doi: 10.1146/annurev.bi.39.070170.002523. [DOI] [PubMed] [Google Scholar]
- Sussman P. M., Tushinski R. J., Bancroft F. C. Pregrowth hormone: product of the translation in vitro of messenger RNA coding for growth hormone. Proc Natl Acad Sci U S A. 1976 Jan;73(1):29–33. doi: 10.1073/pnas.73.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Guttman N., Baltimore D., Lodishi H. F. Complete translation of poliovirus RNA in a eukaryotic cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4157–4161. doi: 10.1073/pnas.72.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Woo S. L., Rosen J. M., Liarakos C. D., Choi Y. C., Busch H., Means A. R., O'Malley Physical and chemical characterization of purified ovalbumin messenger RNA. J Biol Chem. 1975 Sep 10;250(17):7027–7039. [PubMed] [Google Scholar]
- von der Helm K., Duesberg P. H. Translation of Rous sarcoma virus RNA in a cell-free system from ascites Krebs II cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):614–618. doi: 10.1073/pnas.72.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]