Abstract
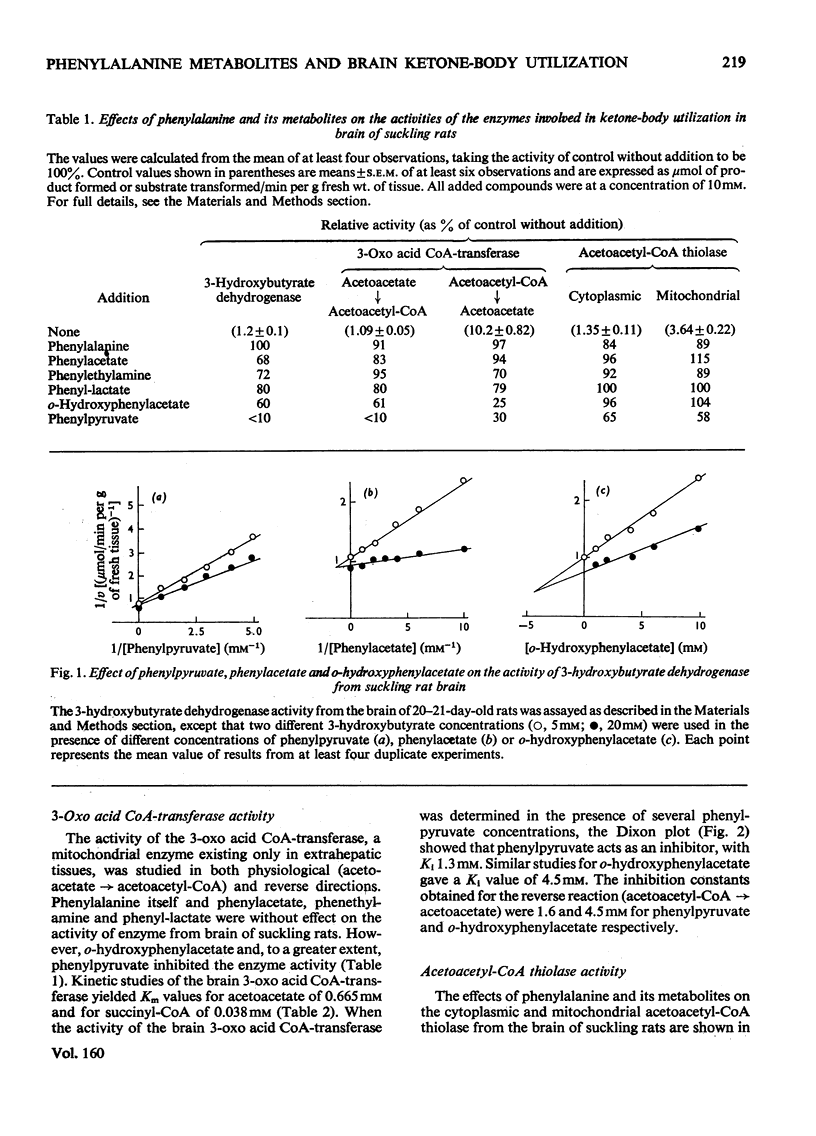
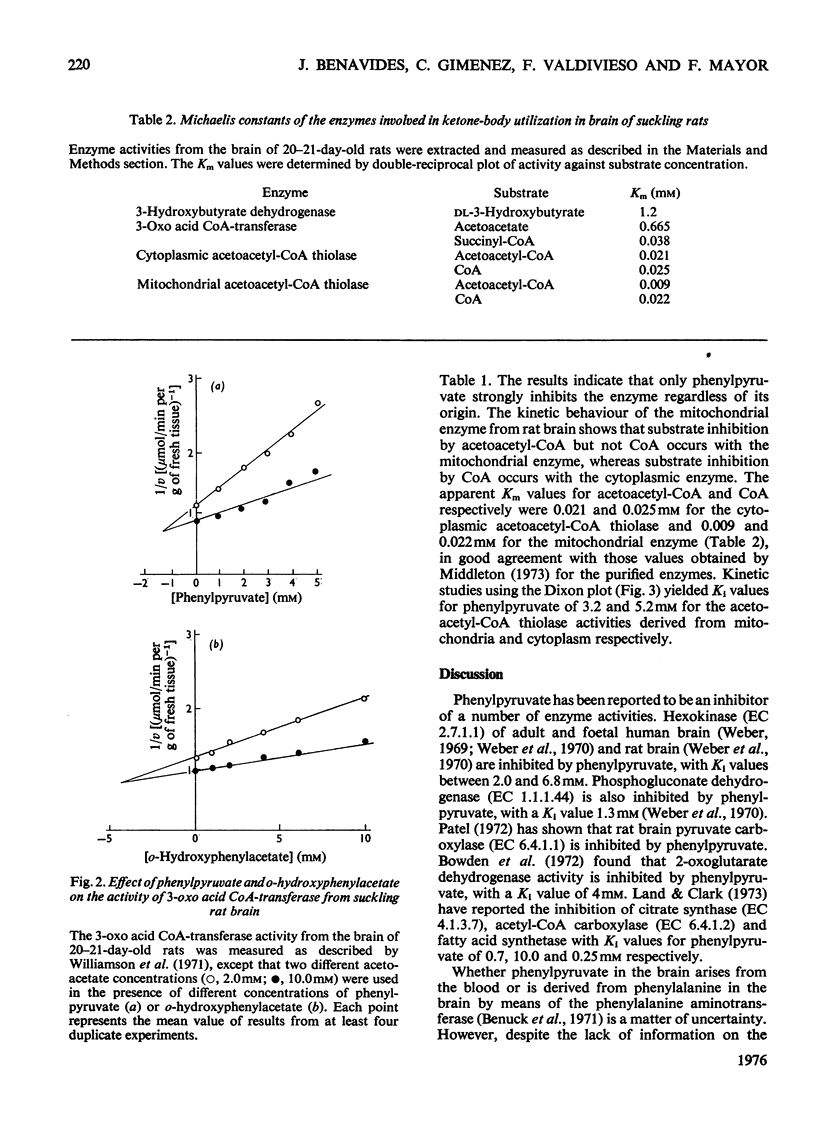
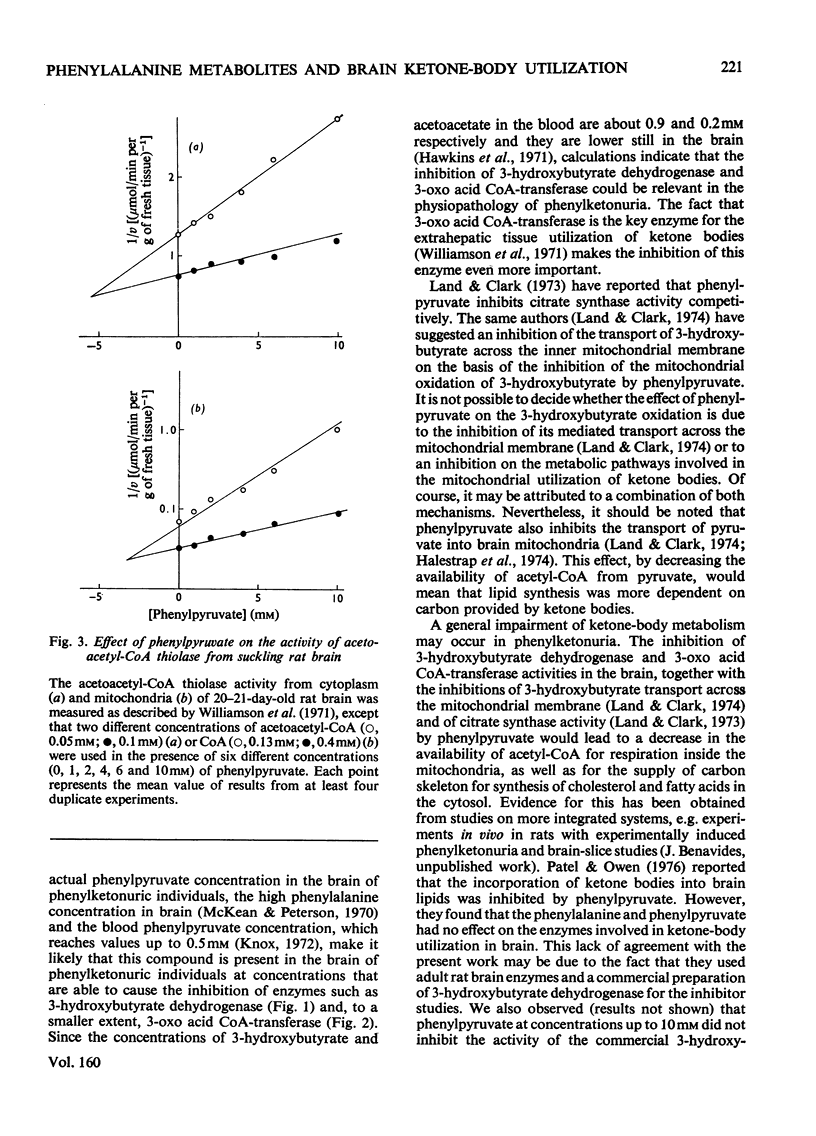
1. The effects of phenylalanine and its metabolites (phenylacetate, phenethylamine, phenyl-lactate, o-hydroxyphenylacetate and phenylpyruvate) on the activity of 3-hydroxybutyrate dehydrogenase (EC 1.1.1.30) 3-oxo acid CoA-transferase (EC 2.8.3.5) and acetoacetyl-CoA thiolase (EC 2.3.1.9) in brain of suckling rats were investigated. 2. The 3-hydroxybutyrate dehydrogenase from the brain of suckling rats had a Km for 3-hydroxybutyrate of 1.2 mM. Phenylpyruvate, phenylacetate and o-hydroxyphenylacetate inhibited the enzyme activity with Ki values of 0.5, 1.3 and 4.7 mM respectively. 3. The suckling-rat brain 3-oxo acid CoA-transferase activity had a Km for acetoacetate of 0.665 mM and for succinyl (3-carboxypropionyl)-CoA of 0.038 mM. The enzyme was inhibited with respect to acetoacetate by phenylpyruvate (Ki equals 1.3 mM) and o-hydroxyphenylacetate (Ki equals 4.5 mM). The reaction in the direction of acetoacetate was also inhibited by phenylpyruvate (Ki equals 1.6 mM) and o-hydroxyphenylacetate (Ki equals 4.5 mM). 4. Phenylpyruvate inhibited with respect to acetoacetyl-CoA both the mitochondrial (Ki equals 3.2 mM) and cytoplasmic (Ki equals 5.2 mM) acetoacetyl-CoA thiolase activities. 5. The results suggest that inhibition of 3-hydroxybutyrate dehydrogenase and 3-oxo acid CoA-transferase activities may impair ketone-body utilization and hence lipid synthesis in the developing brain. This suggestion is discussed with reference to the pathogenesis of mental retardation in phenylketonuria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benuck M., Stern F., Lajtha A. Transamination of amino acids in homogenates of rat brzain. J Neurochem. 1971 Aug;18(8):1555–1567. doi: 10.1111/j.1471-4159.1971.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Edmond J. Ketone bodies as precursors of sterols and fatty acids in the developing rat. J Biol Chem. 1974 Jan 10;249(1):72–80. [PubMed] [Google Scholar]
- Halestrap A. P., Brand M. D., Denton R. M. Inhibition of mitochondrial pyruvate transport by phenylpyruvate and alpha-ketoisocaproate. Biochim Biophys Acta. 1974 Oct 10;367(1):102–108. doi: 10.1016/0005-2736(74)90140-0. [DOI] [PubMed] [Google Scholar]
- JERVIS G. A. Phenylpyruvic oligophrenia deficiency of phenylalanine-oxidizing system. Proc Soc Exp Biol Med. 1953 Mar;82(3):514–515. [PubMed] [Google Scholar]
- Klee C. B., Sokoloff L. Changes in D(--)-beta-hydroxybutyric dehydrogenase activity during brain maturation in the rat. J Biol Chem. 1967 Sep 10;242(17):3880–3883. [PubMed] [Google Scholar]
- LEHNINGER A. L., SUDDUTH H. C., WISE J. B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960 Aug;235:2450–2455. [PubMed] [Google Scholar]
- Land J. M., Clark J. B. Effect of phenylpyruvate on enzymes involved in fatty acid synthesis in rat brain. Biochem J. 1973 Jun;134(2):545–555. doi: 10.1042/bj1340545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land J. M., Clark J. B. Inhibition of pyruvate and beta-hydroxybutyrate oxidation in rat brain mitochondria by phenylpyruvate and alpha-ketoisocaproate. FEBS Lett. 1974 Aug 30;44(3):348–351. [PubMed] [Google Scholar]
- McKean C. M., Peterson N. A. Glutamine in the phenylketonuric central nervous system. N Engl J Med. 1970 Dec 17;283(25):1364–1367. doi: 10.1056/NEJM197012172832503. [DOI] [PubMed] [Google Scholar]
- Middleton B. The acetoacetyl-coenzyme A thiolases of rat brain and their relative activities during postnatal development. Biochem J. 1973 Apr;132(4):731–737. doi: 10.1042/bj1320731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. A., Williamson D. H. Enzymes of ketone-body utilisation in human brain. Lancet. 1971 Jul 10;2(7715):66–68. doi: 10.1016/s0140-6736(71)92044-7. [DOI] [PubMed] [Google Scholar]
- Patel M. S., Owen O. E. Effect of hyperphenylalaninaemia on lipid synthesis from ketone bodies by rat brain. Biochem J. 1976 Feb 15;154(2):319–325. doi: 10.1042/bj1540319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. S. The effect of phenylpyruvate on pyruvate metabolism in rat brain. Biochem J. 1972 Jul;128(3):677–684. doi: 10.1042/bj1280677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. N., Peterson N. A., McKean C. M. Impaired myelin formation in experimental hyperphenylalaninaemia. J Neurochem. 1972 Feb;19(2):479–485. doi: 10.1111/j.1471-4159.1972.tb01357.x. [DOI] [PubMed] [Google Scholar]
- Shah S. N., Peterson N. A., McKean C. M. Lipid composition of human cerebral white matter and myelin in phenylketonuria. J Neurochem. 1972 Oct;19(10):2369–2376. doi: 10.1111/j.1471-4159.1972.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Tildon J. T., Cone A. L., Cornblath M. Coenzyme A transferase activity in rat brain. Biochem Biophys Res Commun. 1971 Apr 2;43(1):225–231. doi: 10.1016/s0006-291x(71)80111-0. [DOI] [PubMed] [Google Scholar]
- Tildon J. T., Sevdalian D. A. CoA transferase in the brain and other mammalian tissues. Arch Biochem Biophys. 1972 Feb;148(2):382–390. doi: 10.1016/0003-9861(72)90155-5. [DOI] [PubMed] [Google Scholar]
- Weber G., Glazer R. I., Ross R. A. Regulation of human and rat brain metabolism: inhibitory action of phenylalanine and phenylpyruvate on glycolysis, protein, lipid, DNA, and RNA metabolism. Adv Enzyme Regul. 1970;8:13–36. doi: 10.1016/0065-2571(70)90006-3. [DOI] [PubMed] [Google Scholar]
- Weber G. Inhibition of human brain pyruvate kinase and hexokinase by phenylalanine and phenylpyruvate: possible relevance to phenylketonuric brain damage. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1365–1369. doi: 10.1073/pnas.63.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


