Abstract
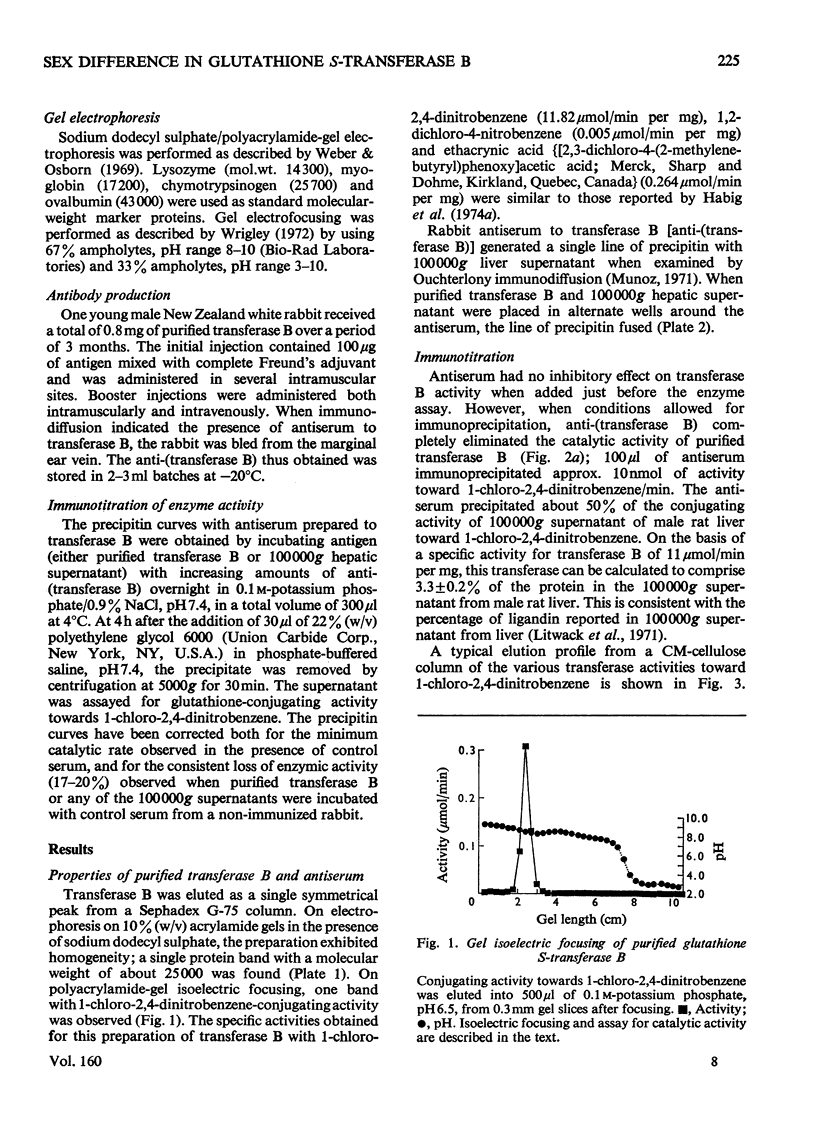
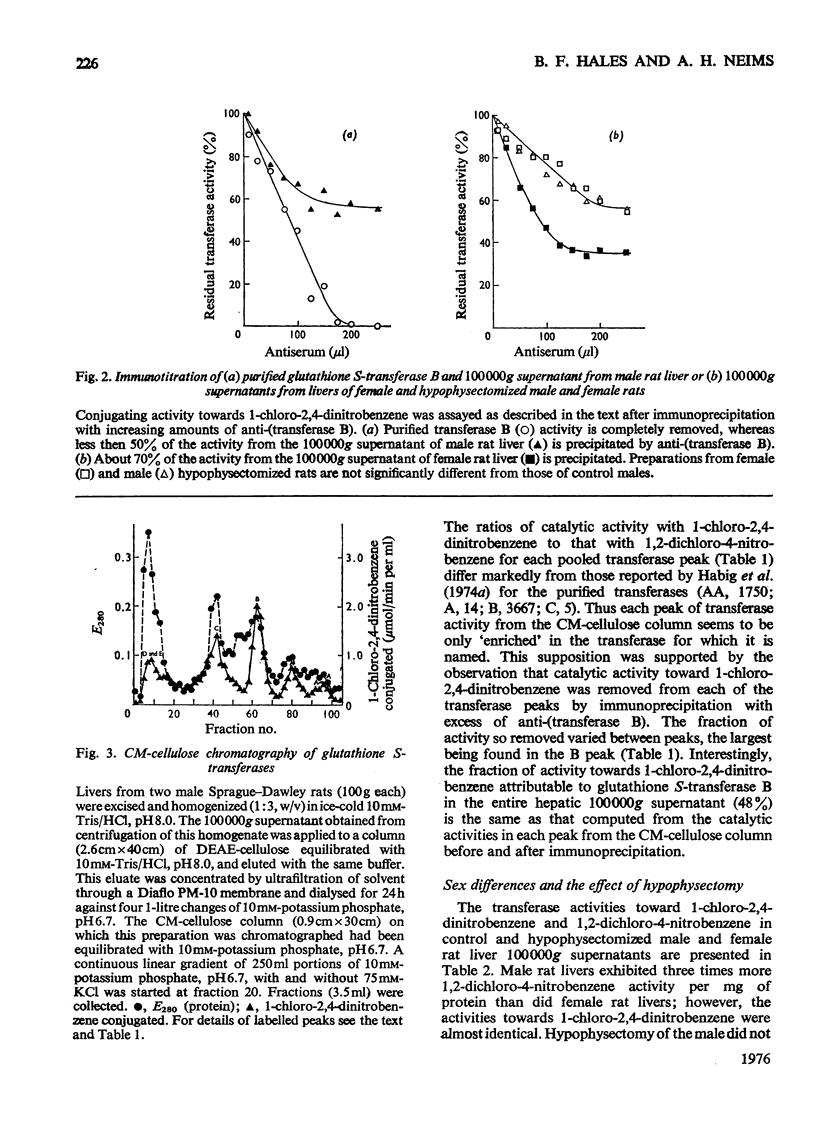
The glutathione S-transferases are a group of proteins with overlapping substrate specificities and ligand-binding capacities. This report examines certain approaches to the measurement of transferase B (ligandin) in the rat liver. The ratio of catalytic activities toward 1-chloro-2,4-dinitrobenzene and 1,2-dichloro-4-nitrobenzene gives some indication of the relative proportions of the various transferases present in 100 000 g supernatants. The fraction of catalytic activity towards 1-chloro-2,4-dinitrobenzene, due to transferase B, was best measured by immunoprecipitation with anti-(transferase B). Male rat liver exhibited three times more activity towards 1,2-dichloro-4-nitrobenzene than female tissue; however, the activities towards 1-chloro-2,4-dinitrobenzene were almost identical. By assuming a specific activity of 11 mumol/min per mg, immunoprecipitable transferase B comprised 4.5 +/- 0.2% of total protein in the 100 000 g supernatant of female rat liver, and 70% of the transferase activity towards 1-chloro-2,4-dinitrobenzene. The amount of transferase B in the 100 000 g supernatant from male rat liver is significantly lower with respect to both fraction of total protein (3.3 +/- 0.2%) and overall transferase activity towards 1-chloro-2,4-dinitrobenzene (48%). Hypophysectomy eliminated this sex difference in the hepatic concentration of glutathione S-transferase B.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darby F. J., Grundy R. K. Glutathione S-aryltransferase: the effect of treating male and female rats with phenobarbitone on the apparent kinetic parameters for the conjugation of 1,2-dichloro-4-nitrobenzene and 1-chloro-2,4-dinitrobenzene with glutathione. Biochem J. 1972 Jun;128(1):175–177. doi: 10.1042/bj1280175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjellstedt T. A., Allen R. H., Duncan B. K., Jakoby W. B. Enzymatic conjugation of epoxides with glutathione. J Biol Chem. 1973 May 25;248(10):3702–3707. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Fleischner G., Gatmaitan Z., Arias I. M., Jakoby W. B. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Kaplowitz N., Kuhlekamp J., Clifton G. Drug induction of hepatic glutathione S-transferases in male and female rats*. Biochem J. 1975 Feb;146(2):351–356. doi: 10.1042/bj1460351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketley J. N., Habig W. H., Jakoby W. B. Binding of nonsubstrate ligands to the glutathione S-transferases. J Biol Chem. 1975 Nov 25;250(22):8670–8673. [PubMed] [Google Scholar]
- Ketterer B., Ross-Mansell P., Whitehead J. K. The isolation of carcinogen-binding protein from livers of rats given 4-dimethylaminoazobenzene. Biochem J. 1967 May;103(2):316–324. doi: 10.1042/bj1030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- Morey K. S., Litwack G. Isolation and properties of cortisol metabolite binding proteins of rat liver cytosol. Biochemistry. 1969 Dec;8(12):4813–4821. doi: 10.1021/bi00840a024. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Habig W. H., Jakoby W. B. Glutathione S-transferase A. A novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J Biol Chem. 1974 Nov 25;249(22):7140–7147. [PubMed] [Google Scholar]
- Reyes H., Levi A. J., Gatmaitan Z., Arias I. M. Studies of Y and Z, two hepatic cytoplasmic organic anion-binding proteins: effect of drugs, chemicals, hormones, and cholestasis. J Clin Invest. 1971 Nov;50(11):2242–2252. doi: 10.1172/JCI106721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]




