Abstract
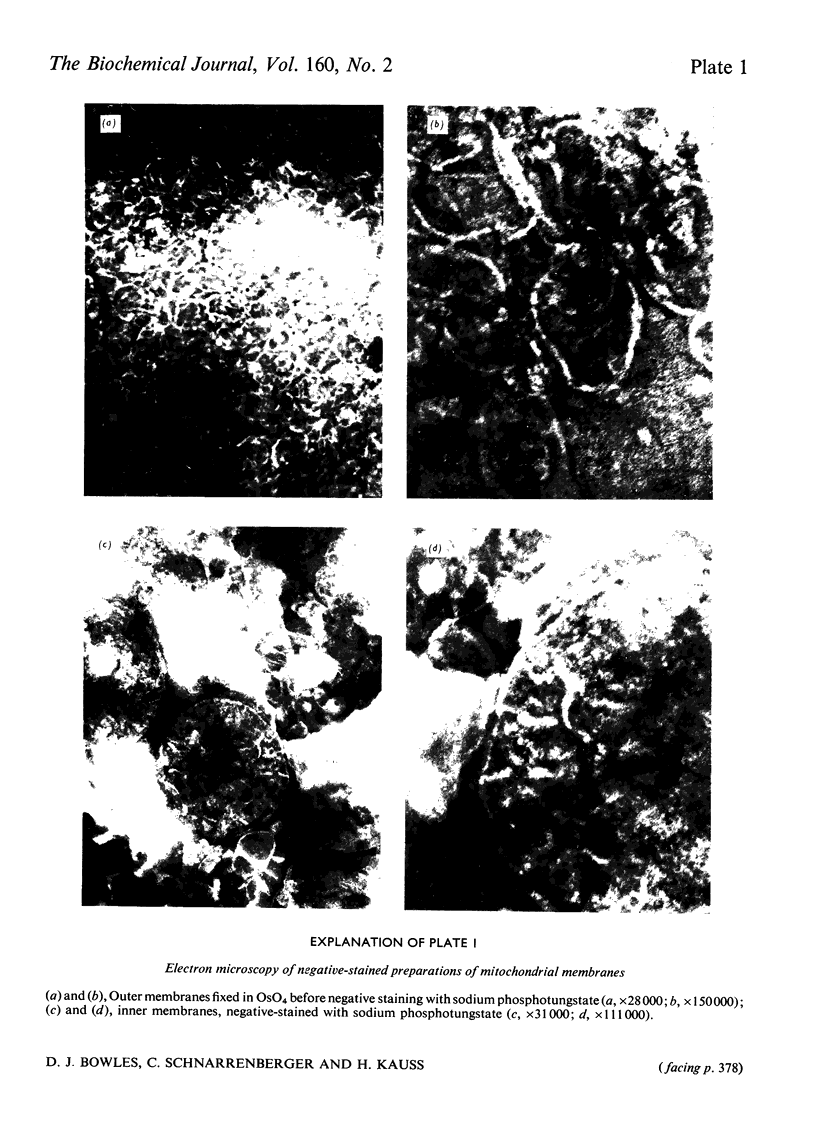
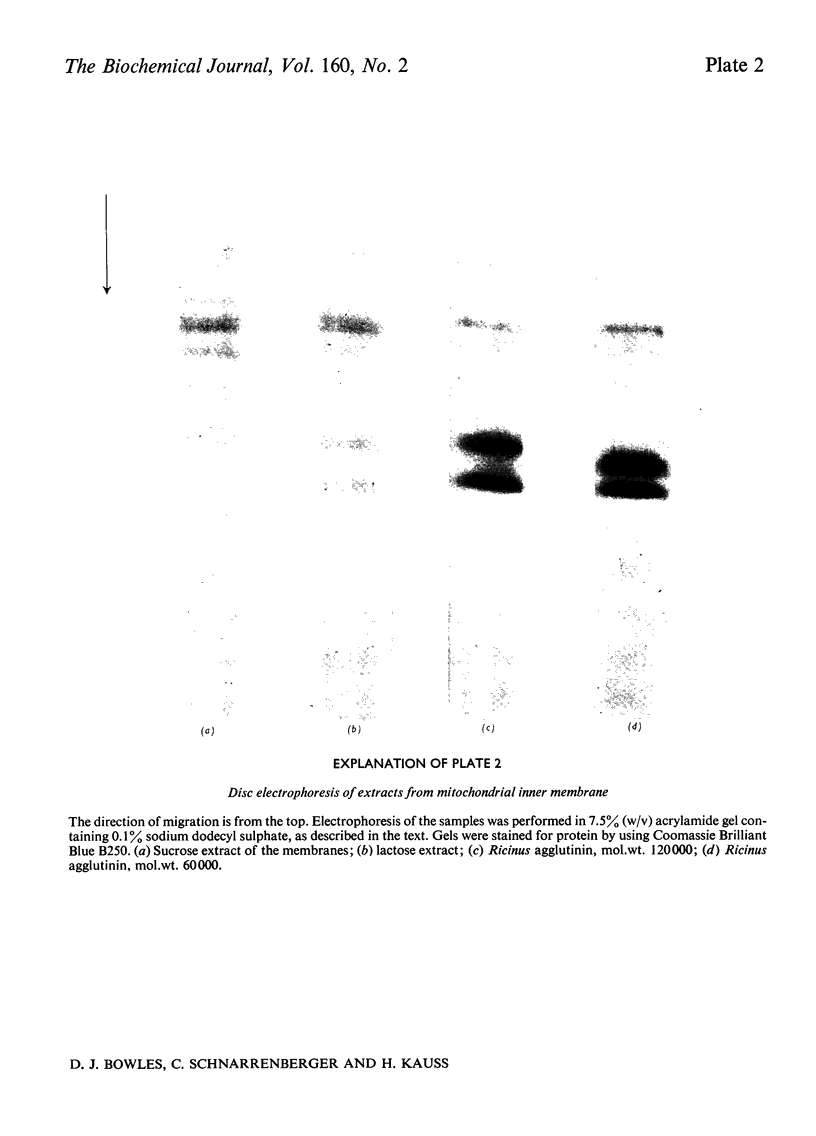
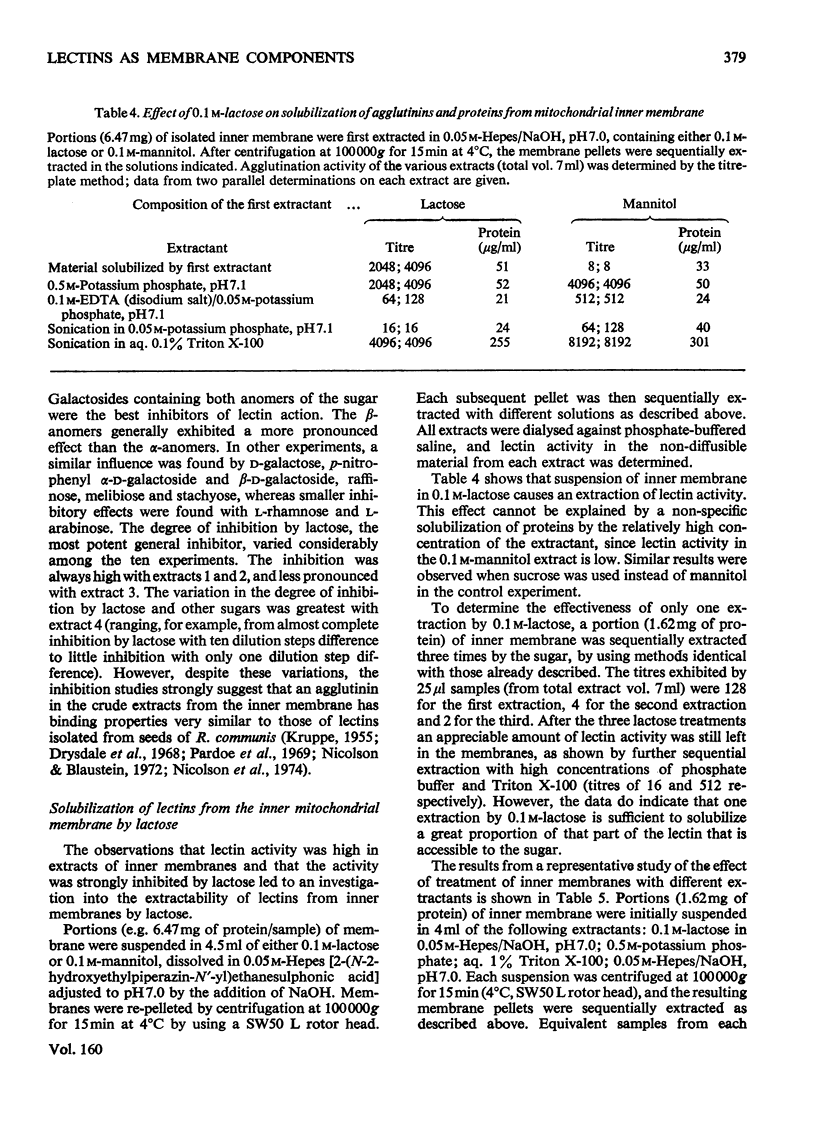
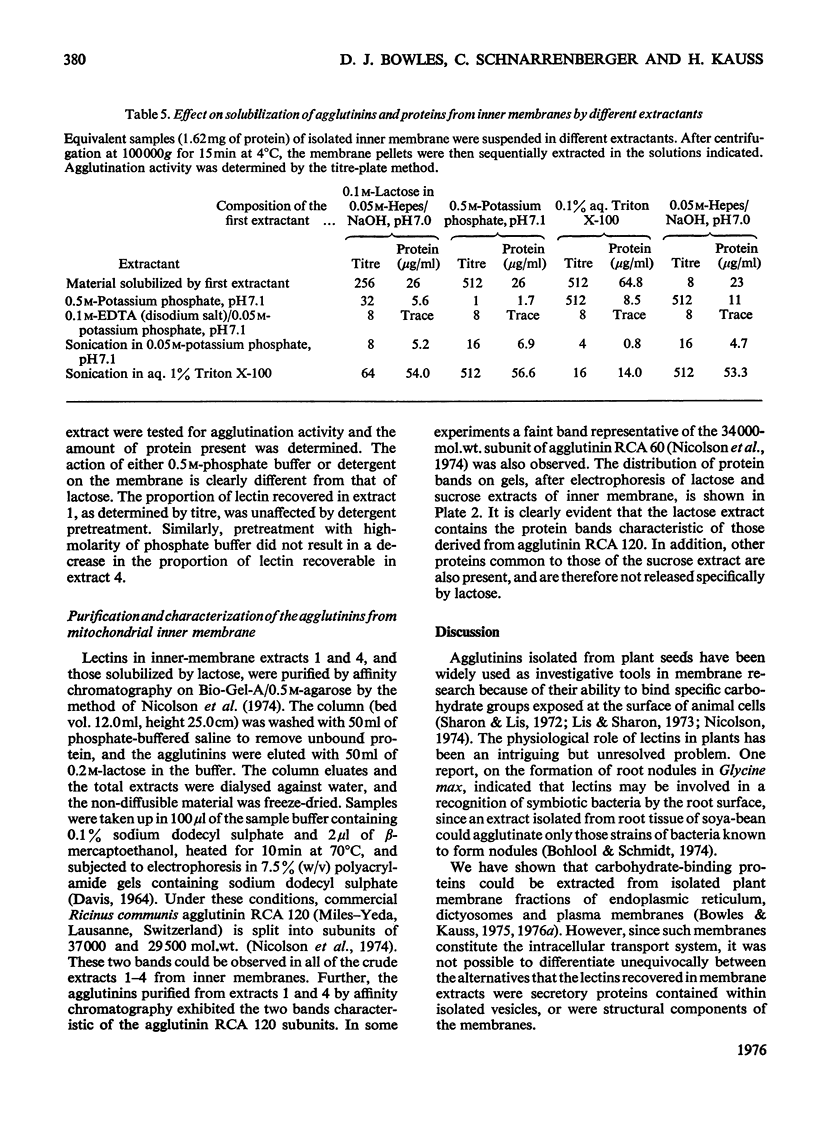
1. Mitochondria were isolated from developing endosperm of Ricinus communis and were fractionated into outer membrane and inner membrane. The relative purity of the two membrane fractions was determined by marker enzymes. The fractions were also examined by negative-stain electron microscopy. 2. Membrane fractions were sequentially extracted in the following way. (a) Suspension in 0.5M-potassium phosphate, pH7.1; (b)suspension in 0.1M-EDTA (disodium salt)/0.05M-potassium phosphate, pH7.1; (c) sonication in 0.05M-potassium phosphate, pH7.1;(d)sonication in aq. Triton X-100 (0.1%). The membranes were pelleted by centrifugation at 100 000g for 15 min, between each step. Agglutination activity in the extracts was investigated by using trypsin-treated rabbit erythrocytes. 3. The addition of lactose to inner mitochondrial membrane resulted in the solubilization of part of the lectin activity, indicating that the protein was attached to the membrane via its carbohydrate-binding site. Pretreatment of the membranes with lactose before tha usual extraction procedure showed that lactose could extract lectins that normally required more harsh treatment of the membrane for solubilization. 4. Lectins extracted from inner membranes were purified by affinity chromatography on agarose gel. Polyacrylamide-gel electrophoresis of purified samples in sodium dodecyl sulphate indicated that at least part of the lectin present in inner mitochondrial membrane was identical with the R. communis agglutinin of mol.wt. 120 000.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Bowles D. J., Kauss H. Isolation of a lectin from liver plasma membrane and its binding to cellular membrane receptors in vitro. FEBS Lett. 1976 Jul 1;66(1):16–19. doi: 10.1016/0014-5793(76)80574-1. [DOI] [PubMed] [Google Scholar]
- Bowles D. J., Northcote D. H. The amounts and rates of export of polysaccharides found within the membrane system of maize root cells. Biochem J. 1974 Jul;142(1):139–144. doi: 10.1042/bj1420139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Northcote D. H. The sites of synthesis and transport of extracellular polysaccharides in the root tissues of maize. Biochem J. 1972 Dec;130(4):1133–1145. doi: 10.1042/bj1301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. Membrane-bound enzymes and membrane ultrastructure. Biochim Biophys Acta. 1973 Apr 3;300(1):1–30. doi: 10.1016/0304-4157(73)90010-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Drysdale R. G., Herrick P. R., Franks D. The specificity of the haemagglutinin of the Castor bean, Ricinus communis. Vox Sang. 1968;15(3):194–202. doi: 10.1111/j.1423-0410.1968.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Zahler W. L., Ozawa H. The extraction of structural protein from submitochondrial vesicles. Biochem Biophys Res Commun. 1968 Sep 30;32(6):1031–1038. doi: 10.1016/0006-291x(68)90133-2. [DOI] [PubMed] [Google Scholar]
- Gulik-Krzywicki T. Structural studies of the associations between biological membrane components. Biochim Biophys Acta. 1975 Mar 25;415(1):1–28. doi: 10.1016/0304-4157(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Structures and organization of cell surface glycolipids dependency on cell growth and malignant transformation. Biochim Biophys Acta. 1975 Mar 20;417(1):55–89. doi: 10.1016/0304-419x(75)90008-6. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Kauss H., Glaser C. Carbohydrate-binding proteins from plant cell walls and their possible involvement in extension growth. FEBS Lett. 1974 Sep 1;45(1):304–307. doi: 10.1016/0014-5793(74)80867-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Maisterrena B., Comte J., Gautheron D. C. Purification of pig heart mitochondrial membranes. Enzymatic and morphological characterization as compared to microsomes. Biochim Biophys Acta. 1974 Oct 29;367(2):115–126. doi: 10.1016/0005-2736(74)90036-4. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Nowak T. P., Haywood P. L., Barondes S. H. Developmentally regulated lectin in embryonic chick muscle and a myogenic cell line. Biochem Biophys Res Commun. 1976 Feb 9;68(3):650–657. doi: 10.1016/0006-291x(76)91195-5. [DOI] [PubMed] [Google Scholar]
- Pardoe G. I., Bird G. W., Uhlenbruck G. On the specificity of lectins with a broad agglutination spectrum. I. The nature of the specific receptors for Ricinus communis and Solanum tuberosum Lectins. Z Immunitatsforsch Allerg Klin Immunol. 1969 May;137(5):442–457. [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion. Biochim Biophys Acta. 1973 Dec 28;300(4):421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]