Abstract
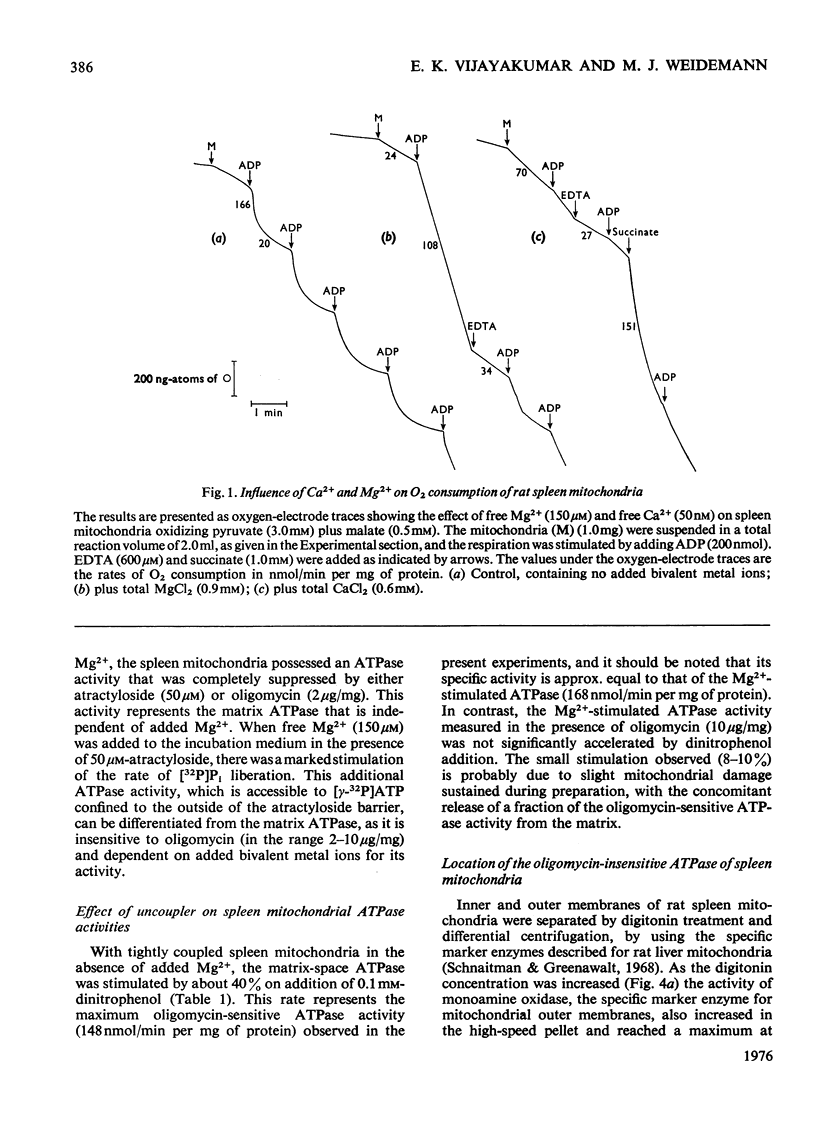
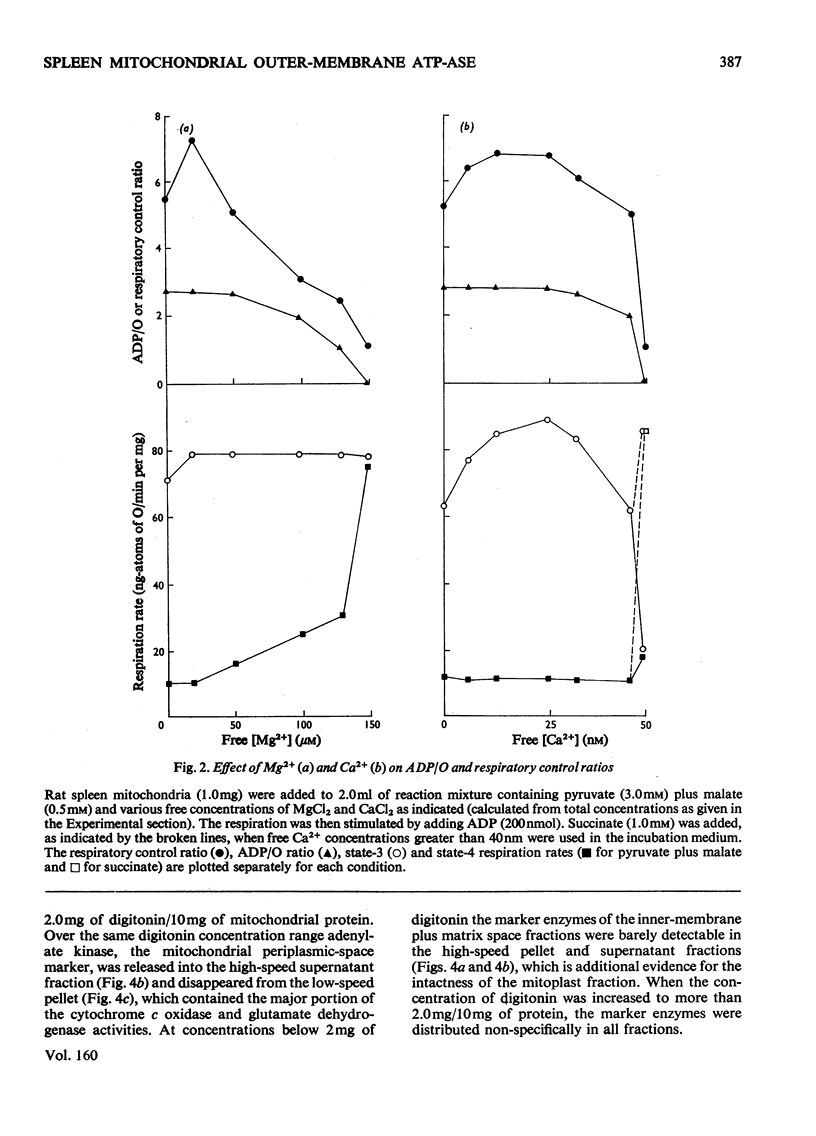
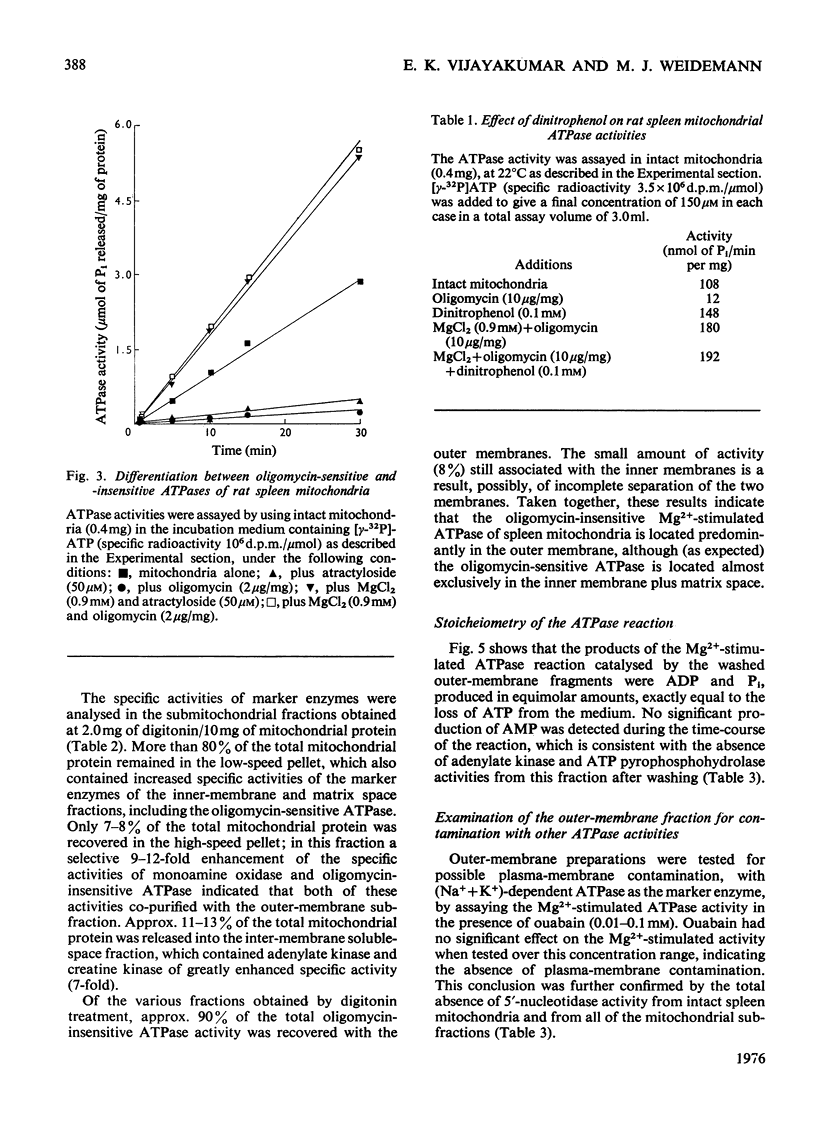
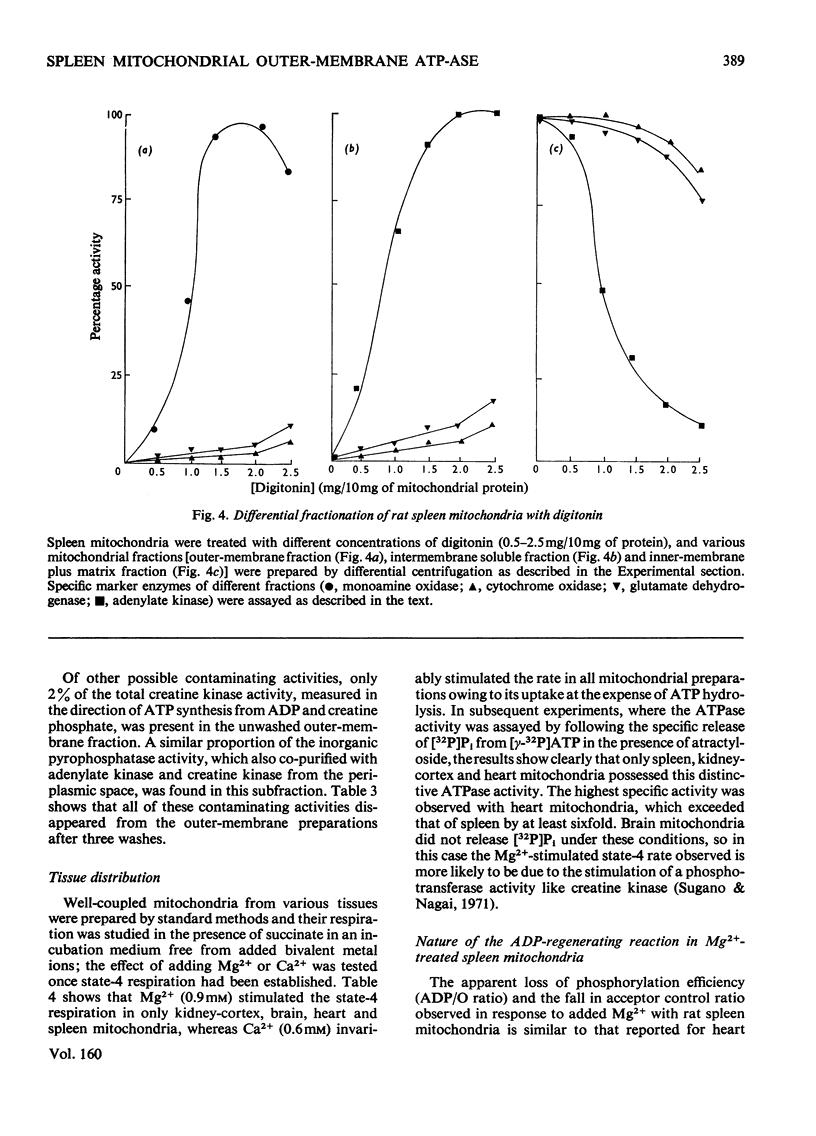
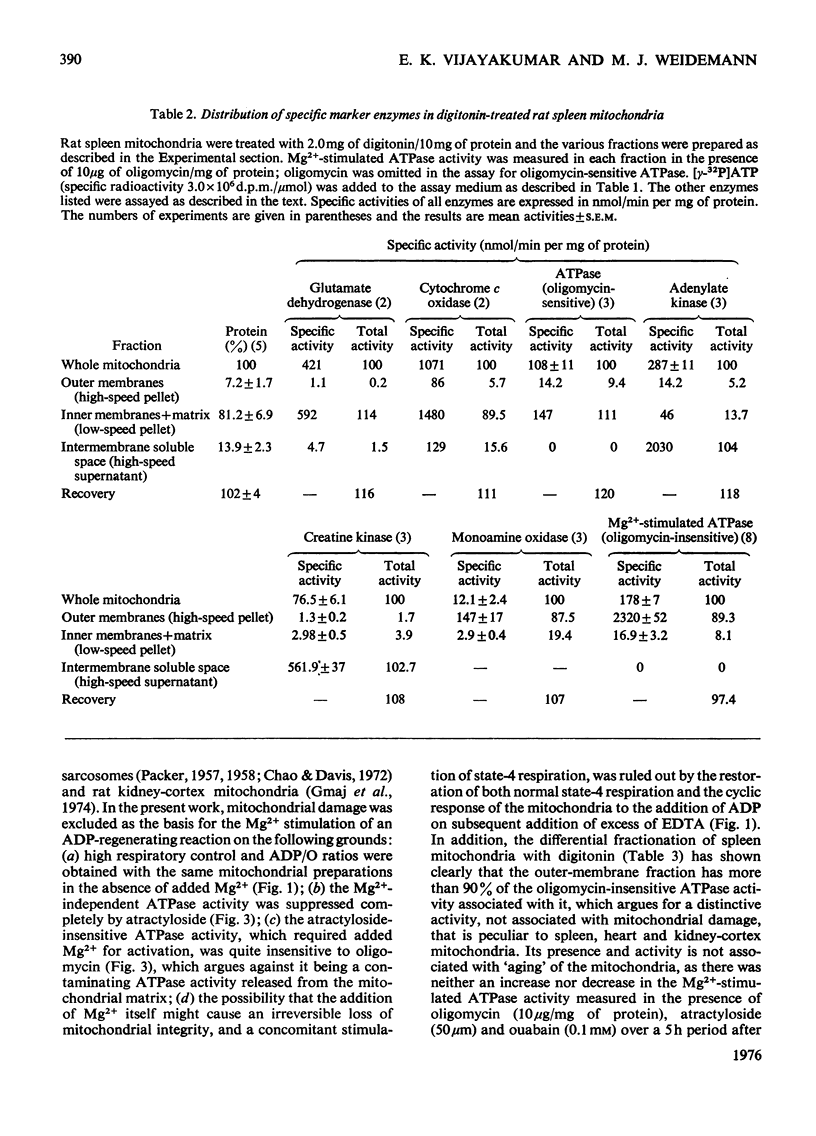
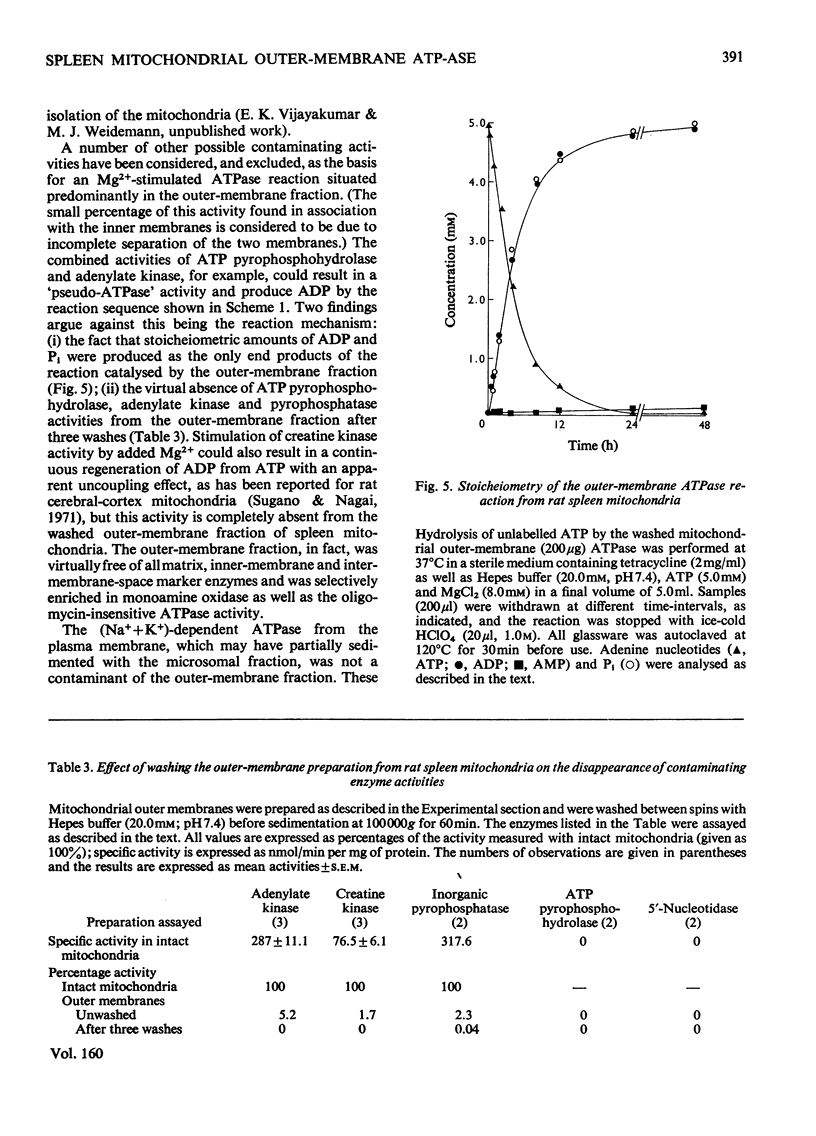
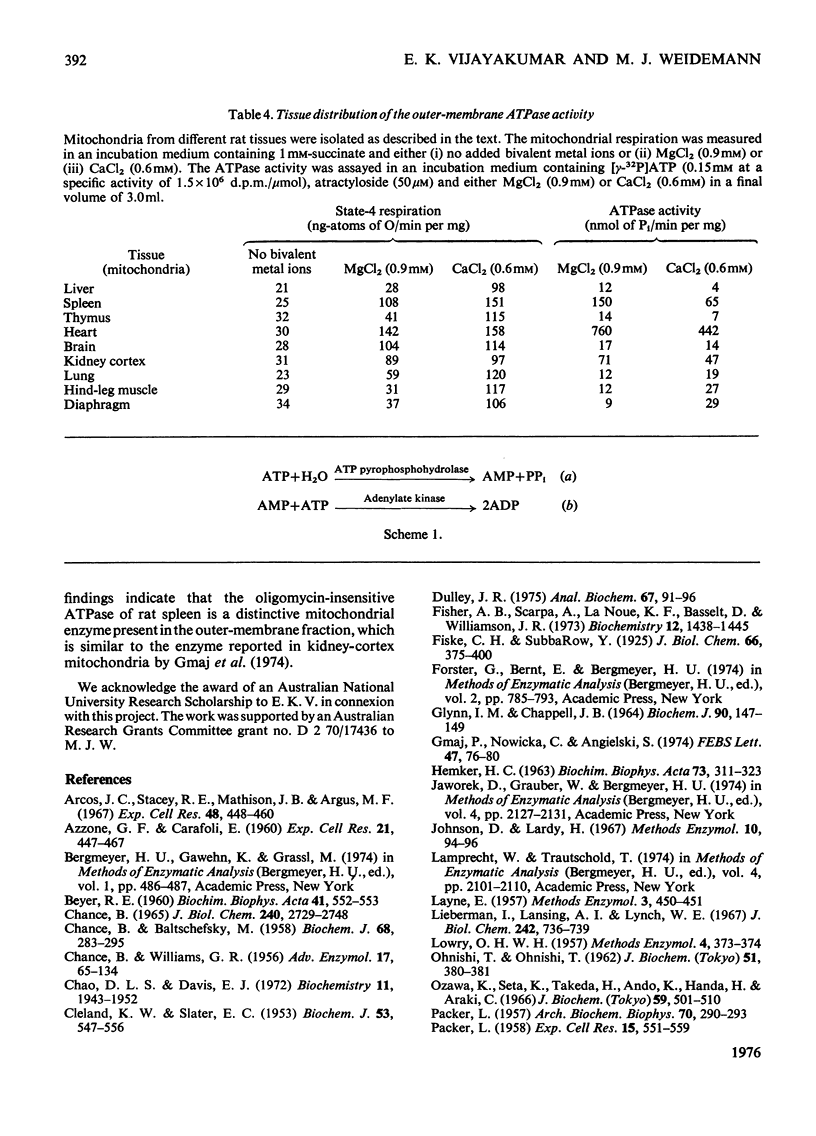
1. When rat spleen mitochondria are incubated with oxidizable substrates, added MgCl2 (greater than 150 muM free concentration) markedly stimulates state-4 respiration and lowers both the respiratory control and ADP/O ratios; this effect is reversible on addition of excess of EDTA. 2. With [gamma-32P]ATP as substrate, an Mg2+-stimulated ATPase (adenosine triphosphate) was identified in the atractyloside-insensitive and EDTA-accessible space of intact rat spleen mitochondria. 3. Oligomycin has no effect on the activity of the Mg2+-stimulated ATPase at a concentration (2.0mug/mg of protein) that completely inhibits the atractyloside-sensitive reaction. Of the two ATPase activities, only the atracytoloside sensitive reaction is stimulated (approx. 40%) by dinitrophenol. 4. On digitonin fractionation the atractyloside-insensitive Mg2+-stimulated ATPase co-purifies with the outer membrane-fraction of rat spleen mitochondria, whereas (as expected) the atractylosidesensitive activity co-purifies with the inner-membrane plus matrix fraction. 5. Stoicheiometric amounts of ADP and Pi are produced as the end products of ATP hydrolysis by purified outer-membrane fragments; no significant AMP production is detected during the time-course of the reaction. 6. The outer-membrane ATPase is present in rat kidney cortex and heart mitochondria as well as in spleen, but is absent from rat liver, thymus, brain, lung, diaphragm and skeletal muscle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZZONE G. F., CARAFOLI E., MUSCATELLO U. Biochemical properties of skeletal muscle mitochondria. II. The ATPase activity and the albumin effect. Exp Cell Res. 1960 Dec;21:456–467. doi: 10.1016/0014-4827(60)90279-2. [DOI] [PubMed] [Google Scholar]
- Arcos J. C., Stacey R. E., Mathison J. B., Argus M. F. Kinetic parameters of mitochondrial swelling. Effect of animal age. Tissue distribution of the mitochondrial "contractile protein". Exp Cell Res. 1967 Nov;48(2):448–460. doi: 10.1016/0014-4827(67)90368-0. [DOI] [PubMed] [Google Scholar]
- BEYER R. E. The release of adenosinetriphosphatases from rat-liver mitochondria during treatment with sound. Biochim Biophys Acta. 1960 Jul 15;41:552–553. doi: 10.1016/0006-3002(60)90064-0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., BALTSCHEFFSKY M. Spectroscopic effects of adenosine diphosphate upon the respiratory pigments of rat-heart-muscle sarcosomes. Biochem J. 1958 Feb;68(2):283–295. doi: 10.1042/bj0680283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. THE ENERGY-LINKED REACTION OF CALCIUM WITH MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2729–2748. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- CLELAND K. W., SLATER E. C. Respiratory granules of heart muscle. Biochem J. 1953 Mar;53(4):547–556. doi: 10.1042/bj0530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao D. L., Davis E. J. Studies on the role of Mg 2+ and the Mg 2+ -stimulated adenosine triphosphatase in oxidative phosphorylation. Biochemistry. 1972 May 9;11(10):1943–1952. doi: 10.1021/bi00760a032. [DOI] [PubMed] [Google Scholar]
- Dulley J. R. Determination of inorganic phosphate in the presence of detergents or protein. Anal Biochem. 1975 Jul;67(1):91–96. doi: 10.1016/0003-2697(75)90275-4. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Scarpa A., LaNoue K. F., Bassett D., Williamson J. R. Respiration of rat lung mitochondria and the influence of Ca 2+ on substrate utilization. Biochemistry. 1973 Mar 27;12(7):1438–1445. doi: 10.1021/bi00731a026. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmaj P., Nowicka C., Angielski S. Oligomycin-insensitive ATPase and calcium transport in rat kidney cortex mitochondria. FEBS Lett. 1974 Oct 1;47(1):76–80. doi: 10.1016/0014-5793(74)80429-1. [DOI] [PubMed] [Google Scholar]
- HEMKER H. C. The contribution of the various phosphorylating steps in the respiratory chain to the dinitrophenol-induced ATPase of rat-liver mitochondria. Biochim Biophys Acta. 1963 Jun 11;73:311–323. doi: 10.1016/0006-3002(63)90316-0. [DOI] [PubMed] [Google Scholar]
- Lieberman I., Lansing A. I., Lynch W. E. Nucleoside triphosphate pyrophosphohydrolase of the plasma membrane of the liver cell. J Biol Chem. 1967 Feb 25;242(4):736–739. [PubMed] [Google Scholar]
- OHNISHI T., OHNISHI T. Extraction of contractile protein from liver mitochondria. J Biochem. 1962 May;51:380–381. [PubMed] [Google Scholar]
- Ozawa K., Seta K., Takeda H., Ando K., Handa H., Araki C. On the isolation of mitochondria with high respiratory control from rat brain. J Biochem. 1966 May;59(5):501–510. doi: 10.1093/oxfordjournals.jbchem.a128334. [DOI] [PubMed] [Google Scholar]
- PACKER L. Coupled phosphorylation in rat heart muscle sarcosomes. Arch Biochem Biophys. 1957 Jul;70(1):290–293. doi: 10.1016/0003-9861(57)90105-4. [DOI] [PubMed] [Google Scholar]
- PACKER L. Regulation of respiration in heart sarcosomes. Exp Cell Res. 1958 Dec;15(3):551–559. doi: 10.1016/0014-4827(58)90103-4. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- ROSSI C. S., LEHNINGER A. L. STOICHIOMETRY OF RESPIRATORY STIMULATION, ACCUMULATION OF CA++ AND PHOSPHATE, AND OXIDATIVE PHOSPHORYLATION IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1964 Nov;239:3971–3980. [PubMed] [Google Scholar]
- Ray T. K. A modified method for the isolation of the plasma membrane from rat liver. Biochim Biophys Acta. 1970 Jan 6;196(1):1–9. doi: 10.1016/0005-2736(70)90159-8. [DOI] [PubMed] [Google Scholar]
- Reed K. C. An oxygen polarograph designed for undergraduate use. Anal Biochem. 1972 Nov;50(1):206–212. doi: 10.1016/0003-2697(72)90500-3. [DOI] [PubMed] [Google Scholar]
- Scaife J. F. The effect of lethal doses of x-irradiation on the enzymatic activity of mitochondrial cytochrome c. Can J Biochem. 1966 Apr;44(4):433–448. doi: 10.1139/o66-053. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano T., Nagai O. Effect of magnesium ion on brain mitochondrial respiration. I. Activation of brain mitochondrial phosphotransferases by magnesium ion. J Biochem. 1971 Sep;70(3):417–427. doi: 10.1093/oxfordjournals.jbchem.a129656. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Vinogradov A., Scarpa A., Chance B. Calcium and pyridine nucleotide interaction in mitochondrial membranes. Arch Biochem Biophys. 1972 Oct;152(2):646–654. doi: 10.1016/0003-9861(72)90261-5. [DOI] [PubMed] [Google Scholar]


