Abstract
Selenium disulfide (often referred to as SeS2) encompasses a family of mixed selenium-sulfide eight-membered rings, traditionally used as an anti-dandruff agent in shampoos. SeS2 can be produced by reacting hydrogen sulfide (H2S) with selenite (SeO32−) under acidic conditions. This chemistry is also possible with natural spring waters that are rich in H2S, thus providing an avenue for the more sustainable, green production of high-quality SeS2 particles from an abundant natural source. The orange material obtained this way consists of small globules with a diameter in the range of 1.1 to 1.2 µm composed of various SexS8−x chalcogen rings. It shows the usual composition and characteristics of a Se-S interchalcogen compound in EDX and Raman spectroscopy. Since the mineral water from Bad Nenndorf is also rich in salts, the leftover brine has been evaporated to yield a selenium-enriched salt mixture similar to table salt. As the water from Bad Nenndorf—in comparison to other bodies of water around the world—is still rather modest in terms of its H2S content, especially when compared with volcanic waters, this approach may be refined further to become economically and ecologically viable, especially as a regional business model for small and medium-sized enterprises.
Keywords: hydrogen sulfide, local economy, nanomaterials, selenium disulfide, spring water, sustainable synthesis, waste-to-value strategy
1. Introduction
Selenium and sulfur are two redox-active non-metals at the heart of many powerful natural redox-modulating compounds, ranging from highly reducing natural products such as ovothiols and selenoneine to oxidizing, electrophilic disulfides and disulfide-S-oxides (e.g., thiosulfinates, thiosulfonates), such as allicin [1,2,3,4,5]. The selenium–sulfur bond is especially redox-active and often catalytic and has been the focus of many studies on biological redox-cycles, for instance in glutathione peroxidase (GPx) enzymes [6,7,8,9]. Notably, selenium and sulfur can also theoretically “do without” a carbon-skeleton as far as their appearances, stabilities and redox chemistries are concerned. Catenation, their ability to form chains and rings with themselves and other elements of the chalcogen group, ensures that they are not “alone”, and some of these inorganic sulfur–selenium molecules can reach sizes of eight or more atoms.
Selenium disulfide, an orange solid composed of a medley of eight member SexS8−x rings, is a prime example of this type of “chalcogen-only” chemistry, devoid of any additional organic ballast [10]. Due to its average chemical composition of around 1:2 for Se and S, it is often referred to somewhat misleadingly as SeS2 and structurally represented as S=Se=S in analogy to SeO2 or SO2, which in fact is structurally incorrect, since SeS2 consists of the eight-membered rings linked via single sulfur–sulfur, sulfur–selenium and selenium–selenium bonds, with sulfur and selenium in the formal oxidation states of zero [11,12].
SeS2 can be synthesized in different ways, ranging from the rather alchemistic melting and mixing of elemental selenium and sulfur in a crucible to more controlled reactions, such as in the commercial method for its synthesis, which employs sodium sulfide (Na2S) solution acidified with glacial acetic acid and SeO2. Although this industrial method already avoids organic solvents, it still relies on commercial Na2S, which itself is produced by the reduction of sodium sulfate (Na2SO4) either at the expense of carbon or hydrogen [13,14,15]. From a green chemistry standpoint, it is therefore tempting to explore alternative, more sustainable methods, which may substitute industrial Na2S with naturally occurring H2S.
Indeed, H2S is quite abundant and common in nature, and a staggering 100–324 million tons of this gas are released from natural sources each year, including inorganic volcanic fumes and waters, as well as organic H2S produced in oceans, swamps, bogs, cesspits and honey-wagons [16,17,18,19]. In the Pacific Ring of Fire, volcanoes such as the Kawah Ijen in the East Java region of Indonesia are famous for producing H2S at a concentration of 15.7 mM in fumarolic discharge in the air [20]. The “Shah Field” in the United Arab Emirates holds approximately 480 billion cubic meters of sour gas reserves with around 23% H2S content [21]. Sulfate-reducing bacteria represent another important source for organic H2S production, especially in anoxic waters [22]. Table 1 provides a list of such sulfur-rich natural springs, which may be considered “accessible” natural sources of H2S.
Table 1.
Natural (re)sources of H2S with relevant sulfide concentrations.
Although Germany possesses no active volcanoes, there are areas of extinct volcanic activity and mineral springs rich in salts and H2S [28,29]. Among them, the “Neue Landgrafenquelle” in the spa town of Bad Nenndorf in the Lower Saxony region of Germany stands out due to its relatively high content of total sulfur of around 140 mg L−1 and a H2S concentration of 2.4 mM and indeed, “Bad” is referring to the German word for ‘spa’, or ‘bath’, which does not necessarily imply any pungent sulfury odor.
Guided by the goal of valorizing this natural H2S-rich water as a “green” supply of a chemical substance in a waste-to-value and zero-waste process, we have explored its practical uses in the synthesis of SeS2 on one hand and Se salt as by-product on the other hand.
2. Materials and Methods
2.1. Collection and Characterization of H2S-Rich Spring Water from Bad Nenndorf
The sulfur-rich water was collected on the early morning of the 6 December 2023 at approximately 8:15 a.m. from the mineral spring in a field near Bad Nenndorf in the Niedersachsen (Lower Saxony) region of Germany. A total of 50 L of this water was stored in ten canisters, each with a capacity of 5 L. The weather at the time of collection was snowy, with a temperature of 0 °C, relative humidity of 96%, and atmospheric pressure of 1011 hPa. The geographic coordinates of the collection site were 52.379947° N, 9.424150° E, and it was 138.4 m above sea level. The geological composition of the ground was primarily Turonian chalks [28]. The freshly collected water samples presented an average pH of 6.6.
Upon arrival at the laboratory of the University of Saarland, the water samples collected were analyzed for their sulfide contents, elemental compositions, and pH values. The Methylene Blue (MB) assay was performed to quantify the sulfide content according to the protocols described in the literature [30,31]. The mineral composition of the water was determined via Atomization Optical Emission Spectrometry with Inductively Coupled Plasma (ICP-OES) using an Ultima 2 tool (Horiba Jobin-Yvon, Longjumenau, France) coupled with a Czerny–Turner-type monochromator with a focal length of 1 m.
2.2. Synthesis of SeS2
SeO2 (1.33 g, 12 mM) was added to the stirred solution of 10 L of sulfide-rich water (24 mM of H2S) previously acidified with concentrated hydrochloric acid (HCl, 37%, 4 mL), which adjusted the pH of the reaction mixture to 4.0. The reaction mixture was stirred for two hours at room temperature to obtain an orange precipitate, which was then collected by vacuum filtration. The precipitate was washed with distilled water (100 mL) to remove any unreacted SeO2 and traces of spring water. The product was dried at room temperature and stored in the dark until further use. The filtrate was used further, as described in Section 3.4.
2.3. Chemical Composition of SeS2
The product was subjected to extensive physico-chemical characterization, including CHNS analysis, Energy-Dispersive X-ray Spectroscopy (EDX), Raman spectroscopy, and ICP-OES. CHNS was performed on a Vario MICRO cube CHN-elemental analyser (Elementar GmbH, Langenselbold, Germany). EDX analysis was carried out using a ZEISS Supra 40 field emitter microscope (Carl Zeiss NTS GmbH, Oberkochen, Germany) attached to a Bruker Quantax EDX system (Bruker Nano GmbH, Berlin, Germany). Raman spectroscopy was performed using a Renishaw InVia microscope (Wotton-under-Edge, Gloucestershire, UK) coupled with an excitation laser adjusted to a 532 nm wavelength. Commercially available SeS2 (Merck, Darmstadt, Germany) served as a reference material for comparing the composition of the material.
2.4. Zetasizer Measurements for Size and Surface Potential
The material was also analyzed using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) to measure size and Zeta potential. The particle sizes were recorded after 1 h and 2 h only, since subsequent readings pointed to the significant sedimentation and aggregation of the particles after 2 h, so the particle size could no longer be recorded by the Zetasizer.
3. Results
In summary, SeS2 was synthesized successfully from spring water from the well at Bad Nenndorf and SeO2 at pH 4.0 under mild reaction conditions. As expected, the resulting orange material consisted of microscopic globular structures composed of various SexS8−x rings. The remaining brine left over during the reaction was converted into selenium-enriched (natural) salt, which could be considered for human consumption. The filtrate comprising water and HCl could be recycled, as anticipated for a zero-waste approach.
3.1. Analysis of Sulfur Rich Water
The spring water collected at the mineral well at Bad Nenndorf was analyzed for its relevant mineral composition, and elemental analysis confirmed the presence of higher amounts of sodium (12.2 g L−1), sulfur (1.552 g L−1), and calcium (1.5 g L−1), as shown in Table 2.
Table 2.
The concentrations of selected inorganic elements found in the mineral water from Bad Nenndorf (see the Supplementary Materials for comparison).
| Elements | B | Ca | Mg | Na | Si | Sr | S |
|---|---|---|---|---|---|---|---|
| Concentration (mg L−1) | 7 | 1500 | 420 | 12,200 | 5 | 34 | 1552 |
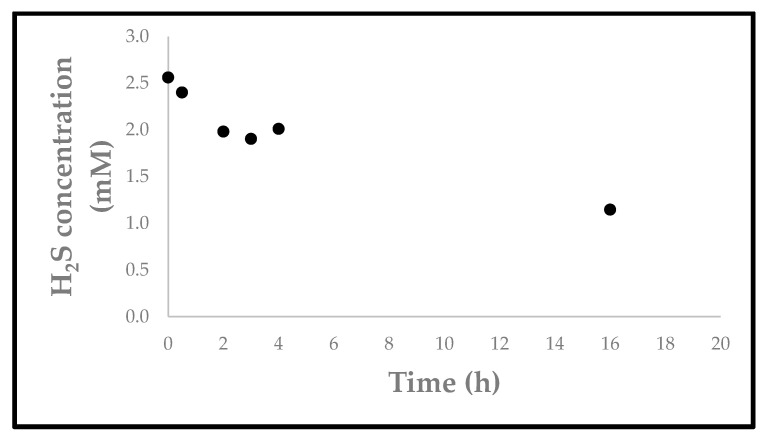
In line with German regulations on spas and water used for human maceration, a detailed analysis of such natural waters must be performed regularly, and the relevant information from the latest certified analysis carried out by Laborunion Prof. Höll & Co. GmbH, Bad Elster, Germany in 2018 is therefore provided in the Supplemental Information. Unlike this certification analysis, the analysis performed as part of this study focuses on the ingredients relevant for the subsequent chemical reaction, which are in line with the certified values as far as sulfide content (2.4 mM) and pH value (6.6) are concerned. Notably, whereas the pH is stable for days, the sulfide content decreases slowly over time, mostly via the escape of H2S, which, for instance, is able to diffuse through such plastic containers (Figure 1).
Figure 1.
The H2S concentration present in the water samples gradually decreases as affirmed using the MB assay.
3.2. Synthesis of SeS2 and Structural Confirmation
Since the H2S content of collected water gradually decreases, as shown in Figure 1, the reaction with SeO2 was performed promptly after collection. The reaction occurred solely at lower pH values; thus, HCl was added to the spring water prior to the addition of SeO2 to acidify the reaction mixture from the natural pH of 6.6 to pH 4.0. As shown in Figure 2, the reaction mixture turned opaque orange almost immediately after the addition of 0.5 equivalents of SeO2, indicating a fast reaction leading to a colloidal product and precipitation. This reaction was highly efficient and could even be performed outside the laboratory in a small Falcon tube “in the field” (Figure 2). It is important to point out that the minerals in the water did not interfere significantly with the reaction. Approximately 2.3 g of the compound was obtained from 20 L of water with a yield of about 66%.
Figure 2.
The water was collected from the underground spring in a field near Bad Nenndorf in northern Germany. A preliminary reaction was carried out “redneck style” at the source of origin, and an immediate change in color confirmed the feasibility of the synthesis. The figure also represents the chemistry carried out in the laboratory and shows a photograph of the orange material obtained.
The SeS2 precipitate was collected by vacuum filtration, washed with distilled water, and dried at 50 °C in the oven. A combination of CHN-S analysis for sulfur and ICP-OES analysis for selenium content confirmed an elemental selenium-to-sulfur ratio of 1:2 (recalculated as an atomic percentage from the mass percentage of the samples), as shown in Table 3. The analytical properties of the SeS2 obtained from spring water therefore did not differ notably from the ones of commercially obtained SeS2, including elemental composition and melting point. This is not trivial, as sulfur and selenium can take positions freely in such eight-membered rings and thus, in theory, many different ratios from 1:7 to 7:1 are possible.
Table 3.
The elemental composition of the selenium disulfide powder obtained compared to a selenium disulfide sample from a commercial supplier.
| Methods | CHN-S | EDX | ICP-OES | |
|---|---|---|---|---|
| Elements | S (%) | S (%) | Se (%) | Se (%) |
| Synthesized compound | 48.25 | 48.70 | 51.30 | 52.90 |
| Reference compound | 43.36 | 43.80 | 56.20 | 56.00 |
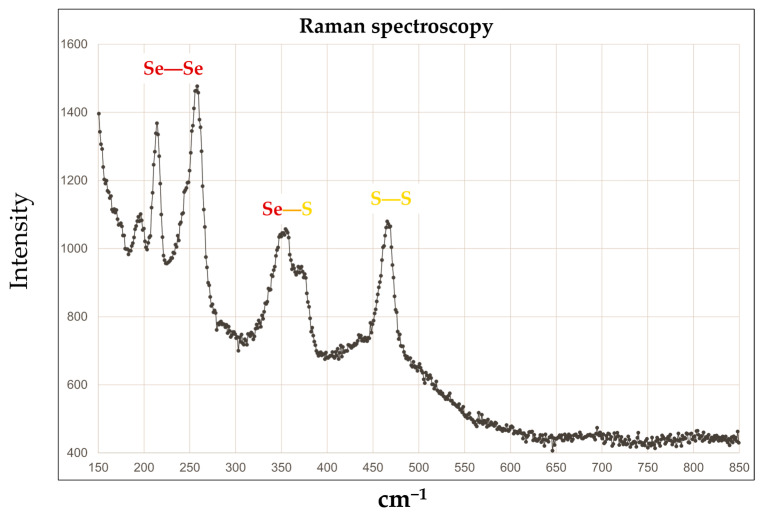
In order to confirm the presence of a genuine selenium disulfide compound, and not simply that of a physical mixture of elemental selenium and sulfur, Raman spectroscopy was employed, and it confirmed the presence of Se-Se, Se-S, and S-S bonds (Figure 3). Based on the Se-S ratios, which were also found by EDX (Table 3), and under the assumption that the material consists of eight-membered rings, finding a plethora of structures in the “circle of eight” was possible, and indeed likely, especially Se2S6 and Se3S5 rings.
Figure 3.
Raman spectroscopy confirmed the presence of S-S, S-Se, and Se-Se bonds.
3.3. Microscopic Properties of the Material
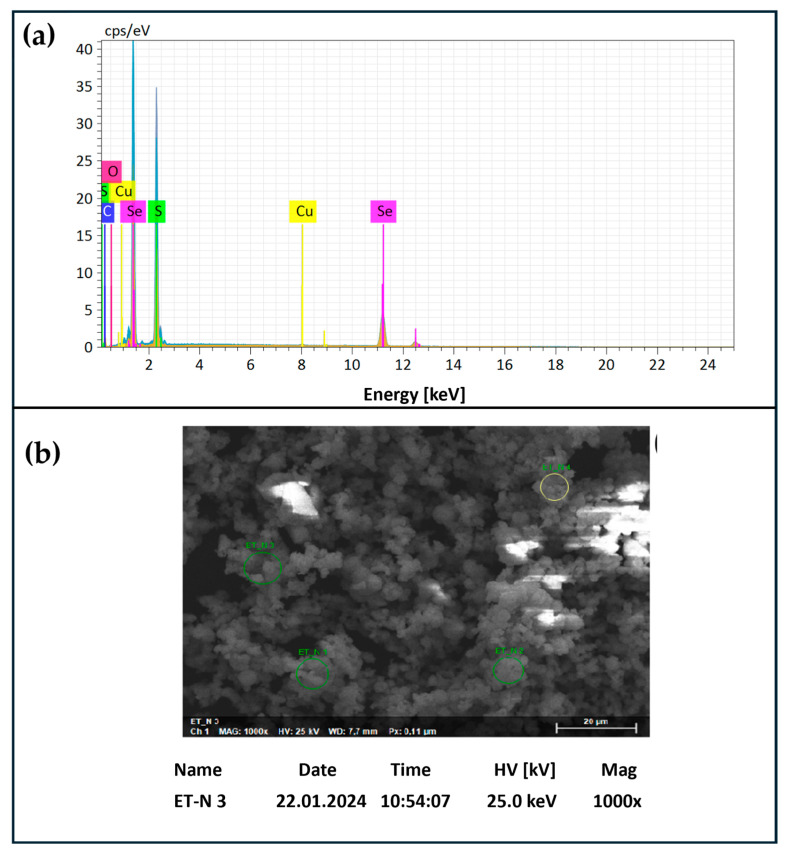
Upon formation, samples of SeS2 particles were analyzed by Dynamic Light Scattering (DLS) using a Zetasizer, which confirmed the formation of a precipitate with an average particle size of around 1 µm in diameter (PDI = 0.180) after one hour of reaction, which continued to increase over time. Notably, the Zeta potential, indicative of the surface charge of the particles, was around −4.77 mV, which may also explain why the material tended to aggregate and precipitate from the solution rather readily. To confirm these DLS measurements and to evaluate the elemental composition of the precipitated materials, SEM, in combination with EDX (Figure 4, Panel a), was utilized and indicated the presence of small globular selenium disulfide-rich objects, with sizes in the high nanometer to low micrometer range (Figure 4, Panel b).
Figure 4.
SeS2 was analyzed to determine chemical composition using EDX coupled to SEM. EDX confirmed the presence of selenium and sulfur at a ratio of around 1:2 (Panel (a)), while the SEM image showed the presence of (aggregated) globular material (Panel (b)).
3.4. Se Salt
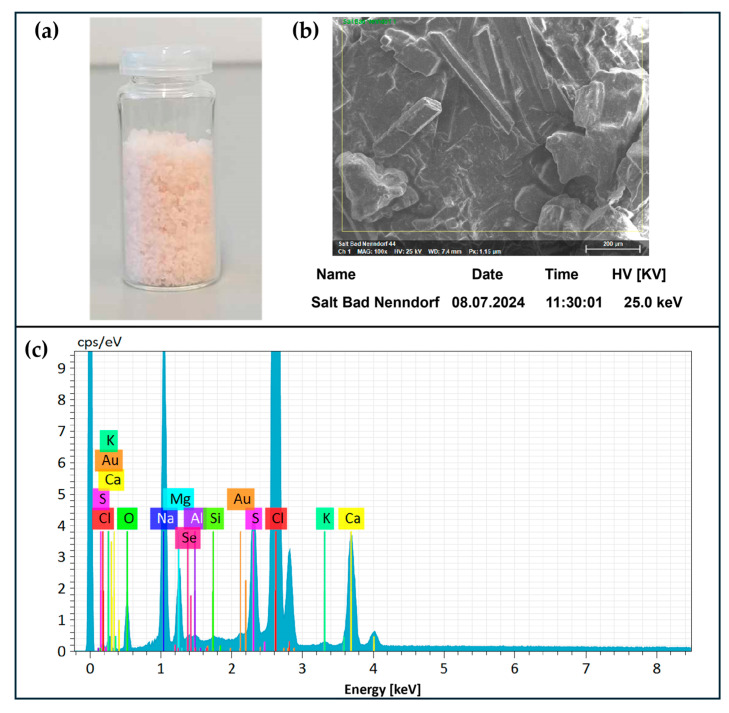
The spring water from Bad Nenndorf is not only rich in H2S but also contains high percentages of salt, namely NaCl. The Mediterranean Sea’s surface water, for instance, comprises around 12.3 g L−1 of sodium, which is remarkably similar to the water sample from Bad Nenndorf, with a sodium content of 12.2 g L−1 (Table 2) [32]. Therefore, the filtrate of the reaction was collected and evaporated in an oven at 50 °C until dryness. A total of 25 mL of this filtrate yielded approximately 3 g (12% w/w) of salt (Figure 5).
Figure 5.
The filtrate was evaporated at 50 °C to obtain salt (Panel (a)), which was analyzed by SEM (Panel (b)) coupled with EDX (Panel (c)) to quantify the elements present in the salt. EDX confirmed the presence of selenium at about 0.40% w/w (dry weight), as compared to the overall salt composition.
This “leftover” salt from the reaction consisted primarily of sodium (20.35% w/w) and chloride (44.76% w/w). Additionally, the resulting salt was rich in calcium (8.78% w/w) and magnesium (3.81% w/w) yet low in sulfide (4.52% w/w for total sulfur). Since the reaction to form SeS2 did not go to completion, traces of selenium in the range of 0.40% w/w (dry weight) were found (Table 4). Apart from this, once dissolved in distilled water, the pH was almost neutral (pH = 7.9 of a 0.9% isotonic salt solution), indicating that the H2S initially present (and not reacted with SeO2) and the HCl added had both escaped.
Table 4.
Elemental composition of Se salt, as estimated by employing EDX.
| Elements found | O | Na | Mg | Al | Si | S | Cl | K | Ca | Se |
|---|---|---|---|---|---|---|---|---|---|---|
| Composition (% w/w) | 16.24 | 20.35 | 3.81 | 0.26 | 0.10 | 4.52 | 44.76 | 0.25 | 8.78 | 0.40 |
4. Discussion
To conserve valuable natural resources and to protect the environment, several industries are currently searching for either renewable, recyclable, or valorizable materials. This also applies to inorganic substances, which in many respects are more difficult to (re)produce than organic ones. For instance, in the European Union (EU), phosphate mining is projected to be substituted by chemical recycling initiatives from 2026 onwards [32,33,34,35]. Nitrogen and sulfur may follow suit; thus, it is imperative to incorporate a greater number of sustainable production methods into the chemical industry to deter waste production and deleterious effects on our environment. Sulfur-rich mineral water may, quite literally, run down the drain if not utilized for its abundant elemental content and would often require steeply priced treatment to remove H2S, for instance by oxidation with H2O2 [36,37]. The mineral water from Bad Nenndorf is such a source - . though its H2S content is modest in comparison to other international sources, it is sufficient to facilitate the reaction with SeO2 in order to produce SeS2 to a good quality and yield.
The SeS2 produced shows a composition and characteristics close to those of its commercial equivalent, ranging from its elemental composition to vibrations in the Raman spectra characteristic of Se-Se, Se-S, and S-S bonds. Although one cannot rule out the presence of additional elemental selenium and sulfur in such mixtures, the SeS2 obtained seems surprisingly pure thanks to its precipitation from the reaction mixture. The compound itself is easy to handle in air and when immersed in water.
SeS2 is commonly considered for its action against scalp irritation and has served as a standard active ingredient in anti-dandruff shampoos for several decades, eventually earning some global prominence as the agent that saves the world from alien invasion in the blockbuster film “Evolution” (2001) [38,39,40].
As for the waste-to-value strategy and its underlying “redneck” chemistry, both may still require optimization; yet, they demonstrate that the quality of the mineral water, while rich in many components aside from sulfide, is still “clean” enough to allow the chemical reaction to proceed. Notably, whereas the purification of the water, performed in order to harvest its individual components, may be tedious and costly, the SeS2 reaction results in precipitation, which is key for purity, as the product can be removed by simple filtration. Furthermore, the reaction does not yield any side products and the remaining filtrate can be valorized further to form a Se salt.
Interestingly, the NaCl content in this Se salt is comparable to sea salt, which contains around 30.6% sodium and 55.2% chloride [41]. As for taste, the corresponding author took the exceptional step to carefully taste a pinch of this salt for the sake of science. The taste is generally amenable and not much different from that of normal table salt. Aside from human consumption, this salt may be more commonly used for bathing and cosmetic purposes, similar to commercially available Dead Sea salts [42,43].
From an ecological perspective, our proposed procedure serves as an attractive, green alternative, since it does not require or generate harmful chemicals, replaces one component of SeS2 production with a natural waste product, and, at the same time, also removes H2S from the water. H2S removal, for instance via oxidation with H2O2, is demanding and often required by law, for instance in H2S-rich water from abandoned coal mines in Germany and abandoned gas wells in Canada [24,44,45,46]. Although it has not yet been possible to replace commercial SeO2 with a natural product, this is a matter that may also be addressed by considering wastes from copper mining and refineries [47,48]. Concerning the HCl employed during SeS2 synthesis, it can be recycled during the process, and the water can eventually be recycled during the drying of Se salt.
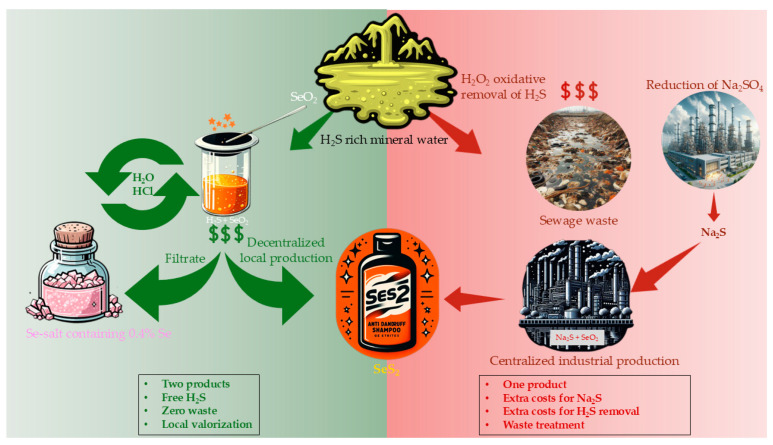
Economically speaking, solely using mineral water from Bad Nenndorf for the large-scale production of SeS2 is, of course, not possible. Here, other richer, more concentrated sources need to be considered, such as the Solec-Zdrój “Malina” spring in Poland, where over 400 kg of H2S can be harvested annually, considering a theoretical flow rate of 1 L min−1. This natural source may be sufficient to replace Na2S, which has a cost of several hundred Euros per ton. Then again, small(er)-scale local production in spa towns with similar mineral springs to Bad Nenndorf, such as Aachen or Bad Wiessee in Germany, may still be attractive, as it could empower local communities and contribute to their overall economic development. These local natural spring spas may, for instance, launch their own proprietary cosmetic products, such as shampoos, or produce their own brands of Se salt on a commercial and larger scale; furthermore, these locations could market these products, allowing for sales to cover production costs and incentivize a small profit margin. This may support the local and regional economy around such spa towns, which are often located in rural areas. Eventually, these smaller and somewhat niche SeS2 business opportunities for small and medium-sized enterprises might become quite lucrative, considering that mineral water is often discarded as waste and must be purified, most commonly via oxidation at an added cost (Figure 6).
Figure 6.
H2S springs may serve as sources for the production of value-added products, such as SeS2, and avoid wasting this natural resource as sewage. This strategy not only opens up the door for boosting local economies but also, as a true “hat trick”, decreases the environmental burden posed by the chemical treatment of H2S-rich water.
5. Conclusions
In summary, our studies have confirmed that it is possible and indeed quite attractive to leverage natural (re)sources in chemical reactions, as illustrated by our method for producing SeS2. Our proposed procedure can be seen as yet another example and proof-of-concept for a carefully devised and designed green and sustainable strategy to transmute waste into value, with the sustainable benefits of protecting the environment and serving as a boon to local economies. Aside from utilizing sources of spring water of inorganic origin, our future directions will focus upon procuring H2S from organic sources, such as wastewater, sewage, and bacterial cultures, which are able to produce H2S either from organic materials during fouling, by reducing sulfite (SO32−), or naturally abundant sulfate (SO42−) [49,50,51,52]. We also intend to look for opportunities to “freeload” SeO2, although this may turn out to be more challenging.
In line with our aims of endorsing sustainability and, at the same time, employing local resources, the strategy of collecting nearby, readily available materials (including, but not limited to, waste bins, sewage treatment plants, soil, and air) and using them as chemical reagents could significantly gain momentum. Yet, this requires a carefully devised “redneck” chemistry equipped to handle initially dirty, impure mixtures of substances, on the one hand, and possible byproducts and “left-overs”, on the other hand. In our example, the precipitation of insoluble SeS2 has done the trick, i.e., it has enabled the separation of considerably pure SeS2 from the minerals in the water and the water itself. Ultimately, our methods have bolstered support for the further use of the Se-rich salt as part of a zero-waste strategy.
In the future, it will prove encouraging to employ similar local (re)sources and a chemistry that is able to blend natural materials with traditional reactions in order to provide further high-value, low-cost products. Precipitation or the production of gases may circumvent some of the issues associated with turning “dirty” waste into clean products. Eventually, these clean products will be obtained from “impure” (re)sources by a cleverly designed yet robust “redneck” chemistry, possibly on smaller scales, for local production and regional waste-to-value chains alike.
Acknowledgments
The authors would like to thank Sodomir Popojuk and Ken Rory for their advice, discussions, and proof-reading of the manuscript. Special thanks go to many other colleagues from the Academiacs International network (www.academiacs.eu) (accessed on 9 May 2024) and “Pharmasophy” for their helpful discussions and advice. The authors extend their gratitude to Anna Elizabeth Schmitz for her invaluable assistance in reviewing and refining the language of the manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma17235733/s1, Table S1: Physical and physico-chemical analysis of the Landgrafen spring from Bad Nenndorf; Table S2: Elemental composition analysis of the Landgrafen spring from Bad Nenndorf; Table S3: Undissociated and gaseous substances found in the Landgrafen spring from Bad Nenndorf.
Author Contributions
Conceptualization, C.J. and M.J.N.; methodology, E.T., R.L. and C.F.-S.; validation, C.J., K.-H.S. and C.F.-S.; formal analysis, M.J.N.; investigation, E.T., S.S. and A.Y.A.; data curation, R.L. and C.F.-S., writing—original draft preparation, C.J.; writing—review and editing, M.J.N., K.-H.S. and C.F.-S.; supervision, M.J.N.; project administration, C.J. and M.J.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The authors acknowledge the financial support provided by the University of Saarland, Saarbruecken, Germany.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Murano C., Zuccarotto A., Leone S., Sollitto M., Gerdol M., Castellano I., Palumbo A. A Survey on the Distribution of Ovothiol and OvoA Gene Expression in Different Tissues and Cells: A Comparative Analysis in Sea Urchins and Mussels. Mar. Drugs. 2022;20:268. doi: 10.3390/md20040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamashita M., Yamashita Y., Suzuki T., Kani Y., Mizusawa N., Imamura S., Takemoto K., Hara T., Hossain M.A., Yabu T., et al. Selenoneine, a Novel Selenium-Containing Compound, Mediates Detoxification Mechanisms against Methylmercury Accumulation and Toxicity in Zebrafish Embryo. Mar. Biotechnol. 2013;15:559–570. doi: 10.1007/s10126-013-9508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhasan R., Nasim M.J., Jacob C., Gaucher C. Selenoneine: A Unique Reactive Selenium Species From the Blood of Tuna With Implications for Human Diseases. Curr. Pharmacol. Rep. 2019;5:163–173. doi: 10.1007/s40495-019-00175-8. [DOI] [Google Scholar]
- 4.Borlinghaus J., Albrecht F., Gruhlke M.C.H., Nwachukwu I.D., Slusarenko A.J. Allicin: Chemistry and Biological Properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allah D.R., Schwind L., Asali I.A., Nasim J., Jacob C., Götz C., Montenarh M. A Scent of Therapy: Synthetic Polysulfanes with Improved Physico-Chemical Properties Induce Apoptosis in Human Cancer Cells. Int. J. Oncol. 2015;47:991–1000. doi: 10.3892/ijo.2015.3093. [DOI] [PubMed] [Google Scholar]
- 6.Sies H., Masumoto H. Ebselen as a Glutathione Peroxidase Mimic and as a Scavenger of Peroxynitrite. In: Sies H., editor. Advances in Pharmacology. Volume 38. Academic Press; Cambridge, MA, USA: 1996. pp. 229–246. [DOI] [PubMed] [Google Scholar]
- 7.Parise A., Romeo I., Russo N., Marino T. The Se–S Bond Formation in the Covalent Inhibition Mechanism of SARS-CoV-2 Main Protease by Ebselen-like Inhibitors: A Computational Study. Int. J. Mol. Sci. 2021;22:9792. doi: 10.3390/ijms22189792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sands K.N., Burman A.L., Ansah-Asamoah E., Back T.G. Chemistry Related to the Catalytic Cycle of the Antioxidant Ebselen. Molecules. 2023;28:3732. doi: 10.3390/molecules28093732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laitinen R., Pakkanen T. A Theoretical Investigation of the Sulfur—Selenium Bond. J. Mol. Struct. Theochem. 1983;91:337–352. doi: 10.1016/0166-1280(83)80079-7. [DOI] [Google Scholar]
- 10.Tiganescu E., Abdin A.Y., Razouk A., Nasim M.J., Jacob C. The Redox Riddle of Selenium Sulfide. Curr. Opin. Chem. Biol. 2023;76:102365. doi: 10.1016/j.cbpa.2023.102365. [DOI] [PubMed] [Google Scholar]
- 11.Laitinen R.S. Selenium Sulfide Ring Molecules. Acta Chem. Scand. 1987;41:361–376. doi: 10.3891/acta.chem.scand.41a-0361. [DOI] [Google Scholar]
- 12.Steudel R., Laitinen R. Inorganic Ring Systems. Springer; Berlin, Heidelberg, Germany: 1982. Cyclic Selenium Sulfides; pp. 177–197. [DOI] [PubMed] [Google Scholar]
- 13.Iran C. Sodium Sulfide Manufacturing Methods and Applications. Chemical Iran; Tehran, Iran: 2023. [(accessed on 30 October 2024)]. Available online: https://www.chemicaliran.com/sodium-sulfide-manufacturing-methods-and-applications/ [Google Scholar]
- 14.Sodium Sulfide Wikipedia 2024. [(accessed on 30 October 2024)]. Available online: https://en.wikipedia.org/wiki/Sodium_sulfide.
- 15.Heinz B. Process for the Production of Sodium Sulphide 1958. [(accessed on 30 October 2024)]. Available online: https://patents.google.com/patent/US2838374A/en.
- 16.Malone Rubright S.L., Pearce L.L., Peterson J. Environmental Toxicology of Hydrogen Sulfide. Nitric Oxide. 2017;71:1–13. doi: 10.1016/j.niox.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L., De Schryver P., De Gusseme B., De Muynck W., Boon N., Verstraete W. Chemical and Biological Technologies for Hydrogen Sulfide Emission Control in Sewer Systems: A Review. Water Res. 2008;42:1–12. doi: 10.1016/j.watres.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Devereux R., Delaney M., Widdel F., Stahl D.A. Natural Relationships among Sulfate-Reducing Eubacteria. J. Bacteriol. 1989;171:6689–6695. doi: 10.1128/jb.171.12.6689-6695.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driver L., Freedman E. Report to Congress on Hydrogen Sulfide Air Emissions Associated with the Extraction of Oil and Natural Gas. Final Report. Environmental Protection Agency; Research Triangle Park, NC, USA: 1993. Office of Air Quality Planning and Standards. [Google Scholar]
- 20.Delmelle P., Bernard A., Kusakabe M., Fischer T.P., Takano B. Geochemistry of the Magmatic–Hydrothermal System of Kawah Ijen Volcano, East Java, Indonesia. J. Volcanol. Geotherm. Res. 2000;97:31–53. doi: 10.1016/S0377-0273(99)00158-4. [DOI] [Google Scholar]
- 21.The World’s Top 10 Sour Gas Fields—Oil & Gas Middle East 2024. [(accessed on 30 October 2024)]. Available online: https://www.oilandgasmiddleeast.com/listing/the-worlds-top-10-sour-gas-fields.
- 22.Volkov I.I., Neretin L.N. Hydrogen Sulfide in the Black Sea. In: Kostianoy A.G., Kosarev A.N., editors. The Black Sea Environment. Springer; Berlin, Heidelberg, Germany: 2008. pp. 309–331. [Google Scholar]
- 23.Sulphide Baths. [(accessed on 28 October 2024)]. Available online: https://www.malinowyraj.com/medical-centre/sulphide-baths?
- 24.Jackson R.E., Dusseault M.B., Frape S., Phan T., Steelman C. Investigating the Origin of Elevated H2S in Groundwater Discharge from Abandoned Gas Wells, Norfolk County, Ontario; Proceedings of the Geoconvention 2020; Virtual Event. 21–23 September 2020. [Google Scholar]
- 25.Pimenov N.V., Kuranov G.V., Bryukhanov A.L., Veslopolova E.F., Koryukina I.P., Maslov Y.N. The Sulfate-Reducing Bacterial Community of Sulfide-Rich Water of the Ust’-Kachka Resort Spring, Perm Krai, Russia. Microbiology. 2012;81:721–726. doi: 10.1134/S0026261712060112. [DOI] [PubMed] [Google Scholar]
- 26.Faulstich L., Griffin S., Nasim M.J., Masood M.I., Ali W., Alhamound S., Omran Y., Kim H., Kharma A., Schäfer K.-H., et al. Nature’s Hat-Trick: Can We Use Sulfur Springs as Ecological Source for Materials with Agricultural and Medical Applications? Int. Biodeterior. Biodegrad. 2017;119:678–686. doi: 10.1016/j.ibiod.2016.08.020. [DOI] [Google Scholar]
- 27.Iurkiewicz A.A., Stevanovic Z.P. Reconnaissance Study of Active Sulfide Springs and Cave Systems in the Southern Part of the Sulaimani Governorate (NE Iraq) Carbonates Evaporites. 2010;25:203–216. doi: 10.1007/s13146-010-0024-3. [DOI] [Google Scholar]
- 28.Wray D.S. Origin of Clay-Rich Beds in Turonian Chalks from Lower Saxony, Germany—A Rare-Earth Element Study. Chem. Geol. 1995;119:161–173. doi: 10.1016/0009-2541(94)00089-Q. [DOI] [Google Scholar]
- 29.Rachold V., Brumsack H.-J. Inorganic Geochemistry of Albian Sediments from the Lower Saxony Basin NW Germany: Palaeoenvironmental Constraints and Orbital Cycles. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001;174:121–143. doi: 10.1016/S0031-0182(01)00290-5. [DOI] [Google Scholar]
- 30.Moest R.R. Hydrogen Sulfide Determination by the Methylene Blue Method. Anal. Chem. 1975;47:1204–1205. doi: 10.1021/ac60357a008. [DOI] [Google Scholar]
- 31.Li Z.-G. Chapter Six—Quantification of Hydrogen Sulfide Concentration Using Methylene Blue and 5,5′-Dithiobis(2-Nitrobenzoic Acid) Methods in Plants. In: Cadenas E., Packer L., editors. Methods in Enzymology. Volume 554. Academic Press; Waltham, MA, USA: 2015. pp. 101–110. Hydrogen Sulfide in Redox Biology, Part A. [DOI] [PubMed] [Google Scholar]
- 32.Nessim R.B., Tadros H.R.Z., Abou Taleb A.E.A., Moawad M.N. Chemistry of the Egyptian Mediterranean Coastal Waters. Egypt. J. Aquat. Res. 2015;41:1–10. doi: 10.1016/j.ejar.2015.01.004. [DOI] [Google Scholar]
- 33.Federal Office for the Environment FOEN Phosphorrecycling. [(accessed on 28 October 2024)]. Available online: https://www.bafu.admin.ch/bafu/en/home/themen/thema-abfall/abfall--fachinformationen/abfallpolitik-und-massnahmen/phosphorrecycling.html.
- 34.Serrano-Gomez J., Metson G.S., Neset T.-S., Santner J., Hermann L., Zessner M. EU-Compliant Wastewater Recycled Phosphorus: How Much National Cereal Demand Can It Meet? J. Clean. Prod. 2023;429:139482. doi: 10.1016/j.jclepro.2023.139482. [DOI] [Google Scholar]
- 35.European Sustainable Phosphorus Platform—The Phosphorus Challenge. [(accessed on 28 October 2024)]. Available online: https://www.phosphorusplatform.eu/links-and-resources/p-facts.
- 36.El Brahmi A., Abderafi S. Hydrogen Sulfide Removal from Wastewater Using Hydrogen Peroxide In-Situ Treatment: Case Study of Moroccan Urban Sewers. Mater. Today Proc. 2021;45:7424–7427. doi: 10.1016/j.matpr.2021.01.641. [DOI] [Google Scholar]
- 37.Ahmad N., Maitra S., Dutta B.K., Ahmad F. Remediation of Sulfidic Wastewater by Catalytic Oxidation with Hydrogen Peroxide. J. Environ. Sci. 2009;21:1735–1740. doi: 10.1016/S1001-0742(08)62481-X. [DOI] [PubMed] [Google Scholar]
- 38.Cohen P.R., Anderson C.A. Topical Selenium Sulfide for the Treatment of Hyperkeratosis. Dermatol. Ther. 2018;8:639–646. doi: 10.1007/s13555-018-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godse G., Godse K. Safety, Efficacy and Attributes of 2.5% Selenium Sulfide Shampoo in the Treatment of Dandruff: A Single-Center Study. Cureus. 2024;16:e57148. doi: 10.7759/cureus.57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evolution. The Montecito Picture Company; Culver City, CA, USA: 2001. Columbia Pictures, Dreamworks Pictures. [Google Scholar]
- 41.Galvis-Sánchez A.C., Lopes J.A., Delgadillo I., Rangel A.O.S.S. Chapter 26—Sea Salt. In: de la Guardia M., Gonzálvez A., editors. Comprehensive Analytical Chemistry. Volume 60. Elsevier; Amsterdam, The Netherlands: 2013. pp. 719–740. Food Protected Designation of Origin. [Google Scholar]
- 42.Sukenik S., Neumann L., Buskila D., Kleiner-Baumgarten A., Zimlichman S., Horowitz J. Dead Sea Bath Salts for the Treatment of Rheumatoid Arthritis. Clin. Exp. Rheumatol. 1990;8:353–357. [PubMed] [Google Scholar]
- 43.Katz U., Shoenfeld Y., Zakin V., Sherer Y., Sukenik S. Scientific Evidence of the Therapeutic Effects of Dead Sea Treatments: A Systematic Review. Semin. Arthritis Rheum. 2012;42:186–200. doi: 10.1016/j.semarthrit.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Es Riecht Nach Faulen Eiern. [(accessed on 28 October 2024)]. Available online: https://www.saarbruecker-zeitung.de/saarland/es-riecht-nach-faulen-eiern_aid-546033.
- 45.Umwelt-Online: AbwV—Abwasserverordnung—Verordnung Über Anforderungen an Das Einleiten von Abwasser in Gewässer—Erläuterungen Zu Anhang 20 (1) [(accessed on 28 October 2024)]. Available online: https://www.umwelt-online.de/regelwerk/cgi-bin/suchausgabe.cgi?pfad=/wasser/abw_vo/an20e.htm&such=Schwefelwasserstoff.
- 46.Tran T.Q., Banning A., Wisotzky F., Wohnlich S. Mine Water Hydrogeochemistry of Abandoned Coal Mines in the Outcropped Carboniferous Formations, Ruhr Area, Germany. Environ. Earth Sci. 2020;79:84. doi: 10.1007/s12665-020-8821-z. [DOI] [Google Scholar]
- 47.Taskinen P., Patana S., Kobylin P., Latostenmaa P. Oxidation Mechanism of Copper Selenide. High. Temp. Mater. Process. 2014;33:469–476. doi: 10.1515/htmp-2013-0097. [DOI] [Google Scholar]
- 48.Okonji S.O., Dominic J.A., Pernitsky D., Achari G. Removal and Recovery of Selenium Species from Wastewater: Adsorption Kinetics and Co-Precipitation Mechanisms. J. Water Process Eng. 2020;38:101666. doi: 10.1016/j.jwpe.2020.101666. [DOI] [Google Scholar]
- 49.Dutta A., Valle F., Goldman T., Keating J., Burke E., Williamson N., Dirmeier R., Bowman J.S. Detection of Sulfate-Reducing Bacteria as an Indicator for Successful Mitigation of Sulfide Production. Appl. Environ. Microbiol. 2021;87:e01748-21. doi: 10.1128/AEM.01748-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng Q., Li S., Yao M., Liu C., Zhang Z., Xiang S. Study on the Factors of Hydrogen Sulfide Production from Lignite Bacterial Sulfate Reduction Based on Response Surface Method. Sci. Rep. 2023;13:20537. doi: 10.1038/s41598-023-47787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim M., Zaman M., Jang E., Nakhla G., Ward M., Gutierrez O., Willis J., Walton J., Santoro D. Experimental Investigation on Hydrogen Sulfide Production, Wastewater Characteristics and Microbial Ecology Profiles in Anaerobic Sewer Lines Using a Sewer Physical Twin. J. Environ. Chem. Eng. 2024;12:111965. doi: 10.1016/j.jece.2024.111965. [DOI] [Google Scholar]
- 52.Yin X., Zhou G., Wang H., Han D., Maeke M., Richter-Heitmann T., Wunder L.C., Aromokeye D.A., Zhu Q.-Z., Nimzyk R., et al. Unexpected Carbon Utilization Activity of Sulfate-Reducing Microorganisms in Temperate and Permanently Cold Marine Sediments. ISME J. 2024;18:wrad014. doi: 10.1093/ismejo/wrad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.