Abstract
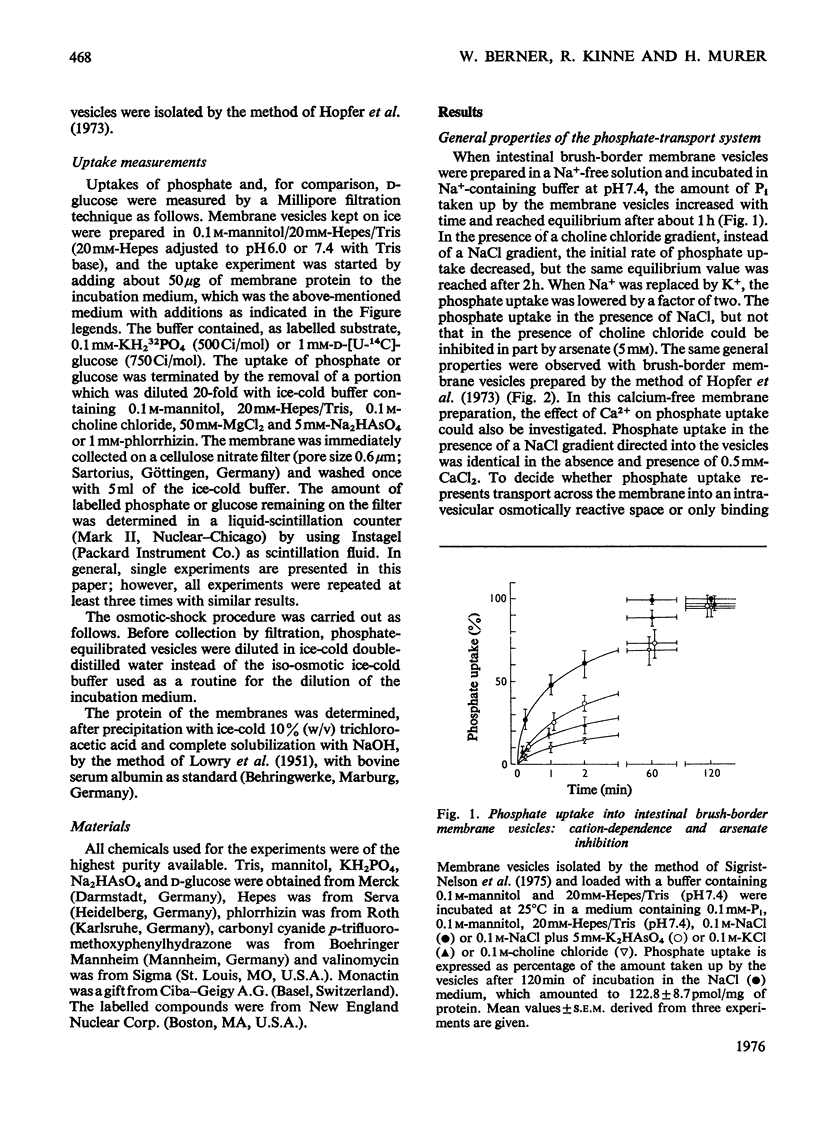
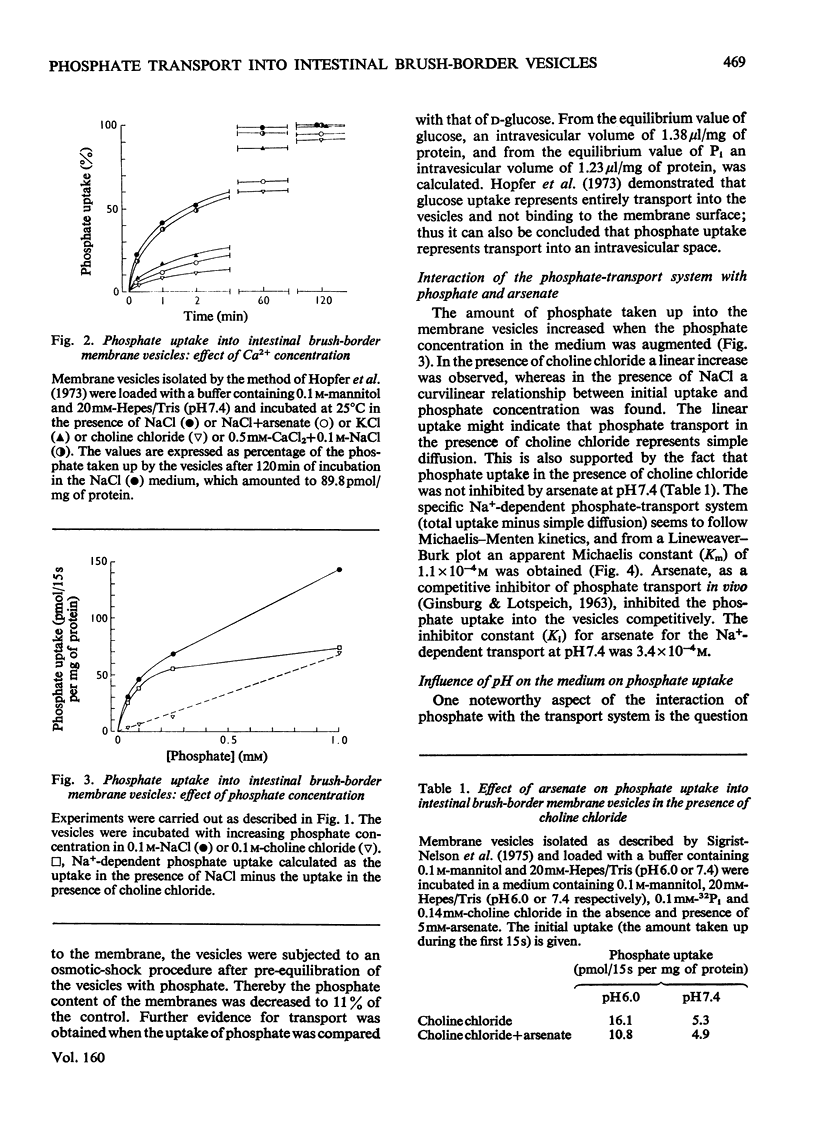
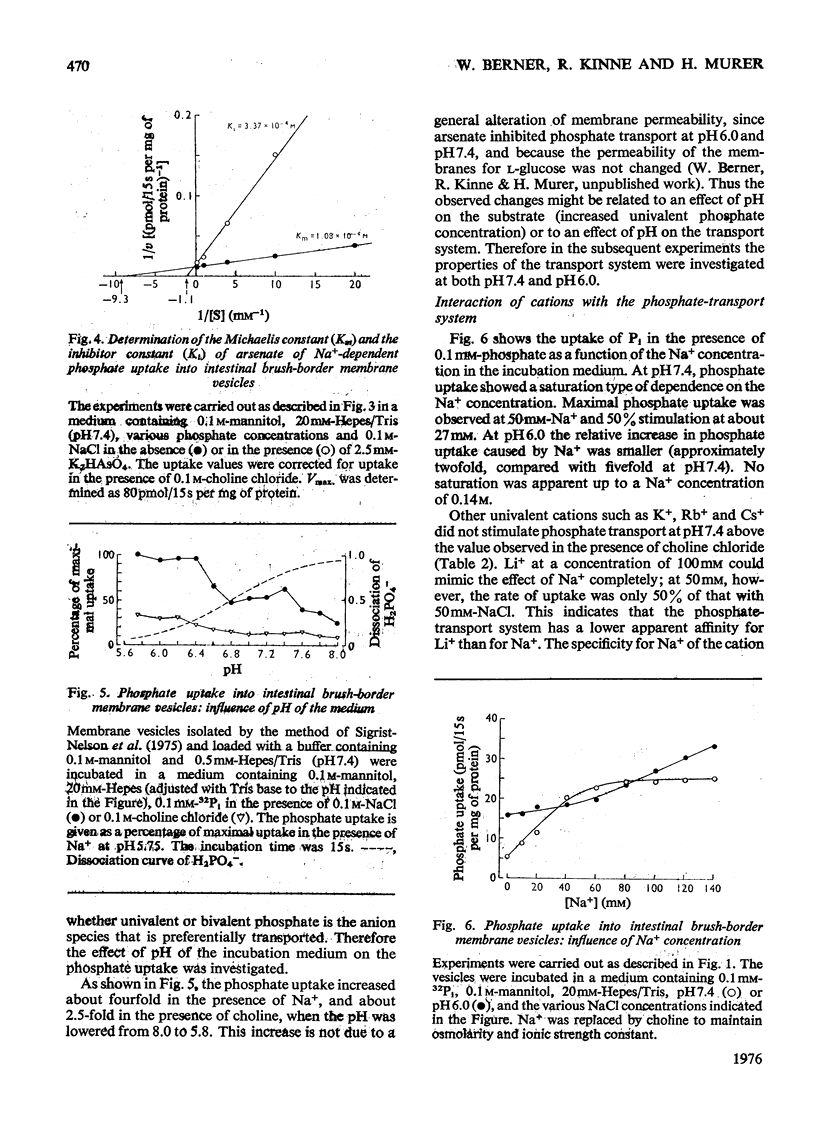
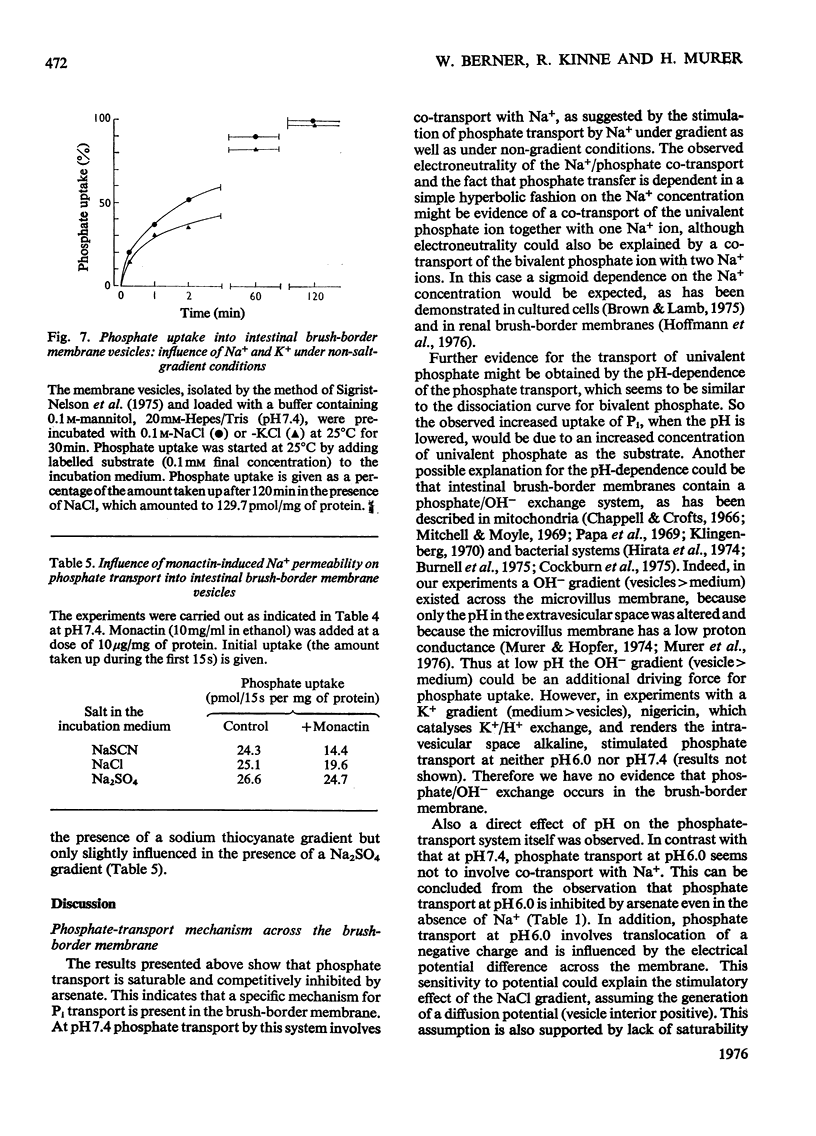
Uptake of Pi into brush-border membrane vesicles isolated from rat small intestine was investigated by a rapid filtration technique. The following results were obtained. 1. At pH 7.4 in the presence of a NaCl gradient across the membrane (sodium concentration in the medium higher than sodium concentration in the vesicles), phosphate was taken up by a saturable transport system, which was competitively inhibited by arsenate. Phosphate entered the same osmotically reactive space as D-glucose, which indicates that transport into the vesicles rather than binding to the membranes was determined. 2. The amount of phosphate taken up initially was increased about fourfold by lowering the pH from 7.4 to 6.0.3. When Na+ was replaced by K+, Rb+ or Cs+, the initial rate of uptake decreased at pH 7.4 but was not altered at pH 6.0.4. Experiments with different anions (SCN-,Cl-, SO42-) and with ionophores (valinomycin, monactin) showed that at pH 7.4 phosphate transport in the presence of a Na+ gradient is almost independent of the electrical potential across the vesicle membrane, whereas at pH 6.0 phosphate transport involves the transfer of negative charge. It is concluded that intestinal brush-border membranes contain a Na+/phosphate co-transport system, which catalyses under physiological conditions an electroneutral entry of Pi and Na+ into the intestinal epithelial cell. In contrast with the kidney, probably univalent phosphate and one Na+ ion instead of bivalent phosphate and two Na+ ions are transported together.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann K., de Rouffignac C., Roinel N., Rumrich G., Ullrich K. J. Renal phosphate transport: inhomogeneity of local proximal transport rates and sodium dependence. Pflugers Arch. 1975;356(4):287–298. doi: 10.1007/BF00580003. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., John P., Whatley F. R. Phosphate transport in membrane vesicles of Paracoccus denitrificans. FEBS Lett. 1975 Oct 15;58(1):215–218. doi: 10.1016/0014-5793(75)80262-6. [DOI] [PubMed] [Google Scholar]
- Cockburn M., Earnshaw P., Eddy A. A. The stoicheiometry of the absorption of protons with phosphate and L-glutamate by yeasts of the genus Saccharomyces. Biochem J. 1975 Mar;146(3):705–712. doi: 10.1042/bj1460705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers J., Murer H., Kinne R. Phenylalanine uptake in isolated renal brush border vesicles. Biochim Biophys Acta. 1976 Apr 5;426(4):598–615. doi: 10.1016/0005-2736(76)90124-3. [DOI] [PubMed] [Google Scholar]
- Frömter E., Rumrich G., Ullrich K. J. Phenomenologic description of Na+, Cl- and HCO-3 absorption from proximal tubules of rat kidney. Pflugers Arch. 1973 Oct 22;343(3):189–220. doi: 10.1007/BF00586045. [DOI] [PubMed] [Google Scholar]
- GINSBURG J. M., LOTSPEICH W. D. INTERRELATIONS OF ARSENATE AND PHOSPHATE TRANSPORT IN THE DOG KIDNEY. Am J Physiol. 1963 Oct;205:707–714. doi: 10.1152/ajplegacy.1963.205.4.707. [DOI] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. Intestinal transport of phosphate: action of vitamin D, calcium, and potassium. Am J Physiol. 1961 Dec;201:1007–1012. doi: 10.1152/ajplegacy.1961.201.6.1007. [DOI] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. Sodium, potassium, and intestinal transport of glucose, 1-tyrosine, phosphate, and calcium. Am J Physiol. 1963 Jul;205:107–111. doi: 10.1152/ajplegacy.1963.205.1.107. [DOI] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Energy coupling in membrane vesicles of Escherichia coli. I. Accumulation of metabolites in response to an electrical potential. J Biol Chem. 1974 May 10;249(9):2939–2945. [PubMed] [Google Scholar]
- Hoffmann N., Thees M., Kinne R. Phosphate transport by isolated renal brush border vesicles. Pflugers Arch. 1976 Mar 30;362(2):147–156. doi: 10.1007/BF00583641. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Klingenberg M. Mitochondria metabolite transport. FEBS Lett. 1970 Feb 16;6(3):145–154. doi: 10.1016/0014-5793(70)80044-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Murer H., Hopfer U. Demonstration of electrogenic Na+-dependent D-glucose transport in intestinal brush border membranes. Proc Natl Acad Sci U S A. 1974 Feb;71(2):484–488. doi: 10.1073/pnas.71.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne-Saffran E., Kinne R. Glucose transport in isolated brush-border and lateral-basal plasma-membrane vesicles from intestinal epithelial cells. Biochim Biophys Acta. 1974 Apr 29;345(2):170–179. doi: 10.1016/0005-2736(74)90256-9. [DOI] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976 Mar 15;154(3):597–604. [PMC free article] [PubMed] [Google Scholar]
- Papa S., Lofrumento N. E., Loglisci M., Quagliariello E. On the transport of inorganic phosphate and malate in rat-liver mitochondria. Biochim Biophys Acta. 1969 Oct 21;189(2):311–314. doi: 10.1016/0005-2728(69)90060-7. [DOI] [PubMed] [Google Scholar]
- Rose R. C., Schultz S. G. Studies on the electrical potential profile across rabbit ileum. Effects of sugars and amino acids on transmural and transmucosal electrical potential differences. J Gen Physiol. 1971 Jun;57(6):639–663. doi: 10.1085/jgp.57.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Siegenthaler P. A., Belsky M. M., Goldstein S. Phosphate uptake in an obligately marine fungus: a specific requirement for sodium. Science. 1967 Jan 6;155(3758):93–94. doi: 10.1126/science.155.3758.93. [DOI] [PubMed] [Google Scholar]
- Sigrist-Nelson K., Murer H., Hopfer U. Active alanine transport in isolated brush border membranes. J Biol Chem. 1975 Jul 25;250(14):5674–5680. [PubMed] [Google Scholar]
- Taylor A. N. In vitro phosphate transport in chick ileum: effect of cholecalciferol, calcium, sodium and metabolic inhibitors. J Nutr. 1974 Apr;104(4):489–494. doi: 10.1093/jn/104.4.489. [DOI] [PubMed] [Google Scholar]


